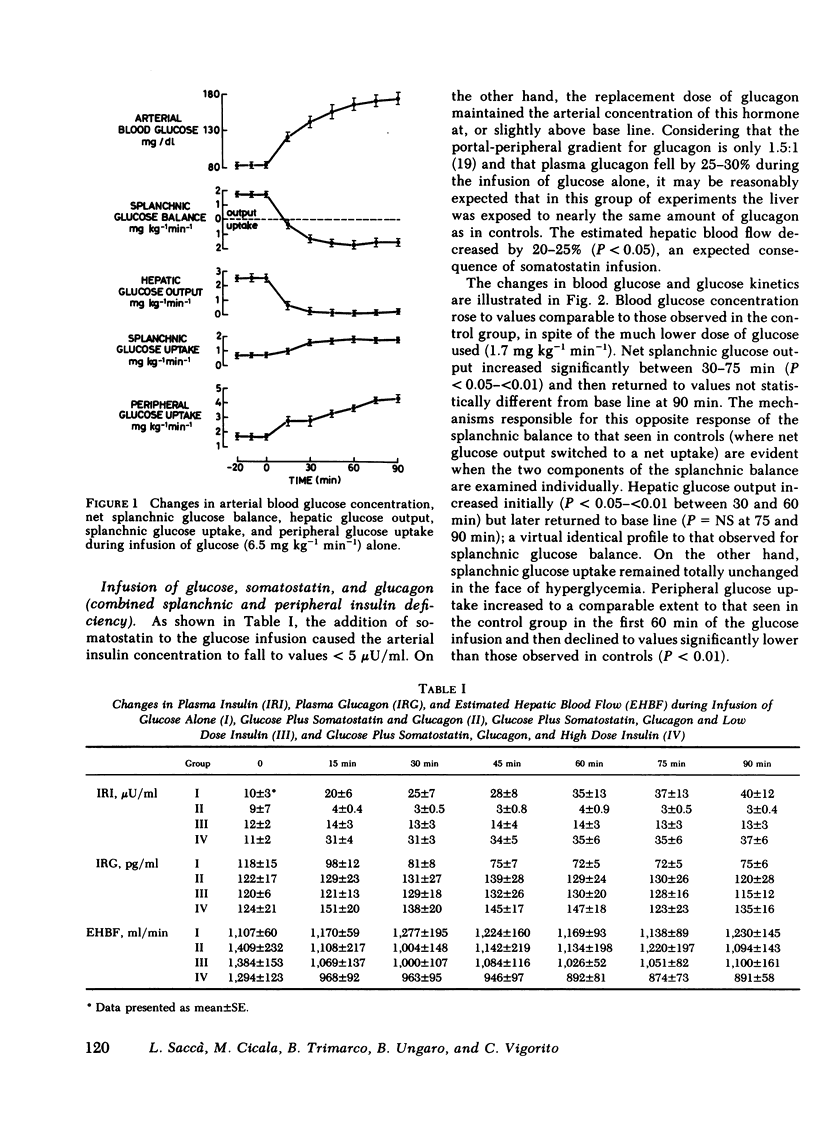
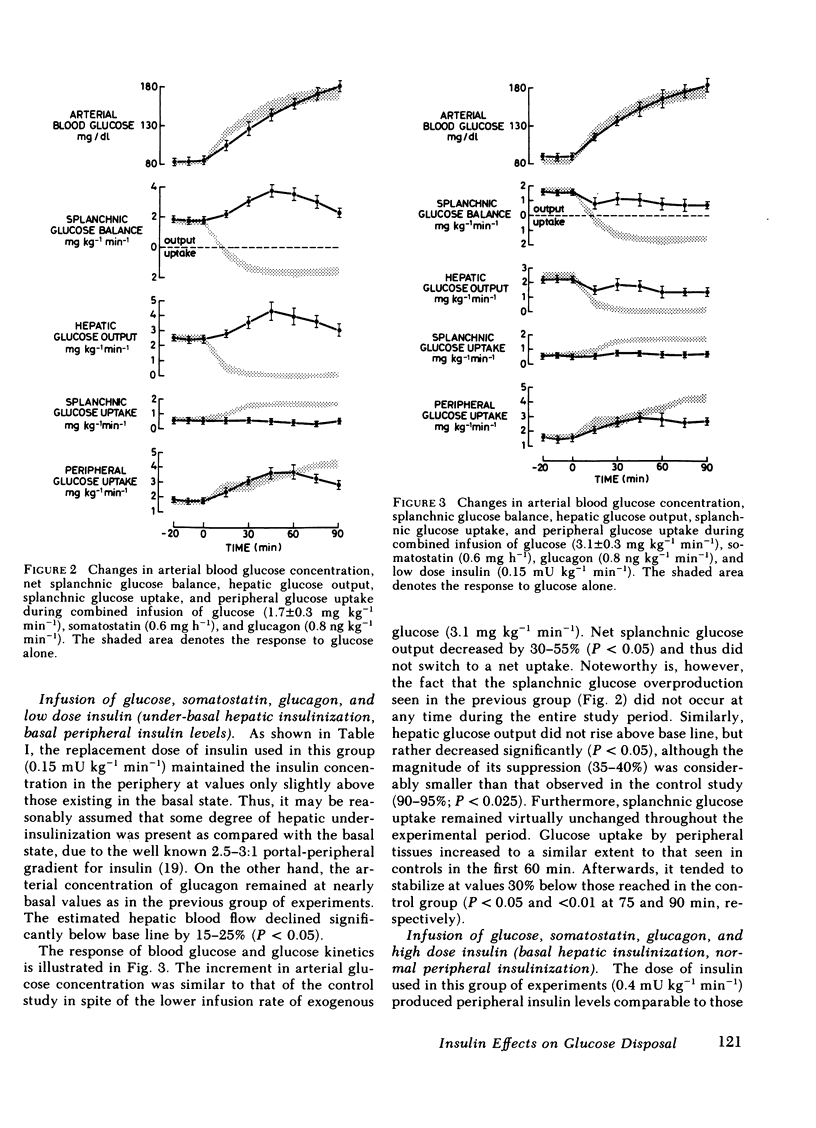
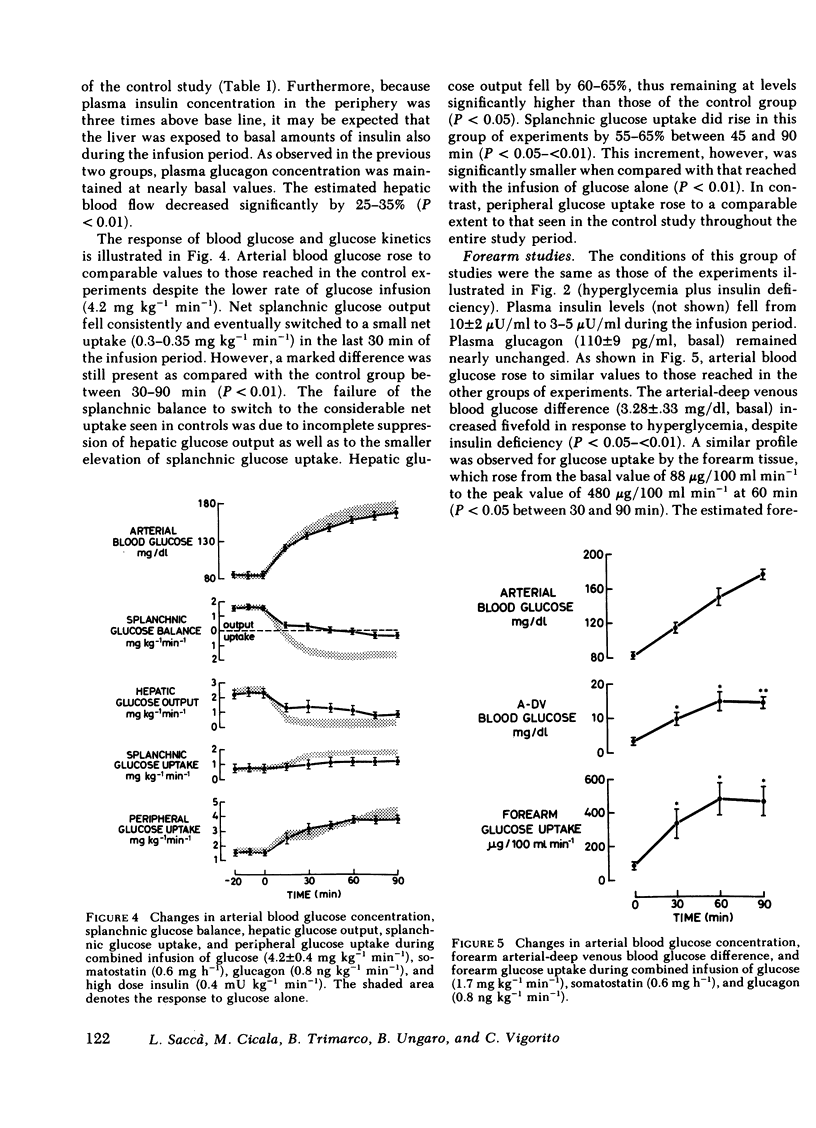
Abstract
The present study was designed to investigate the mechanisms by which insulin regulates the disposal of an intravenous glucose load in man. A combined tracer-hepatic vein catheter technique was used to quantitate directly the components of net splanchnic glucose balance (NSGB), i.e., splanchnic glucose uptake and hepatic glucose output, and peripheral (extrasplanchnic) glucose uptake. Four different protocols were performed: (a) intravenous infusion of glucose alone (6.5 mg kg−1 min−1) for 90 min (control group); (b) glucose plus somatostatin (0.6 mg/h) and glucagon (0.8 ng kg−1 min−1; (c) glucose plus somatostatin, glucagon, and insulin (0.15 mU kg−1 min−1); and (d) glucose plus somatostatin, glucagon, and insulin (0.4 m U kg−1 min−1). In groups 2-4, arterial blood glucose was raised to comparable levels to those of controls (≃170 mg/dl) by a variable glucose infusion. In the control group, plasma insulin levels reached 40 μU/ml at 90 min. NSGB switched from a net output of 1.71±0.13 to a net uptake of 1.5-1.6 mg kg−1 min−1 due to a 90-95% suppression of hepatic glucose output (P < 0.01) and a 105-130% elevation of splanchnic glucose uptake (from 0.78±0.13 to 1.6-1.8 mg kg−1 min−1; P < 0.01). Peripheral glucose uptake rose by 150-160% (P < 0.01). In group 2, plasma insulin fell to <5 μU/ml. Net splanchnic glucose output initially rose twofold but later returned to basal values. This response was entirely accounted for by similar changes in hepatic glucose output since splanchnic glucose uptake remained totally unchanged in spite of hyperglycemia. In contrast, peripheral glucose uptake rose consistently by 100% (P < 0.01) despite insulin deficiency. In an additional group of experiments, glucose metabolism by the forearm muscle tissue was quantitated during identical conditions to those of group 2 (hyperglycemia plus insulin deficiency). Both the arterial-deep venous blood glucose difference and forearm glucose uptake increased markedly by 300-400% (P < 0.05 - <0.01). In group 3, plasma insulin was maintained at near-basal, peripheral levels (12-14 μU/ml). Hepatic glucose output decreased slightly by 35-40% (P < 0.05) while splanchnic glucose uptake remained unchanged. Consequently, the net glucose overproduction seen in group 2 was totally prevented although NSGB still remained as a net output. In group 4, peripheral insulin levels were similar to those of the control group (35-40 μU/ml). The suppression of hepatic glucose output was more pronounced (60-65%) and splanchnic glucose uptake rose consistently by 65% (P < 0.01). Consequently, NSGB did not remain as a net output but eventually switched to a small uptake (0.3 mg kg−1 min−1). Peripheral glucose uptake rose to the same extent as in controls.
It is concluded that: (a) the suppressive effect of hyperglycemia on hepatic glucose output is strictly dependent on the degree of hepatic insulinization; (b) insulin plays an essential role in promoting splanchnic glucose uptake after an intravenous glucose load whereas hyperglycemia per se is totally unable to activate this process; (c) peripheral glucose uptake is markedly stimulated by hyperglycemia even in the face of insulin deficiency. Direct evidence also demonstrates that the skeletal muscle is involved in this response. Our data, thus, indicate that insulin rather than hyperglycemia regulates splanchnic glucose disposal in man. On the other hand, hyperglycemia per se appears to be an important regulator of glucose disposal by peripheral tissues.
Full text
PDF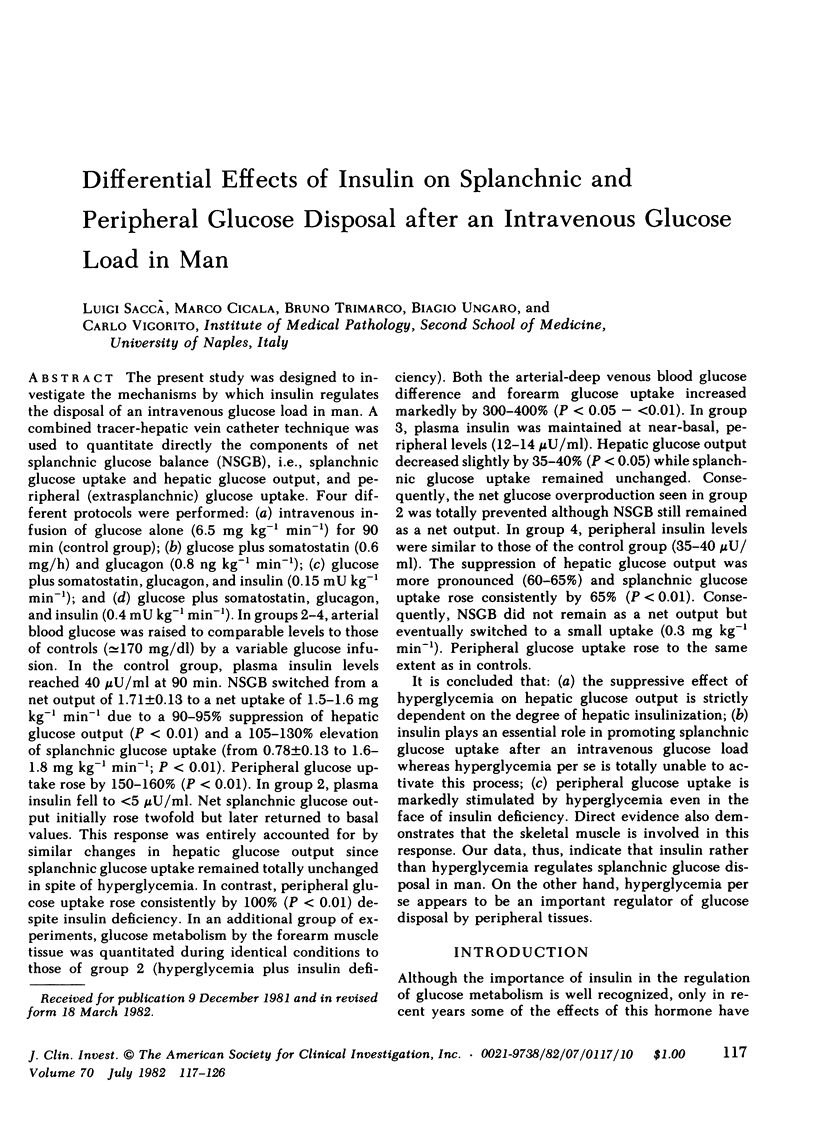






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., ZIERLER K. L., ANDERSON H. M., STAINSBY W. N., CADER G., GHRAYYIB A. S., LILIENTHAL J. L., Jr Measurement of blood flow and volume in the forearm of man; with notes on the theory of indicator-dilution and on production of turbulence, hemolysis, and vasodilatation by intra-vascular injection. J Clin Invest. 1954 Apr;33(4):482–504. doi: 10.1172/JCI102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D., Jr, Terris S., Steiner D. F. Characterization of insulin-like actions of anti-insulin receptor antibodies. Effects on insulin binding, insulin degradation, and glycogen synthesis in isolated rat hepatocytes. J Biol Chem. 1980 May 10;255(9):4028–4034. [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo R. J., Bergman R. N., Marsh D. J., Yates F. E. Dynamics of glucose autoregulation in the isolated, blood-perfused canine liver. Am J Physiol. 1974 Jul;227(1):209–217. doi: 10.1152/ajplegacy.1974.227.1.209. [DOI] [PubMed] [Google Scholar]
- Buschiazzo H., Exton J. H., Park C. R. Effects of glucose on glycogen synthetase, phosphorylase, and glycogen deposition in the perfused rat liver. Proc Natl Acad Sci U S A. 1970 Feb;65(2):383–387. doi: 10.1073/pnas.65.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Caldwell M. D., Dietz M. R., Exton J. H., Crofford O. B. The effect of somatostatin on glucose uptake and production by rat tissues in vitro. Diabetes. 1977 Aug;26(8):740–748. doi: 10.2337/diab.26.8.740. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Chiasson J. L., Liljenquist J. E., Jennings A. S., Keller U., Lacy W. W. The role of insulin and glucagon in the regulation of basal glucose production in the postabsorptive dog. J Clin Invest. 1976 Dec;58(6):1407–1418. doi: 10.1172/JCI108596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Gusberg R., Hendler R., Gump F. E., Kinney J. M., Mulrow P. J. Concentrations of glucagon and the insulin:glucagon ratio in the portal and peripheral circulation. Proc Soc Exp Biol Med. 1974 Oct;147(1):88–90. doi: 10.3181/00379727-147-38286. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Glinsmann W. H., Hern E. P., Lynch A. Intrinsic regulation of glucose output by rat liver. Am J Physiol. 1969 Apr;216(4):698–703. doi: 10.1152/ajplegacy.1969.216.4.698. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEEVY C. M., MENDENHALL C. L., LESKO W., HOWARD M. M. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962 May;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- Madison L. L. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969 Mar;123(3):284–292. [PubMed] [Google Scholar]
- Miller T. B., Jr, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem. 1973 May 25;248(10):3483–3488. [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Saccà L., Trimarco B., Perez G., Rengo F. Studies on the mechanism underlying the influence of alanine infusion on glucose dynamics in the dog. Diabetes. 1977 Apr;26(4):262–270. doi: 10.2337/diab.26.4.262. [DOI] [PubMed] [Google Scholar]
- Saccà L., Vigorito C., Cicala M., Ungaro B., Sherwin R. S. Mechanisms of epinephrine-induced glucose intolerance in normal humans. J Clin Invest. 1982 Feb;69(2):284–293. doi: 10.1172/JCI110451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà L., Vitale D., Cicala M., Trimarco B., Ungaro B. The glucoregulatory response to intravenous glucose infusion in normal man: roles of insulin and glucose. Metabolism. 1981 May;30(5):457–461. doi: 10.1016/0026-0495(81)90180-3. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Liljenquist J. E., Williams P. E., Lacy W. W., Cherrington A. D. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest. 1978 Aug;62(2):487–491. doi: 10.1172/JCI109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R., Rostami H., Altszuler N. A two-compartment calculator for the dog glucose pool in the nonsteady state. Fed Proc. 1974 Jul;33(7):1869–1876. [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Avruch J. Insulin regulation of hepatic glycogen synthase and phosphorylase. Biochemistry. 1978 Feb 7;17(3):406–410. doi: 10.1021/bi00596a004. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. ROLES OF INSULIN AND GROWTH HORMONE, BASED ON STUDIES OF FOREARM METABOLISM IN MAN. Medicine (Baltimore) 1963 Nov;42:385–402. doi: 10.1097/00005792-196311000-00002. [DOI] [PubMed] [Google Scholar]


