Abstract
Background and Objectives
β3-deficient(−/−) megakaryocytes were modified by human β3-lentivirus transduction and transplantation to express sufficient levels of a C560Rβ3 amino acid substitution to investigate how an activated αIIbβ3 conformation affects platelets in vivo in mice.
Patient/Methods
Identical to our previous report of a R560β3 mutation in a patient with Glanzmann thrombasthenia, R560β3 murine platelets spontaneously bound antibody that only recognizes activated αIIbβ3 bound to its ligand, fibrinogen.
Results
With this murine model, we now show that αIIb-R560β3 mutation mediated continuous binding of fibrinogen occurs in the absence of P-selectin surface expression indicating that the integrin was in an active conformation, although the platelets circulated in a quiescent manner. Remarkably, only 35% of R560β3 “mutant” mice survived six months after transplant while 87% of C560β3 “wildtype” mice remained alive. Pathological examination revealed that R560β3 mice had enlarged spleens with extramedullary hematopoiesis and increased hemosiderin indicating haemorrhage. R560β3 megakaryocytes and platelets displayed abnormal morphology and irregular granule distribution. Interestingly, R560β3 washed platelets could aggregate upon simultaneous addition of fibrinogen and physiological agonists, but aggregation failed when platelets were exposed to fibrinogen before activation in vitro and in vivo.
Conclusions
Thus, the results demonstrate that continuous occupancy of αIIbβ3 with fibrinogen disrupts platelet structure and function leading to haemorrhagic death consistent with Glanzmann thrombasthenia rather than a thrombotic state.
Keywords: Integrin αIIbβ3, Lentivirus Gene Transfer, Hematopoietic Stem cells, Megakaryocytes, Platelets, Glanzmann Thrombasthenia
Introduction
Glanzmann thrombasthenia (GT) is a rare bleeding disorder resulting from ITGA2B or ITGB3 gene defects leading to integrin αIIbβ3 abnormalities that prevent platelet aggregation.[1] Previous reports showed that altered αIIbβ3 conformation permitted spontaneous binding of activation-dependent monoclonal antibodies (Ab) or its principle ligand, fibrinogen, with high-affinity. Enigmatically, this situation does not confer a thrombotic but a prolonged bleeding phenotype and abnormal platelet morphology. For example, activating mutations affecting transmembrane or cytoplasmic domains perturbed the R995αIIb-D723β3 salt-bridge leading to a GT-like phenotype and/or macrothrombocytopenia.[2–5] Genetic mutations (e.g. C542R, C549R, C560R, C560F) disrupting disulfides within the ITGB3 extracellular EGF domains also led to reduced expression of a constitutively active αIIbβ3 in GT patients.[6–12] The present study was established to gain an in-depth in vivo analysis of the physiological effect of a single amino acid substitution of C560Rβ3 that places αIIbβ3 in an active conformation with a high-affinity for fibrinogen.
Materials and Methods
Mice
β3(−/−) mice (R.O.Hynes, Howard Hughes Medical Institute,Cambridge,MA) studies approved by Medical College of Wisconsin’s Animal Care Committee.
Lentivirus
β3-WPTS lentivirus encoding C560β3 was described.[13] cDNA encoding R560β3 was constructed by mutagenesis of C560β3 cDNA at g1776 T>C as described.[8]
β3(−/−) Murine Bone Marrow Isolation, Transduction and Transplantation (tx)
Each mouse received lethal irradiation prior to tx with 9.0×106 β3(−/−) bone marrow cells untransduced (as a negative control) or transduced with β3-lentivirus.[13] Survival studies represent observations of 23 C560β3 tx mice, 20 R560β3 tx mice and 10 β3(−/−) mice tx with untransduced β3(−/−) bone marrow.
Blood Collection
Murine tail-vein bled into 0.13M sodium citrate anti-coagulant as described.[13] Blood cells were counted on a Vet ABC hematology analyzer (scil animal care company, Gurnee, IL). Platelets were isolated with Fico/Lite™ (Atlanta Biologicals, Norcross, GA) as described.[13]
Antibodies
PE-conjugated Ab to human β3, and FITC-conjugated Ab to murine αIIb (BD Biosciences, San Jose, CA); monoclonal Ab (D3) to the high-affinity conformation of human β3 (L. Jennings, University of Tennessee, Memphis, TN)[14]; FITC-rabbit anti-murine fibrinogen Ab and FITC-rat anti-murine P-selectin (Emfret Analytics, Würzburg, Germany); FITC-F(ab’)2 goat anti-murine IgG Fc (Jackson ImmunoResearch, West Grove, PA).
Flow Cytometry
Platelets labeled with PE-and/or FITC-conjugated Abs were analyzed as described.[13] Some platelets were pretreated with the fibrinogen mimetic peptide, GRGDW (2mM). Cytofix™ and PERM/WASH™ reagents (BD Biosciences) were used for intracellular detection of fibrinogen.
Tissue Analysis
Organs were isolated from paraformaldehyde perfused mice as described.[15] Tissue sections were stained with hematoxylin and eosin as described.[16] Bone sections were analyzed with αIIb(1:325) or β3(1:2,000) 1°Ab (P.Newman, Blood Research Institute,Milwaukee,WI) and biotinylated-goat-anti-rabbit 2°Ab as described.[15]
Platelet Morphology
Wright/Giemsa stained blood smears underwent microscopic analysis.
Electron Microscopy (EM)
Fixed platelet sections stained with uranyl acetate and osmium and frozen-thin sections were prepared and analyzed by EM as described.[17] Platelets sections on collodion-coated nickel grids were incubated with rabbit-anti-murine Ab to fibrinogen (10µg/ml, Emfret Analytics, Würzburg, Germany) and goat-anti-rabbit 2°Ab adsorbed onto AuroProbe EM G10 gold particles as described.[17]
Platelet Aggregation
PAP-4 platelet aggregation profile (BioData Corporation) analysis was performed as described.[13] Fibrinogen (0.15mg/ml; Enzyme Research Laboratories, South Bend, IN) was added alone or simultaneously with platelet activation agonist, 100µM adenosine diphosphate (ADP) alone or in mixture with 20µM epinephrine (BioData Corporation, Horsham, PA) and 250µM of thrombin receptor activating-peptide (PAR4,GYPGKF-NH2).
Pulmonary Thrombi
Mice were injected intravenously with 0.35mg ADP/g, sacrificed after 10 minutes, lung tissue isolated and Gomori’s trichrome stained lung sections were analyzed for platelet aggregation as described.[13]
Results
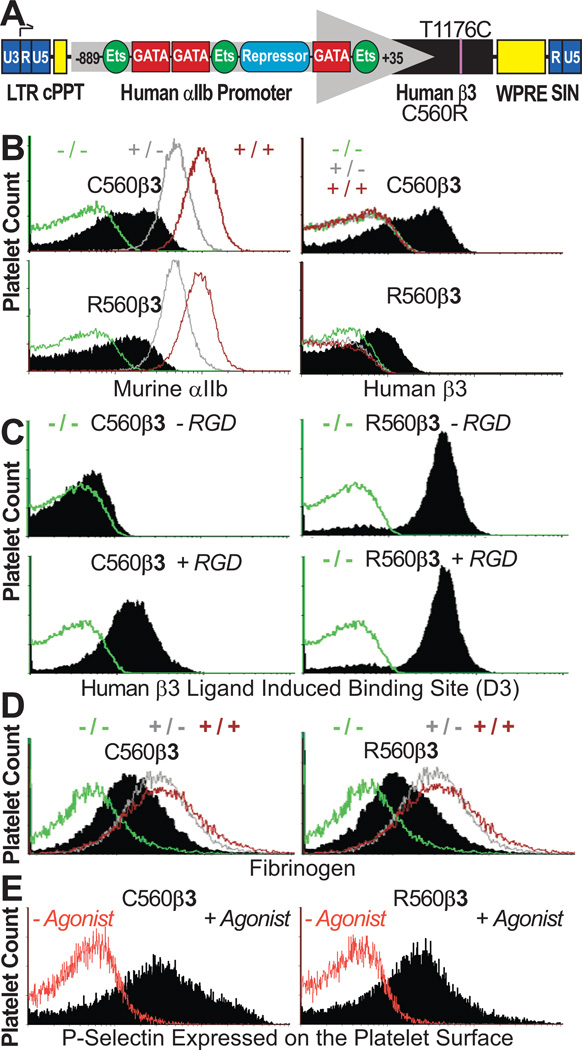
We previously observed restored platelet function following human β3-lentivirus transduction and transplantation (tx) of β3(−/−) murine bone marrow into β3(−/−) littermates.[13] Currently, β3(−/−) marrow was transduced with a C560R “activated” form of human β3 to investigate the effect of this mutation on integrin function and platelet survival. As shown, the lentivirus vector contained GATA-1 and Ets elements of the human αIIb gene promoter essential for high level transcription of either form of β3 within the megakaryocyte lineage (Fig1A). Immunocytometry (Fig1B) showed that a typical tx recipient had platelets that expressed moderate levels of endogenous αIIb complexed with R560β3 on murine platelets. Tx mice that expressed human C560β3 at comparable receptor levels on their platelet surface were used as positive controls to permit objective comparison of receptor and platelet function for this study (Fig 1B). As anticipated, platelets expressing C560β3 (Fig1C;Left), only bound the “D3” Ab when its epitope was exposed on activated αIIbβ3 bound to a fibrinogen mimetic peptide (+RGD), while R560β3 spontaneously bound “D3” (±RGD) (Fig1C;Right).[14] Identical results have been shown for human GT platelets that naturally express moderate levels of R560β3.[8] This outcome suggests feasibility for this murine model to uncover the pathophysiologic consequences of platelets expressing R560β3. Immunocytometry of fixed/permeabilized platelets (Fig1D) indicated that surface expression of C560β3 and R560β3 restored the ability of β3(−/−) tx platelets to bind fibrinogen and execute receptor-mediated endocytosis of fibrinogen. Immunocytometry (Fig1E;+agonist) showed that R560β3 platelets circulated normally in a quiescent “unactivated” manner because the α-granule protein, P-selectin, appeared only on the surface of platelets treated with agonists of platelet activation. This outcome is consistent with the observation that outside-in signaling (e.g. FAK phosphorylation) was not spontaneously induced by the C506R amino acid substitution within human platelets, which retained their ability to spread on a Fibrinogen-covered surface.[8] Thus this aspect was not studied further.
Figure 1. C560β3 & R560β3 tx Recipient Platelets Expressed αIIbβ3.
(A) β3-WPT Diagram. Viral 3'-long terminal repeat (LTR) enhancer/promoter was removed to self-inactivate vector (SIN) and αIIb gene promoter (nucleotides −889 to +35) directed megakaryocyte-specific transcription of cDNA with either nucleotide C1776T encoding a Cys (C) or Arg (R) at amino acid 560 of human β3. αIIb promoter binds GATA and Ets for high-level gene transcription in megakaryocytes while a repressor inhibits gene transcription in other lineages. The woodchuck hepatitis virus postregulatory element (WPRE) and central polypurine tract (cPPT) enhance transgene expression.
(B) αIIbβ3 was Detected on Platelets Tranduced with C560Rβ3. Left, immunocytometric analysis showed the mean fluorescence intensity (MFI) for αIIb(+) platelets in C560Rβ3 mice (black) appeared at moderate levels compared to β3(−/− green; +/− grey; +/+ brown) controls. Right, only C560Rβ3 tx mice displayed detectable levels of human β3 on platelets (black) compared to MFI levels of platelet controls. Results represent 15 experiments analyzing platelets from β3(−/−,+/−,+/+) controls and 23 C560β3 mice and 20 R560β3.
(C) αIIbR560β3 was Activated Continuously. Left, immunocytometric analysis revealed that C560β3 platelets (shaded peak) bound an Ab “D3” (recognizing an epitope exposed on high-affinity conformation of human β3 bound to ligand) only in the presence of a fibrinogen mimetic peptide containing Arg-Gly-Asp (+RGD). Right, in contrast, R560β3 platelets bound “D3” in the absence (−RGD) and presence (+RGD) of the peptide indicating that αIIbR560β3 was in an activated conformation. Negative control β3(−/−) platelets (green) failed to bind “D3”. Results represent four experiments using platelets from β3(−/−,+/−,+/+) controls and three C560β3 and R560β3 mice.
(D) Immunocytometric Analysis of Fixed/Permeabilized Platelets. Fibrinogen was absent from β3(−/−) platelets, although β3(+/−;+/+) controls displayed appreciable levels of platelet fibrinogen. C560Rβ3 platelets bound, endocytosed, and stored fibrinogen (shaded peak). Results represent ten experiments using β3(−/−,+/−,+/+) controls and three C560β3 and R560β3 mice.
(E) Immunocytometric Analysis Detected Activation of Platelets Treated with a mixture of ADP, epinephrine, and PAR4. The α-granule protein, P-selectin, was detected on the surface only after activation of C560β3 and R560β3 platelets (shaded peak) demonstrating that platelets expressing either form of β3 circulated normally in a quiescent manner. Results represent three experiments using platelets from β3(−/−,+/−,+/+) controls and three C560β3 and R560β3 mice.
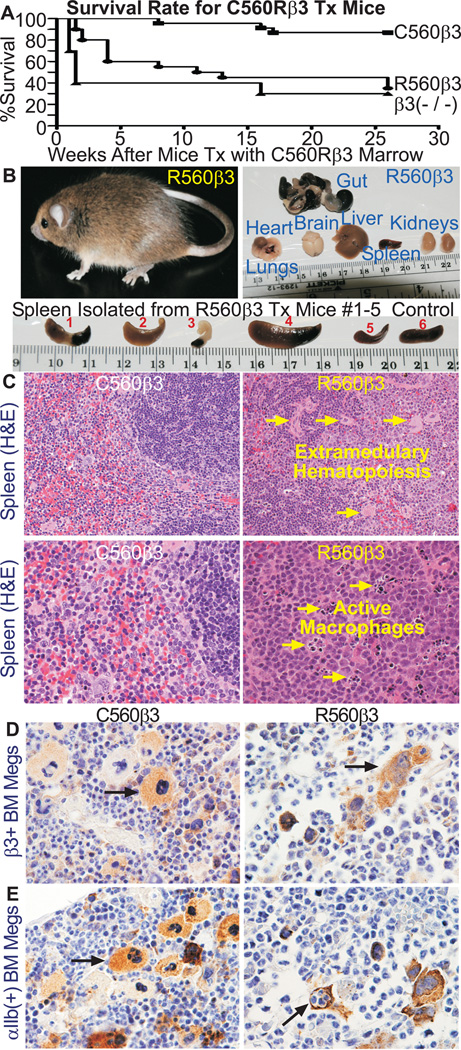
Of Significance, 87% of C560β3 mice survived at least 26 weeks after tx (Fig2A). In contrast, 50% of R560β3 mice died by week 11 and only 35% survived until the experimental endpoint. This remarkable result resembled the 30% survival rate at the experimental endpoint recorded for β3(−/−) mice tx with non-transduced β3(−/−) marrow. Note that post mortem examination was not performed on mice tx with non-transduced marrow that died within 2 weeks after tx. These events were attributed to hemorrhage due to the inability of β3(−/−) platelets to repair vascular injury related to intense lethal irradiation of the pre-tx conditioning regimen. In contrast, several (≈50%) of the R560β3 tx mice deaths occurred at a more staggered rate over the first 12 weeks after tx. This result indicates that the initial presence of R560β3 may have conferred some ability of R560β3 platelets to alleviate the effect of the pre-tx conditioning regimen. Since the difference in mortality between C560β3 and R560β3 was highly significant (P=0.0002,Gehan-Breslow-Wilcoxon Test), R560β3 mice were examined for a cause of death. Each affected R560β3 mouse displayed a sudden onset of illness characterized by pale extremities (feet, tail, ears) accompanied with lethargy and hunched body usually producing death within 48 hours (Fig2B,Top Left). Autopsy was performed on nine affected R560β3 mice to detect tissue pathology. Gut, heart, lungs, brain, liver, spleen, kidney and bone were analyzed (Top Right). One R560β3 mice displayed gross intestinal bleeding (Gut), a finding confirmed by positive hemoccult tests and histochemical analysis identical to results reported for β3(−/−) mice in hemorrhagic crisis.[18] Four representative R560β3 mice demonstrated that the spleen appeared abnormally large or necrotic (Fig2B;Bottom,#1–4). Histochemistry further revealed that R560β3 spleen displayed extramedullary hematopoiesis (Fig2C;Top Arrows) and activated macrophages were present (Fig2C;Bottom Arrows). This result is consistent with spleens isolated from β3(−/−) mice affected with prolonged bleeding associated with GT.[18]
Figure 2. R560β3 Mice had a Significantly High Mortality Rate.
(A) Graph of Survival Rates for C560β3 and R560β3 tx Mice. Results demonstrate that 87% of C560β3 mice survived for at least 26 weeks post tx (n=23), while in contrast, 50% R560β3 animals died by 11 weeks after tx and only 35% of R560β3 mice survived until the experimental endpoint (n=20). Results with β3(−/−) mice tx with untransduced β3(−/−) bone marrow as a negative control (n=10) revealed that nearly 60% of β3(−/−) tx mice died by 1.5 weeks after lethal irradiation and bone marrow tx. Although, the endpoint survival of R560β3 mice was nearly identical to the 30% survival rate of β3(−/−) mice tx with untransduced marrow.
(B) Tissue Analysis Revealed R560β3 Mice in Pathological Crisis. Top Left, shown is one of thirteen R560β3 mice that displayed pathological crisis, which usually resulted in death 48 hours after sudden onset of a hunched appearance and pale-white extremities. Top right, intact organs were isolated from R560β3 mice in pathological crisis. Analysis showed heart, lungs, brain, liver, and kidneys appeared normal in all mice, although gut appeared black and necrotic in one mouse which is consistent with results from hemocrlt counts indicating severe gastrointestinal bleeding. Tissues were fixed, sectioned, stained and analyzed as described in methods from nine R560β3 mice in crisis, nine R560β3 mice that appeared well, and three each of C560β3 and β3(−/−,+/−,+/+) controls. Bottom, R560β3 mice spleens (#1–5) appeared mostly abnormal in size and shape (large and/or necrotic) compared to a healthy spleen from a normal control (#6). Shown is spleen from five of nine R560β3 affected mice that underwent autopsy and tissue analysis.
(C) R560β3 Mice had Abnormal Spleens. Top Left, H&E staining of spleen tissue indicated the C560β3 mice displayed normal spleen morphology at 200× magnification. In contrast (Top Right) extramedullary hematopoiesis (arrows) was detected in spleen sections from several R560β3 mice in crisis. Bottom Left, H&E staining indicated C560β3 mice displayed normal spleen morphology at 400× magnification. Bottom Right, in contrast numerous activated macrophages (arrows) were detected in the process of repair of pathological conditions within R560β3 murine spleens.
(D) R560β3 Megakaryocytes were Shaped Abnormally within the Bone Marrow. Immunohistochemical brown staining with Ab recognizing integirn β3 showed megakaryocytes expressing C560Rβ3 in vivo (Magnification, 400×). Left panel shows that C560β3 megakaryocytes appeared normal in shape and size (arrow), while in contrast, the right panel revealed that R560β3 megakaryocytes appeared abnormal in shape and clustered within the bone marrow (arrow). Results represent observations of bone sections from β3(−/−,+/−,+/+) controls and three mice expressing either C560β3 or R560β3.
(E) R560β3 Megakaryocytes Were in a Pathological State. As described in Part D, an Ab to integrin αIIb showed C560β3 megakaryocytes appeared healthy (top left arrow), while in contrast R560β3 Megakaryocytes appeared abnormal in shape (top right arrow). Interestingly, occasional R560β3 megakaryocytes had engulfed neutrophils by emperiopoiesis (bottom right arrow) indicating that the megakaryocytes were activated atypically. Result represents observations of bone sections from β3(−/−,+/−,+/+) controls and three C560β3 or R560β3 mice. (Magnification, 400×)
Immunohistochemistry of bone with Ab to β3 (Fig2D) and αIIb (Fig2E) showed that C560β3 megakaryocytes appeared normal in size and shape (Left). Yet, R560β3 megakaryocytes were morphologically abnormal and appeared as clusters within the bone (Right Top Arrow). This analysis was not performed with the human GT patient R560β3 megakaryocytes;[8] however, the outcome is consistent with results obtained in previous studies that expressed activating mutations of αIIbβ3 (R995WαIIb,T562Nβ3) on the surface of tissue-cultured cells.[5] Thus, the single amino acid substitution of C560Rβ3 may have also permitted bone marrow megakaryocytes to spontaneously bind fibrinogen and ultimately causing a profound effect on their morphology. Emperiopoiesis of neutrophils by R560β3 megakaryocytes was also detected frequently (Right Bottom Arrow) indicating that the C560Rβ3 amino acid substitution affected the function of bone marrow megakaryocytes.[19, 20]
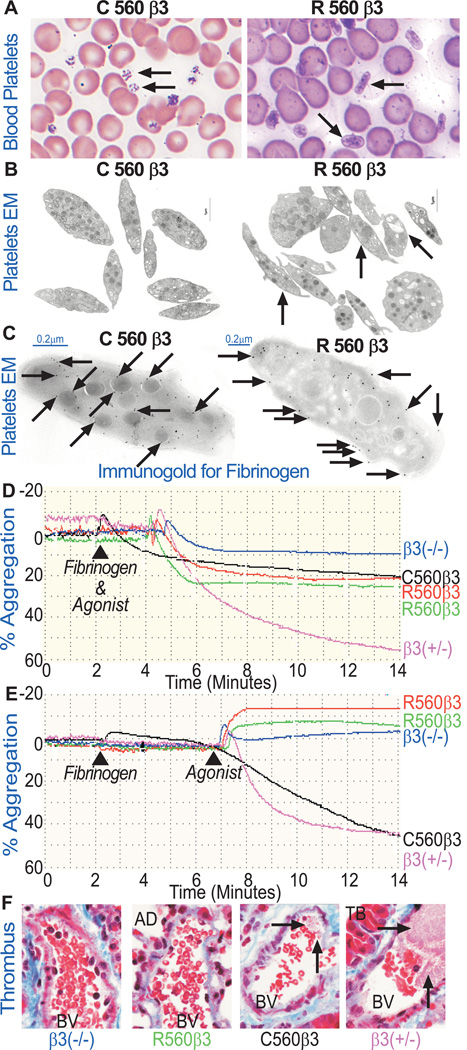
Platelet counts were within normal limits (140–600×103platelets/mm3) since R560β3 showed a mean platelet count of 367±109×103platelets/mm3 for nine mice and C560β3 mice had approximately 432±160×103platelets/mm3 in blood analyzed from fourteen mice. Although, Wright/Giemsa stained peripheral blood smears revealed that R560β3 platelets showed anisotrophy compared to C560β3 platelets (Fig3A, Arrows). Electron microscopic (EM) analysis of platelets revealed that size measurements of ≈100 platelets collected from each tx group suggested that most tx platelets were similar in size, although R560β3 platelets frequently had an increased length to width ratio (SupTable I). Remarkably, EM also showed that R560β3 platelets displayed an abnormal heterogeneous distribution of granules and increased number of vacuoles (Fig3B, Arrows). Thus, R560β3 affected megakaryocytopoiesis and platelet genesis in vivo as is consistent with abnormalities observed with human R560β3 platelets.[8] As anticipated, EM of frozen-thin sections employing immunogold beads (Fig3C) revealed that fibrinogen was localized within α-granules and internal membrane systems of C560β3 platelets (Left Arrows). In contrast, an increased presence of fibrinogen was detected on the surface of R560β3 platelets as well as within the internal membranes (Right Arrows).
Figure 3. R560β3 Platelet Structure, Granular Content, and Function were Abnormal.
(A) Light Microscopic Analysis of Wright/Giemsa Stained Peripheral Blood Smears. C560β3 platelets were normal in shape and size (left arrow). In contrast, R560β3 mice had some abnormally shaped platelets (right arrow). Results represent observations of six experiments using β3(−/−,+/−,+/+) controls and C560β3 or R560β3 tx mice. (Magnification 200×).
(B) Electron Microscopy of Blood Platelets. Ultrathin sections were examined for platelet ultrastructure and granular distribution and content. High magnification images of C560β3 platelets displayed normal size, shape, and granule distribution. In contrast, R560β3 platelets were abnormally shaped with uneven granule and vacuole size and distribution (right arrows). A minimum of 100 sections were examined from each platelet sample. bar=1.0 µm.
(C) Immunogold Labeling to Detect Platelet Fibrinogen by Electron Microscopy. Ultrathin sections of platelets were prepared and examined for surface and granular distribution of murine fibrinogen. Sections were placed on grids that were incubated with a rabbit Ab to murine fibrinogen and a goat anti-rabbit secondary antibody conjugated to (10nm) gold particles (arrows). Shown are representative sections of platelets derived from C560β3 (left panel) and R560β3 (right panel) tx mice depicting mature α-granules, vacuoles, and vesicular canals. These high magnification images showed that platelets expressing C560β3 bound, endocytosed and stored fibrinogen within α-granule in a fairly normal distribution pattern (left arrows). In striking contrast, R560β3 platelets show that fibrinogen was detected in an uneven distribution pattern bound mainly to the platelet surface (right arrows) with some endocytosis of fibrinogen present within canals and α-granules. A minimum of 100 sections were examined from each platelet sample. Isotype-matched IgG was used as a negative control for staining (not shown). Each sample was cut on a minimum of 3 separate occasions with a series of ultrathin sections subjected to immunogold labeling. bar=0.2 µm.
(D) R560β3 Washed Platelets Aggregated with the Simultaneous Addition of Fibrinogen and a Mixture of Activation Agonists (ADP, epinephrine, and PAR4). C560β3 and R560β3 platelets aggregated appreciably in comparison to β3(−/−) negative control platelets, which failed to aggregate. Aggregation of β3(+/−) platelets was used as a positive control using identical conditions. The mean fluorescence intensity (MFI) of FITC-conjugated moAb to αIIb revealed that the receptor levels were similar for tx mice C560β3 (black 7±3%) and R560β3 (red and green 5±2%) compared to β3(−/−,+/−,+/+) controls assigned an arbitrary value of 0%, 50%, and 100%, respectively. Results represent platelet aggregation profiles under constant stirring from five β3(−/−,+/−) controls and eight C560β3 and six R560β3 mice. Slight variations in % aggregation profiles between R560β3 and C560 β3 samples may be due to minor differences in receptor levels.
(E) Aggregation Failed when R560β3 Platelets were Incubated First with Fibrinogen for Four Minutes and Then Treated with Activation Agonists (ADP, epinephrine, and PAR4). Remarkably, with this slight change in protocol, R560β3 platelets changed shape but failed to aggregate following addition of activation agonists. This result suggests that pre-treating platelets with fibrinogen allowed immediate binding of the ligand to its receptor αIIbR560β3, thus saturating them with fibrinogen and preventing platelet-platelet aggregates from forming upon agonist simulation. As anticipated, using identical conditions, β3(−/−) platelets failed to aggregate, while C560β3 and β3(+/−) positive control platelets aggregated appreciably. The MFI at saturating concentrations of moAb to αIIb revealed that the receptor levels were similar for tx mice R560β3 (red 6±1%, green 9±1%), C560β3 (black 10±1%) when compared to β3(−/−,+/−,+/+) controls assigned an arbitrary value of (0%,50%,100%) respectively. Results represent aggregation profiles using constant stirring of platelets in a minimum of two experiments using β3(−/−,+/−,+/+) controls and one C560β3 and three R560β3 mice. Slight variations in % aggregation profiles between the two R560 samples may be due to minor differences in receptor levels, although the overall result of shape change without aggregation is consistent.
(F) In vivo platelet function was examined by light microscopic analysis of fixed lung tissue stained with Trichrome following IV injection of a platelet agonist (ADP) into mice (Magnification, 400×). Platelets that aggregated into a thrombus were not detected within the pulmonary blood vessels (BV) of β3(−/−) negative control and R560β3 tx mice following infusion with 0.35 mg ADP/g body weight. In contrast, C560β3 platelets aggregated appreciably to form thrombi that were detected within the pulmonary blood vessels similar in content to the prominent thrombi that formed in the pulmonary blood vessels (BV) of β3(+/−) controls (see arrows). Trichrome stain differentially displays protein fibers (fibrotic tissue: muscle, collagen fibers, fibrin clots) and erythrocytes to allow identification of platelet aggregates within BV among the terminal bronchiole (TB), and alveolar ducts (AD). This result demonstrates that the single amino acid substitution of C560Rβ3 prevented activated platelets from undergoing normal aggregation in vivo. This result represents the outcome observed after viewing 10 sections of each lung from seven controls (four β3(−/−), three β3(+/−) mice) as well as two C560β3 and two R560β3 experimental mice 5 weeks post tx. The MFI at saturating concentrations of moAb to αIIb revealed that the receptor levels were similar for tx mice R560β3 (6±1%), C560β3 (8±2%) when compared to β3(−/−,+/,+/+) controls assigned an arbitrary value of (0%,50%,100%) respectively.
In vitro analysis of platelet function was performed with Ficoll purified platelets washed in PBS/EDTA to chelate calcium and cause dissociation of the integrin complex prior to incubation in Tyrode’s buffer. The washed platelets were then placed in buffer containing calcium to help the integrin subunits to re-associate on the platelet surface. This strategy revealed that simultaneous treatment of washed platelets with fibrinogen and a mixture of platelet activation agonists induced appreciable aggregation of platelets from C560β3 and R560β3 mice compared to the β3(−/−,+/−) controls (Fig3D). However, pre-incubation of washed platelets with fibrinogen for four minutes followed by agonist stimulation induced R560β3 platelets to change shape but aggregation failed (Fig3E). In contrast, C560β3 platelets aggregated successfully using identical conditions (Fig3E). This outcome suggests that R560β3’s altered conformation induced atypical occupancy of αIIbβ3 with fibrinogen on the surface of resting platelets that prevented aggregation of agonist-activated platelets.
Platelet function was examined in vivo to determine if expression of R560β3 affected platelet aggregation within the dynamic environment of the vasculature. ADP was injected intravenously to induce formation of pulmonary thrombi as previously described.[13, 21] Platelet aggregates were absent within pulmonary blood vessels of β3(−/−) and R560β3 mice (Fig.3F). Although, platelet aggregates were readily detectable within C560β3 and β3(+/−) mice as evidenced by the presence of thrombus formation within the blood vessels (Fig.3F, Arrows). This outcome confirms the basis for a GT phenotype in mice expressing an activated R560 form of β3, which continuously binds fibrinogen.
Discussion
There are 56-paired cysteines clustered within the integrin epidermal growth factor domains (IEGF) of the disulfide-rich core of β3 and these provide structural restraints to β3.[22, 23] In early work, C457Y and C542R mutations in the EGF-1 and EGF-3 domains were shown to prevent more than trace αIIbβ3 expression in platelets and give rise to type I GT.[24] Transfection in COS-7 cells showed that pro-αIIbβ3 formed but failed to mature. Significantly, the Cys560Argβ3 mutation in a French male allowed 20% of the normal surface αIIbβ3 expression in platelets was shown to lock the integrin in a spontaneously activated state.[8] This gain-of-function mutation allowed spontaneous binding of Fg and MoAbs recognizing activation-dependent determinants on αIIbβ3. Despite severely defective platelet aggregation, the patient’s platelets bound to and spread on a fibrinogen-covered surface. In our current murine model, β3(−/−) megakaryocytes were modified by human β3-lentivirus transduction and transplantation to provide sufficient levels of the C560Rβ3 in platelets to investigate how the activated αIIbβ3 conformation affected hemostasis in vivo. In this respect, the results recall platelet-type von Willebrand disease where spontaneous binding of VWF to GPIbα leads to a bleeding syndrome rather than thrombosis.[25]
Mor-Cohen et al,[7] showed that a homozygous β3 C549R mutation in EGF-3 in 6 Jordanian families with severe GT led to low amounts (1–14%) of constitutively active αIIbβ3 spontaneously binding PAC-1 in platelets or after transfection in BHK cells. This mutation broke a conserved C549-C558 bond in EGF-3 with mutant pro-αIIbβ3 largely retained in the ER. Other naturally occurring β3 Cysteine mutations giving rise to activated αIIbβ3 include C374Y, C506Y, C560F and C598Y (data reviewed in reference 23). One approach to investigate the role of β3 C residues has been to systematically mutate them to S; alternatively, others have transfected heterologous cells with αIIb and wild-type β3 or β3 with specific single or double C substitutions in the EGF domains.[10, 12, 23]. Although disulfide disruption mostly resulted in αIIbβ3 activation, PAC-1 binding was not always accompanied by fibrinogen binding implying differences in the activation state. Interestingly, disrupting the bond between C560 (EGF-3) and C583 (EGF-4) activated αIIbβ3 only when C560 was mutated suggesting that C583 plays a unique regulatory role in αIIbβ3 activation, possibly through a disulfide exchange-dependent mechanism.[12] A NMR model contradicted earlier work by suggesting that the C560-C583 bond is positioned within the EGF-4 domain although it continues to play a role in controlling the rigidity of the EGF-3 to EGF-4 interface.[26] The fact that C560R gave the highest expression of αIIbβ3 in human platelets and that it led to spontaneous binding of fibrinogen led us to believe that it was the most appropriate for the study of the pathophysiological effects of activated αIIbβ3 in our conditional mouse model.
Taken collectively, our results indicate that the altered structure of R560β3 expressed on murine platelets contributed to a unique case of GT that adhered ultimately to the classical definition of GT characterized by a severe bleeding phenotype.[1] Previous work showing the ability of human R560β3 platelets to spread on fibrinogen coated plates was not repeated in this study. The current data expands upon the functionality of the mutated integrin receptor by providing survival plots, organ histology, platelet aggregation assays and in vivo thrombus formation; results that mutually demonstrate that R560β3 platelets cannot form sufficient aggregates in vivo. This outcome is consistent with the occurrence of uncontrolled bleeding associated with Glanzmann thrombasthenia in humans and mice. Thus, the current investigation has helped to further define a potential mechanism for GT in one of only a few reported human cases characterized by an activating mutation of integrin αIIbβ3. Together, our data suggests that αIIbR560β3 readily binds fibrinogen resulting in the existence of fibrinogen coated platelets that remain in a resting state. After platelet activation with physiological agonists, platelet-platelet aggregation cannot occur because αIIbR560β3 receptors are already occupied with fibrinogen; therefore, the thrombasthenic phenotype of uncontrolled bleeding results. This outcome is supported by the results of Fig 3D showing that αIIbR560β3 platelet-platelet aggregates can form in vitro when fibrinogen is first washed off the platelets with EDTA/PBS and then the platelets are simultaneously incubated with fibrinogen and agonist. In contrast, αIIbR560β3 washed platelets are not able to form aggregates in vitro when treated with fibrinogen prior to activation with physiological agonists (Fig 3E). This result is consistent with the failure of platelet aggregate to form in vivo when mice are treated with a platelet activation agonist (ADP) as plasma has a high concentration of fibrinogen (Fig 3F) and the occurrence of uncontrolled bleeding and low survival rates for R560β3 mice.
It remains to be determined why ≈30% of R560β3 tx mice managed to survive for 26 weeks after transplantation. Admittedly, uncharacterized genetic and environmental factors may have had an undeterminable effect on platelet function that helped the R560β3 GT patient to reach adulthood. The ability of a heterozygote “carrier” for the R560β3 mutation to have normal platelet function is consistent with our proposed mechanism that integrin receptor occupancy that prevents platelet-platelet aggregates from forming in the homozygote. Since a heterozyogote has a mixture of R560β3 “mutant” fibrinogen occupied receptors and C560β3 “wildtype” fibrinogen unoccupied receptors on the platelet surface that are readily available for mediating platelet-platelet aggregation upon agonist activation.
This study provides further insight for the necessity for maintaining proper integrin structure when utilizing a recombinant lentivirus vector for hematopoietic stem cell gene therapy as recently shown with de novo synthesis αIIbβ3 on the surface of platelets to improve hemostatic function in a dog model of GT.[27] We also propose that the establishment of breeding colonies of a conditional mouse model[11] may enable a comparison of megakaryocyte or myeloid expression of β3R560 to permit a differential evaluation of the effect of this mutation on αIIbβ3 and αvβ3 as well as further investigations on the intriguing possibility that prolonged presence of fibrinogen on αIIbβ3 leads to a loss of adhesive function.
Supplementary Material
Acknowledgements
Histology was performed by MCW and CRI cores.
Sources of Funding: National Heart Lung Blood Institute-NIH HL-68138(DAW), American Heart Association BGIA0160441Z(DAW), and INSERM ANR-08-GENO-028-03(PN,ATN). Generous gifts: John & Judith Gardetto and Glanzmann Research Foundation(DAW).
Footnotes
Disclosures: The authors have no conflict of interest to disclose.
Authorship: J. Fang performed research experiments and analyzed data. P. Nurden performed research experiments and analyzed data. P. North analyzed data. A.T. Nurden analyzed research data and wrote manuscript. L.M. Du performed research experiments. N.V. performed research experiments and analyzed data. D.A. Wilcox designed research study, performed research, analyzed data, and wrote manuscript.
References
- 1.Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118:5996–6005. doi: 10.1182/blood-2011-07-365635. [DOI] [PubMed] [Google Scholar]
- 2.Hardisty R, Pidard D, Cox A, Nokes T, Legrand C, Bouillot C, Pannocchia A, Heilmann E, Hourdille P, Bellucci S, et al. A defect of platelet aggregation associated with an abnormal distribution of glycoprotein IIb-IIIa complexes within the platelet: the cause of a lifelong bleeding disorder. Blood. 1992;80:696–708. [PubMed] [Google Scholar]
- 3.Peyruchaud O, Nurden AT, Milet S, Macchi L, Pannochia A, Bray PF, Kieffer N, Bourre F. R to Q amino acid substitution in the GFFKR sequence of the cytoplasmic domain of the integrin IIb subunit in a patient with a Glanzmann's thrombasthenia-like syndrome. Blood. 1998;92:4178–4187. [PubMed] [Google Scholar]
- 4.Ghevaert C, Salsmann A, Watkins NA, Schaffner-Reckinger E, Rankin A, Garner SF, Stephens J, Smith GA, Debili N, Vainchenker W, de Groot PG, Huntington JA, Laffan M, Kieffer N, Ouwehand WH. A nonsynonymous SNP in the ITGB3 gene disrupts the conserved membrane-proximal cytoplasmic salt bridge in the alphaIIbbeta3 integrin and cosegregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood. 2008;111:3407–3414. doi: 10.1182/blood-2007-09-112615. [DOI] [PubMed] [Google Scholar]
- 5.Kunishima S, Kashiwagi H, Otsu M, Takayama N, Eto K, Onodera M, Miyajima Y, Takamatsu Y, Suzumiya J, Matsubara K, Tomiyama Y, Saito H. Heterozygous ITGA2B R995W mutation inducing constitutive activation of the {alpha}IIb{beta}3 receptor affects proplatelet formation and causes congenital macrothrombocytopenia. Blood. 2011;117:5479–5484. doi: 10.1182/blood-2010-12-323691. [DOI] [PubMed] [Google Scholar]
- 6.Ruan J, Schmugge M, Clemetson KJ, Cazes E, Combrie R, Bourre F, Nurden AT. Homozygous Cys542-->Arg substitution in GPIIIa in a Swiss patient with type I Glanzmann's thrombasthenia. Br J Haematol. 1999;105:523–531. [PubMed] [Google Scholar]
- 7.Mor-Cohen R, Rosenberg N, Peretz H, Landau M, Coller BS, Awidi A, Seligsohn U. Disulfide bond disruption by a beta 3-Cys549Arg mutation in six Jordanian families with Glanzmann thrombasthenia causes diminished production of constitutively active alpha IIb beta 3. Thromb Haemost. 2007;98:1257–1265. [PubMed] [Google Scholar]
- 8.Ruiz C, Liu CY, Sun QH, Sigaud-Fiks M, Fressinaud E, Muller JY, Nurden P, Nurden AT, Newman PJ, Valentin N. A point mutation in the cysteine-rich domain of glycoprotein (GP) IIIa results in the expression of a GPIIb-IIIa (alphaIIbbeta3) integrin receptor locked in a high-affinity state and a Glanzmann thrombasthenia-like phenotype. Blood. 2001;98:2432–2441. doi: 10.1182/blood.v98.8.2432. [DOI] [PubMed] [Google Scholar]
- 9.Ambo H, Kamata T, Handa M, Taki M, Kuwajima M, Kawai Y, Oda A, Murata M, Takada Y, Watanabe K, Ikeda Y. Three novel integrin beta3 subunit missense mutations (H280P, C560F, and G579S) in thrombasthenia, including one (H280P) prevalent in Japanese patients. Biochem Biophys Res Commun. 1998;251:763–768. doi: 10.1006/bbrc.1998.9526. [DOI] [PubMed] [Google Scholar]
- 10.Mor-Cohen R, Rosenberg N, Landau M, Lahav J, Seligsohn U. Specific cysteines in beta3 are involved in disulfide bond exchange-dependent and -independent activation of alphaIIbbeta3. J Biol Chem. 2008;283:19235–19244. doi: 10.1074/jbc.M802399200. [DOI] [PubMed] [Google Scholar]
- 11.Morgan EA, Schneider JG, Baroni TE, Uluckan O, Heller E, Hurchla MA, Deng H, Floyd D, Berdy A, Prior JL, Piwnica-Worms D, Teitelbaum SL, Ross FP, Weilbaecher KN. Dissection of platelet and myeloid cell defects by conditional targeting of the beta3-integrin subunit. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1117–1127. doi: 10.1096/fj.09-138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor-Cohen R, Rosenberg N, Einav Y, Zelzion E, Landau M, Mansour W, Averbukh Y, Seligsohn U. Unique disulfide bonds in epidermal growth factor (EGF) domains of beta3 affect structure and function of alphaIIbbeta3 and alphavbeta3 integrins in different manner. J Biol Chem. 2012;287:8879–8891. doi: 10.1074/jbc.M111.311043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang J, Hodivala-Dilke K, Johnson BD, Du LM, Hynes RO, White GC, 2nd, Wilcox DA. Therapeutic expression of the platelet-specific integrin,αIIbβ3, in a murine model for Glanzmann thrombasthenia. Blood. 2005;106:2671–2679. doi: 10.1182/blood-2004-12-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings LK, Haga JH, Slack SM. Differential expression of a ligand induced binding site (LIBS) by GPIIb-IIIa ligand recognition peptides and parenteral antagonists. Thromb Haemost. 2000;84:1095–1102. [PubMed] [Google Scholar]
- 15.Frei AC, Guo Y, Jones DW, Pritchard KA, Jr, Fagan KA, Hogg N, Wandersee NJ. Vascular dysfunction in a murine model of severe hemolysis. Blood. 2008;112:398–405. doi: 10.1182/blood-2007-12-126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wandersee NJ, Lee JC, Deveau SA, Barker JE. Reduced incidence of thrombosis in mice with hereditary spherocytosis following neonatal treatment with normal hematopoietic cells. Blood. 2001;97:3972–3975. doi: 10.1182/blood.v97.12.3972. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox DA, Shi Q, Nurden P, Haberichter SL, Rosenberg JB, Johnson BD, Nurden AT, White 2nd GC, Montgomery RR. Induction of megakaryocytes to synthesize and store a releasable pool of human factor VIII. J Thromb Haemost. 2003;1:2477–2489. doi: 10.1111/j.1538-7836.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- 18.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. Journal of Clinical Investigation. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmley RT, Kim TH, Austin RL, Alvarado CS, Ragab AH. Emperipolesis of neutrophils by dysmorphic megakaryocytes. Am J Hematol. 1982;13:303–311. doi: 10.1002/ajh.2830130405. [DOI] [PubMed] [Google Scholar]
- 20.de Pasquale A, Paterlini P, Quaglino D. Emperipolesis of granulocytes within megakaryocytes. Br J Haematol. 1985;60:384–386. doi: 10.1111/j.1365-2141.1985.tb07429.x. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao G, Yen MH, Lee YM, Sheu JR. Antithrombotic effect of PMC, a potent alpha-tocopherol analogue on platelet plug formation in vivo. Br J Haematol. 2002;117:699–704. doi: 10.1046/j.1365-2141.2002.03492.x. [DOI] [PubMed] [Google Scholar]
- 22.Calvete JJ, Jurgens M, Marcinkiewicz C, Romero A, Schrader M, Niewiarowski S. Disulphide-bond pattern and molecular modelling of the dimeric disintegrin EMF-10, a potent and selective integrin alpha5beta1 antagonist from Eristocophis macmahoni venom. Biochem J. 2000;345(Pt 3):573–581. [PMC free article] [PubMed] [Google Scholar]
- 23.Kamata T, Ambo H, Puzon-McLaughlin W, Tieu KK, Handa M, Ikeda Y, Takada Y. Critical cysteine residues for regulation of integrin alphaIIbbeta3 are clustered in the epidermal growth factor domains of the beta3 subunit. Biochem J. 2004;378:1079–1082. doi: 10.1042/BJ20031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milet-Marsal S, Breillat C, Peyruchaud O, Nurden P, Combrie R, Nurden A, Bourre F. Two different beta3 cysteine substitutions alter alphaIIb beta3 maturation and result in Glanzmann thrombasthenia. Thromb Haemost. 2002;88:104–110. [PubMed] [Google Scholar]
- 25.Nurden A, Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J Thromb Haemost. 2011;9(Suppl 1):76–91. doi: 10.1111/j.1538-7836.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 26.Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nature structural biology. 2002;9:282–287. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- 27.Fang J, Jensen ES, Boudreaux MK, Du LM, Hawkins TB, Koukouritaki SB, Cornetta K, Wilcox DA. Platelet gene therapy improves hemostatic function for integrin alphaIIbbeta3-deficient dogs. Proc Natl Acad Sci U S A. 2011;108:9583–9588. doi: 10.1073/pnas.1016394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.