Abstract
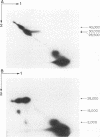
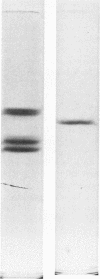
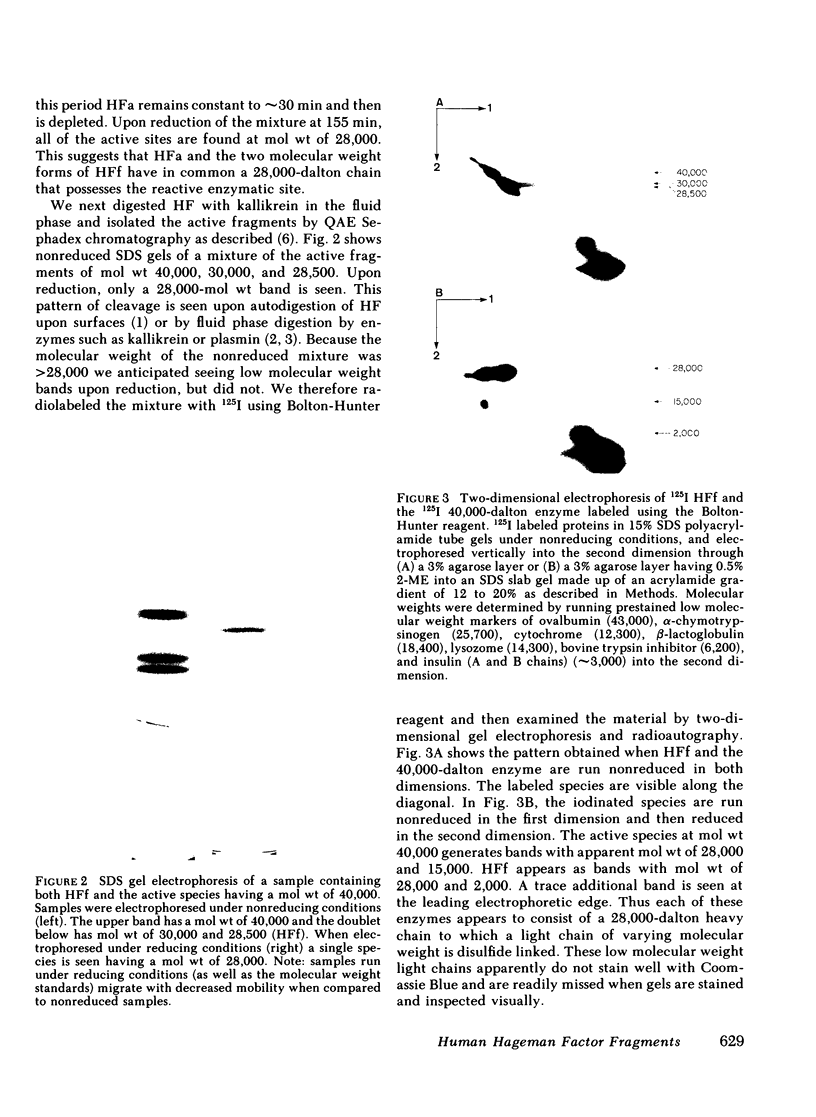
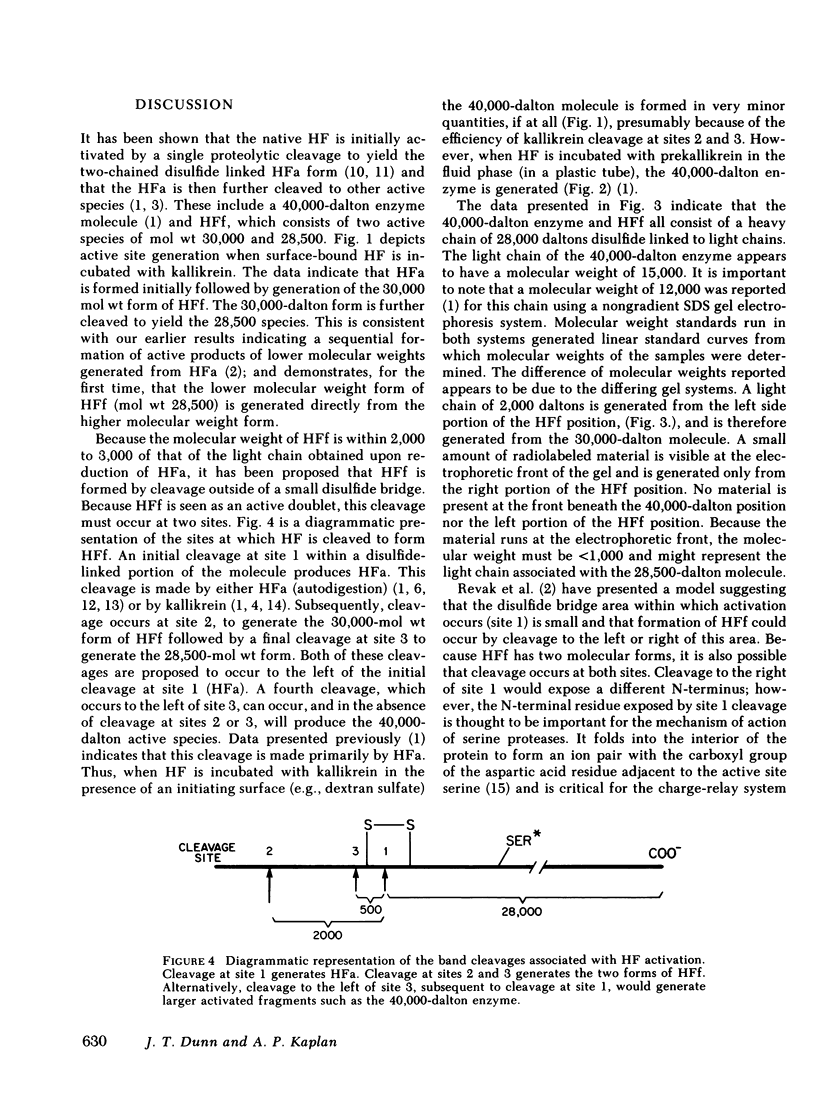
Autodigestion of activated Hageman factor (HFa) yields a 40,000-mol wt activated enzyme as well as Hageman factor fragment (HFf); HFf consists of two molecular weight species of 28,500 and 30,000. We have investigated the structure of these active fragments and demonstrate that upon reduction, each possesses a heavy chain of 28,000. The associated light chains were identified by subjecting iodinated proteins to two-dimensional slab gel electrophoresis in which the second dimension is run reduced. The 40,000-dalton enzyme has a light chain of 15,000, the 30,000-dalton form of HFf has a light chain of 2,000 and we have suggestive evidence of a light chain associated with the 28,500-dalton form of HFf (putative mol wt approximately 500). We also demonstrate that the 30,000-dalton form of HFf precedes the 28,500 form. These data indicate that digestion of native HF to form HFa precedes cleavages that fragment the molecule and diminish its molecular weight. The 28,500-dalton light chain of HFa becomes the heavy chain of each of the fragmentation products while cleavage at different points along the heavy chain of HFa determines which fragments will be produced. In contrast to autoactivation, kallikrein digestion of HFa yields primarily HFf; however, the 40,000-dalton enzyme may be seen when prekallikrein-deficient (Fletcher trait) plasma is activated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. T., Silverberg M., Kaplan A. P. The cleavage and formation of activated human Hageman factor by autodigestion and by kallikrein. J Biol Chem. 1982 Feb 25;257(4):1779–1784. [PubMed] [Google Scholar]
- Fujikawa K., Heimark R. L., Kurachi K., Davie E. W. Activation of bovine factor XII (Hageman factor) by plasma kallikrein. Biochemistry. 1980 Apr 1;19(7):1322–1330. doi: 10.1021/bi00548a010. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Kurachi K., Davie E. W. Characterization of bovine factor XIIa (activated Hageman factor). Biochemistry. 1977 Sep 20;16(19):4182–4188. doi: 10.1021/bi00638a008. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A pre-albumin activator of prekallikrein. J Immunol. 1970 Oct;105(4):802–811. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandle R., Jr, Kaplan A. P. Hageman factor substrates. Human plasma prekallikrein: mechanism of activation by Hageman factor and participation in hageman factor-dependent fibrinolysis. J Biol Chem. 1977 Sep 10;252(17):6097–6104. [PubMed] [Google Scholar]
- Miller G., Silverberg M., Kaplan A. P. Autoactivatability of human Hageman factor (factor XII). Biochem Biophys Res Commun. 1980 Feb 12;92(3):803–810. doi: 10.1016/0006-291x(80)90774-3. [DOI] [PubMed] [Google Scholar]
- Revak S. D., Cochrane C. G., Bouma B. N., Griffin J. H. Surface and fluid phase activities of two forms of activated Hageman factor produced during contact activation of plasma. J Exp Med. 1978 Mar 1;147(3):719–729. doi: 10.1084/jem.147.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revak S. D., Cochrane C. G., Griffin J. H. The binding and cleavage characteristics of human Hageman factor during contact activation. A comparison of normal plasma with plasmas deficient in factor XI, prekallikrein, or high molecular weight kininogen. J Clin Invest. 1977 Jun;59(6):1167–1175. doi: 10.1172/JCI108741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Silverberg M., Dunn J. T., Garen L., Kaplan A. P. Autoactivation of human Hageman factor. Demonstration utilizing a synthetic substrate. J Biol Chem. 1980 Aug 10;255(15):7281–7286. [PubMed] [Google Scholar]
- Watson H. C., Shotton D. M., Cox J. M., Muirhead H. Three-dimensional Fourier synthesis of tosyl-elastase at 3.5 å resolution. Nature. 1970 Feb 28;225(5235):806–811. doi: 10.1038/225806a0. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Cochrane C. C. The autoactivation of rabbit Hageman factor. J Exp Med. 1979 Nov 1;150(5):1122–1133. doi: 10.1084/jem.150.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]