Abstract
Purpose
To report the one-year clinical outcomes of an outbreak of Streptococcus endophthalmitis after intravitreal injection of bevacizumab, including visual acuity outcomes, microbiological testing and compound pharmacy investigations by the Food and Drug Administration (FDA).
Design
Retrospective consecutive case series.
Participants
12 eyes of 12 patients who developed endophthalmitis after receiving intravitreal bevacizumab prepared by a single compounding pharmacy.
Methods
Medical records of patients were reviewed; phenotypic and DNA analyses were performed on microbes cultured from patients and from unused syringes. An inspection report by the FDA based on site-visits to the pharmacy that prepared the bevacizumab syringes was summarized.
Main Outcome Measures
Visual acuity, interventions received, time-to-intervention; microbiological consistency; FDA inspection findings.
Results
Between July 5 and July 8, 2011, 12 patients developed endophthalmitis after intravitreal bevacizumab from syringes prepared by a single compounding pharmacy. All patients received initial vitreous tap and injection, and eight (67%) subsequently underwent pars plana vitrectomy (PPV). After twelve months follow-up, outcomes have been poor: 7 patients (58%) required evisceration or enucleation, and only one patient regained pre-injection visual acuity. Molecular testing using real time polymerase chain reaction, partial sequencing of the groEL gene, and multilocus sequencing of 7 housekeeping genes confirmed the presence of a common strain of Streptococcus mitis/oralis in vitreous specimens and seven unused syringes prepared by the compounding pharmacy at the same time. An FDA investigation of the compounding pharmacy noted deviations from standard sterile technique, inconsistent documentation, and inadequate testing of equipment required for safe preparation of medications.
Conclusions
In this outbreak of endophthalmitis, outcomes have been generally poor and PPV did not improve visual results at one year follow-up. Molecular testing confirmed a common strain of Streptococcus mitis/oralis. Contamination appears to have occurred at the compounding pharmacy, where numerous problems in sterile technique were noted by public health investigators.
Introduction
Endophthalmitis after intravitreal injection is a rare but feared complication, occurring once every 2,000 – 5,000 injections.1–3 Many reports have focused on the peri-injection period, including the use of a topical povidone-iodine preparation and sterile lid speculums, and have debated the role for face masks and topical antibiotics, to reduce the risk of endophthalmitis.4–7
Recently, several outbreaks of bacterial and fungal infections, or sterile inflammations, have raised concern about the safety of the drug-preparation process.8–16 Included among these was a report on a series of 12 cases of Streptococcus endophthalmitis after intravitreal injection of bevacizumab (Avastin, Genentech/Roche, South San Francisco, California, USA).8 To summarize, 12 patients received injections from four community retina specialists in early July, 2011, in south Florida. Treating physicians all reported the use of topical povidone-iodine, sterile lid speculums, and fourth-generation fluoroquinolone eye drops at the time of injection. All affected patients received bevacizumab prepared by the same compounding pharmacy. Ten vitreous specimens, as well as seven unused syringes prepared at that time were culture-positive for Streptococcus mitis/oralis.
In the current study, the one-year clinical outcomes of affected patients are reported. Additionally, the results of microbiological testing confirming a common source contamination are described, as are the findings of a Food and Drug Administration (FDA) inspection of the compounding pharmacy that prepared the syringes in this outbreak.
Methods
After approval of the Institutional Review Board of the University of Miami Human Subjects Research Office, and in cooperation with local and state Departments of Health, the charts of all patients in this outbreak of endophthalmitis after intravitreal injection were retrospectively reviewed. The data collection methods have been previously published,8 and results were updated to reflect the 12-month post-injection clinical outcomes. A report issued on September 27, 2011, by the FDA reflecting the observations of their inspections of the compounding pharmacy over multiple days in July and September, 2011, was reviewed.17 These inspections led the FDA to send a Warning Letter to the implicated pharmacy dated July 30, 2012, which was subsequently made publicly available.18
Microbiological evaluation was performed using both conventional and molecular techniques. VITEK 2 (BioMeriéux, Inc, Durham NC) and E tests (BioMerieux, Inc., Durham, NC) were used for conventional identification and in vitro susceptibility testing. A combination of real time polymerase chain reaction (PCR), partial sequencing of the groEL gene and multilocus sequencing (7 housekeeping genes) were used for molecular identification and characterization.
Results
Twelve patients were included in this study; baseline characteristics including age, lens status, indications for bevacizumab therapy, and pre-injection visual acuity have been previously reported.8
Clinical course and outcomes
The average time from bevacizumab injection to endophthalmitis presentation was 2.7 days (range 1–6 days). At presentation, all patients were treated with initial vitreous tap and injection, including intravitreal vancomycin 1.0 mg/0.1 mL. Eight patients (67%) underwent subsequent pars plana vitrectomy (PPV); five of these patients received 5,000-centistoke silicone oil infusion at the time of PPV. The average time from injection to PPV was 30 days (range 6–42 days). Extensive corneal infiltrates and anterior chamber opacities often delayed PPV. See Figure 1 for representative examples of patient presentations and outcomes.
Figure 1.
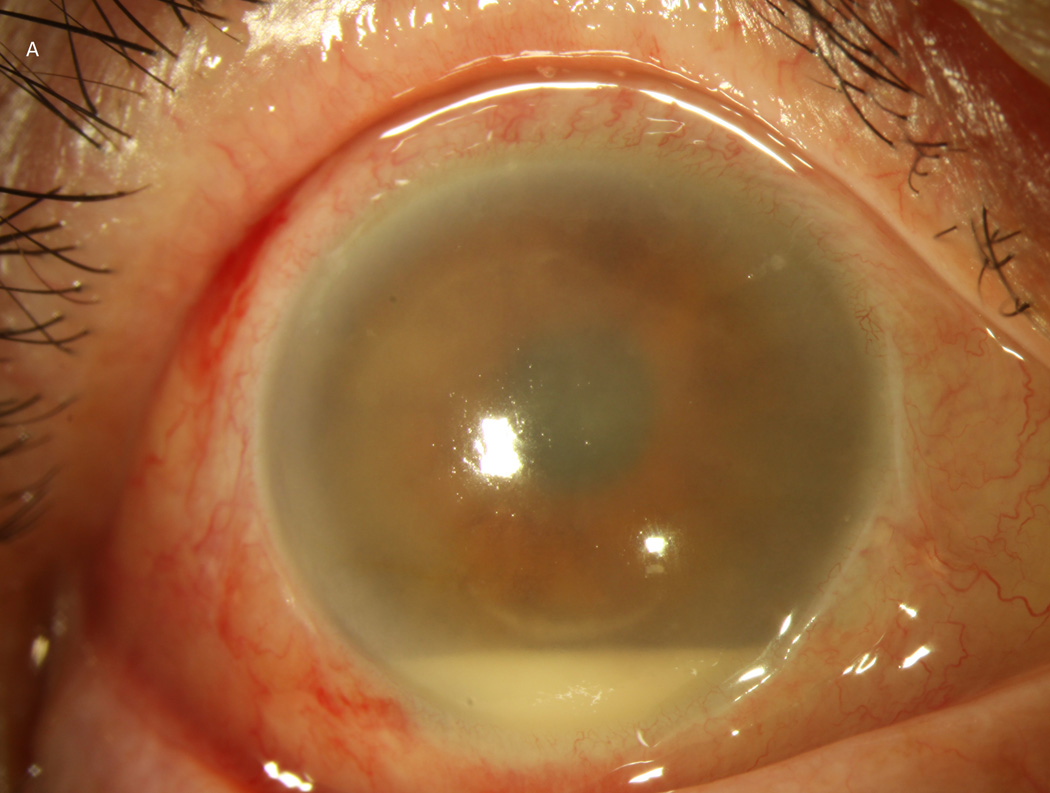
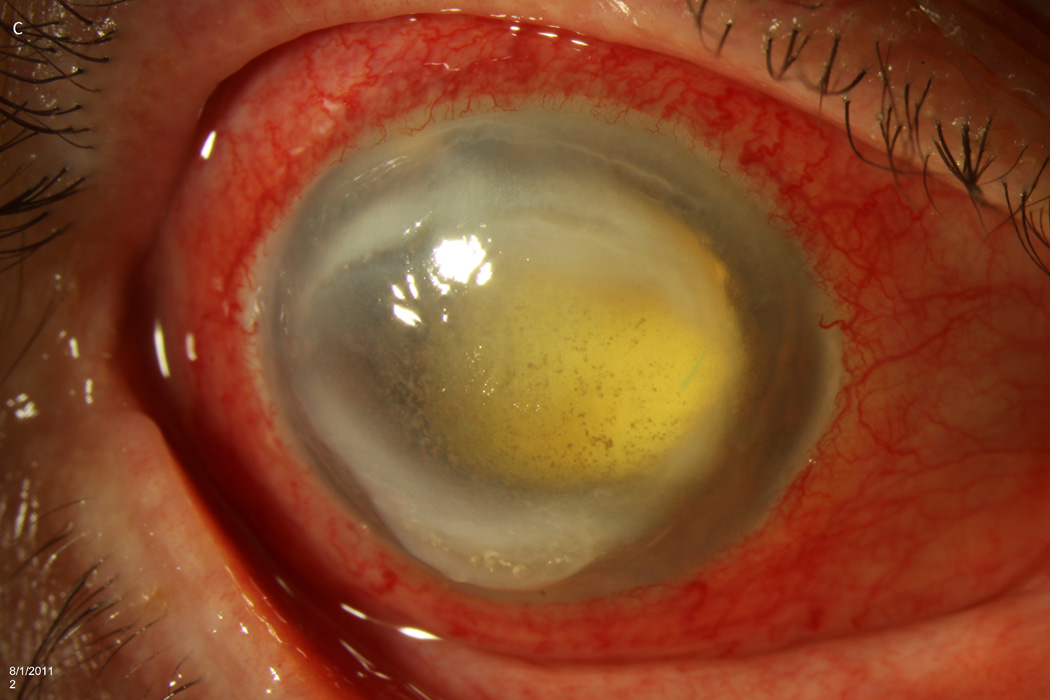

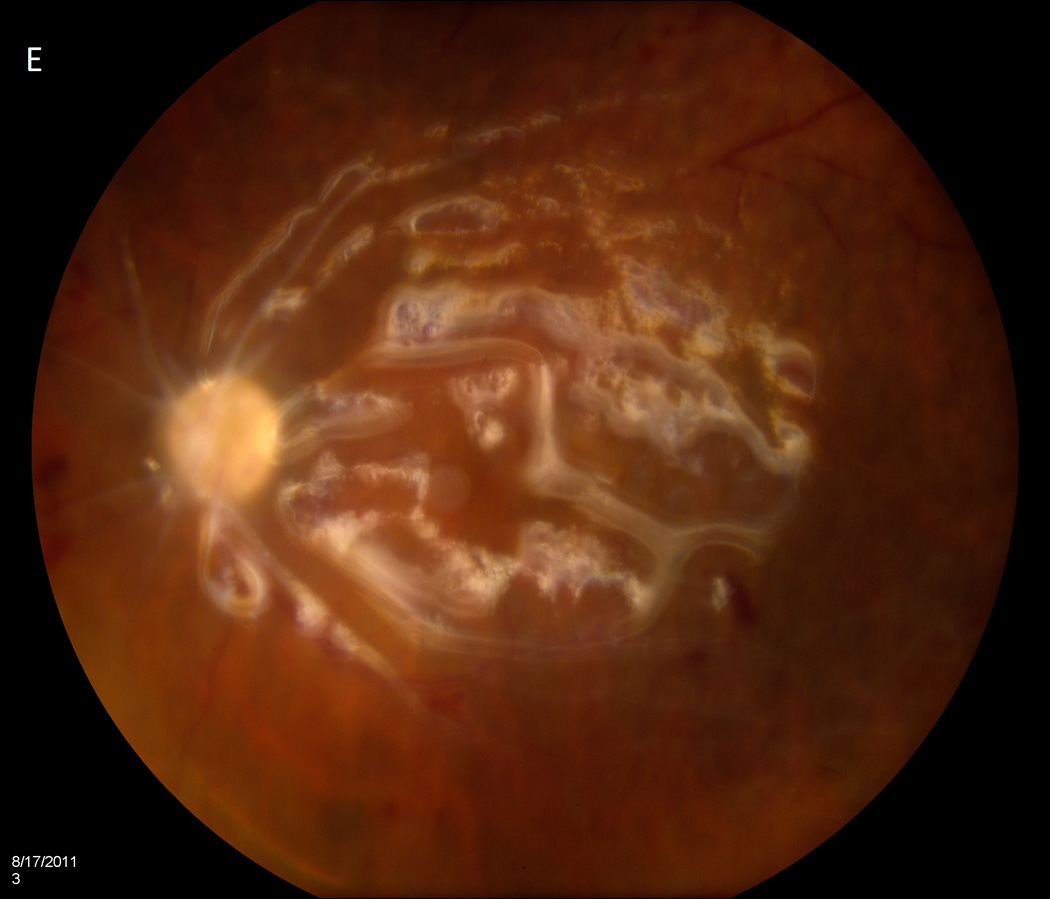
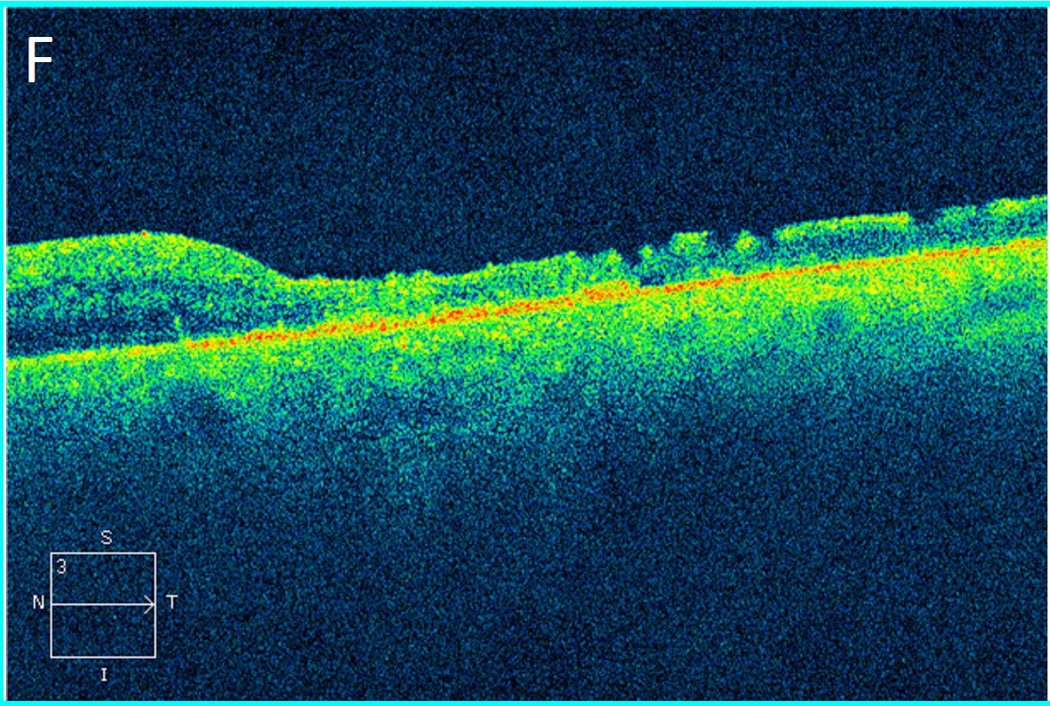
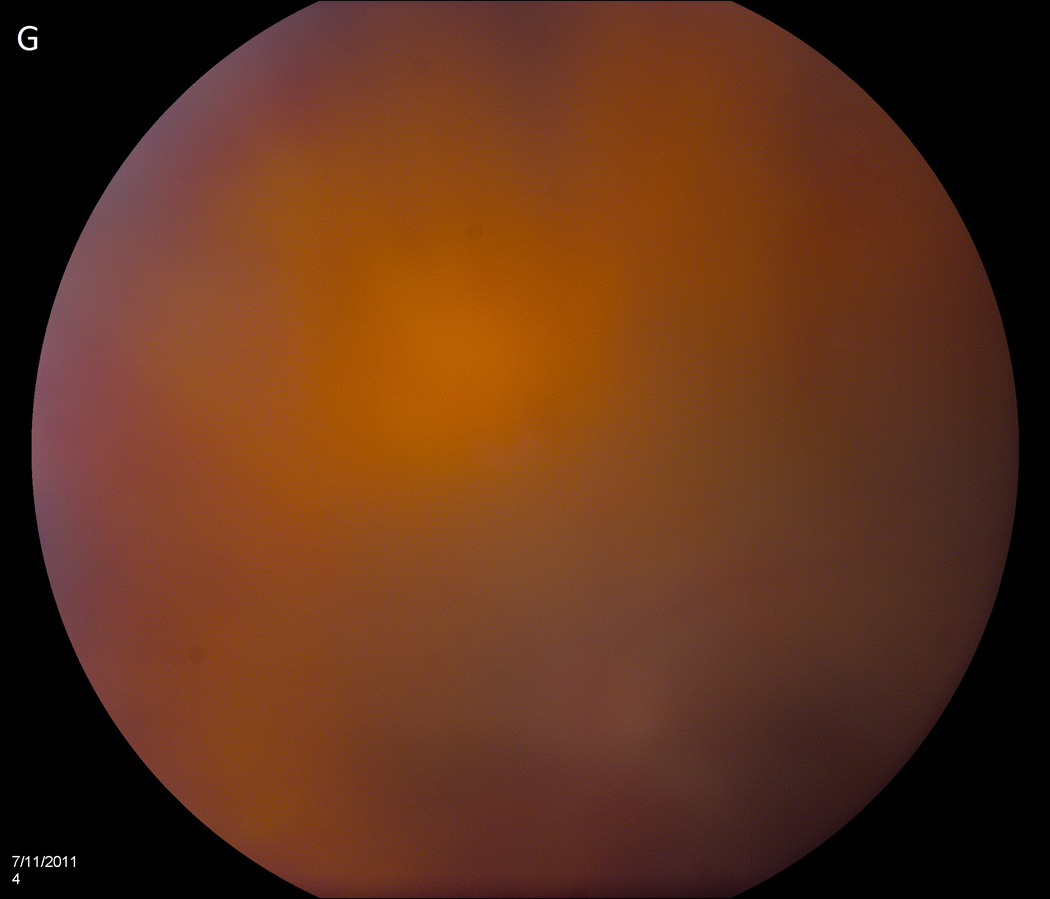
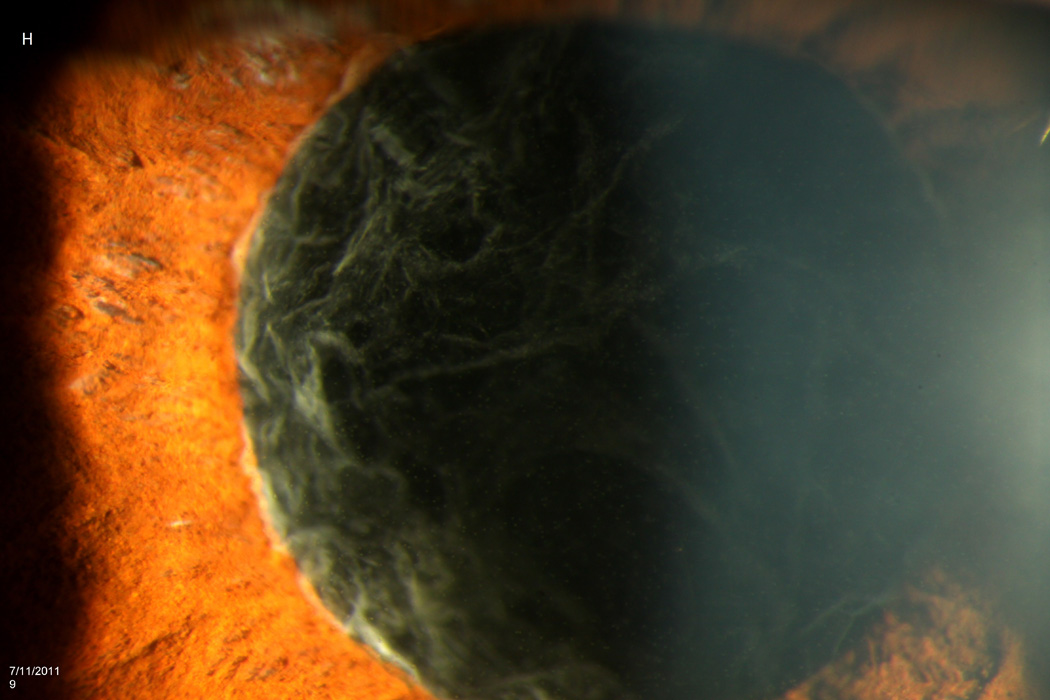
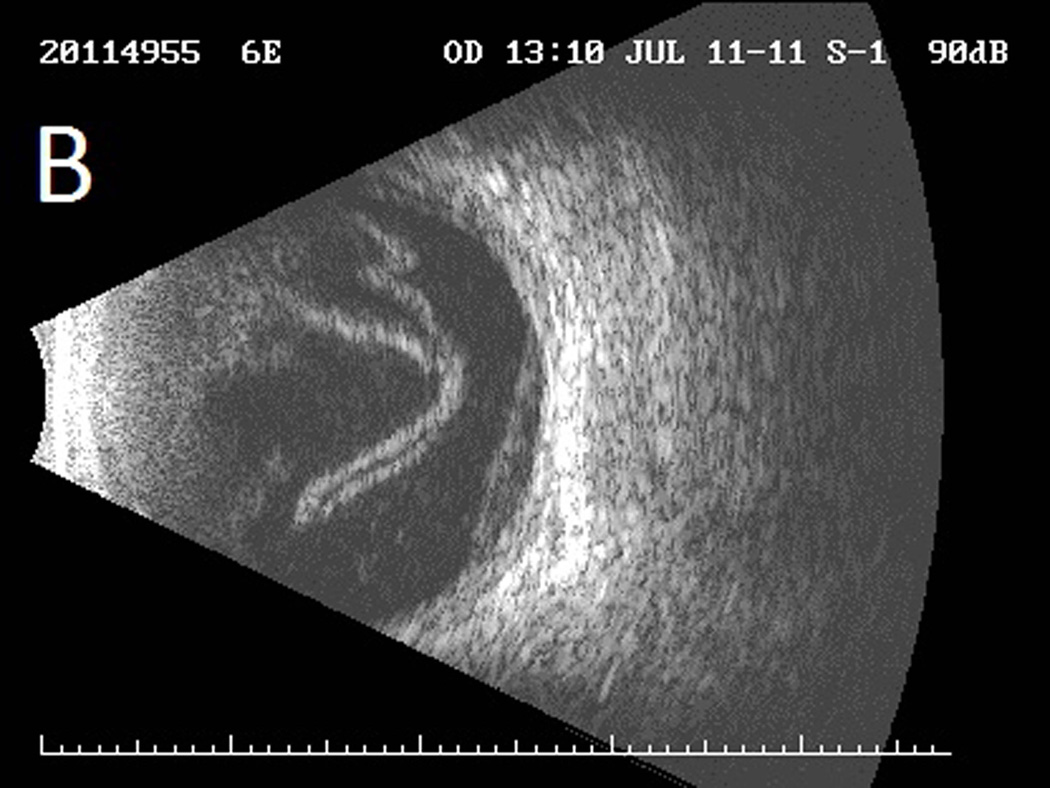
Case 2 demonstrates the marked anterior chamber reaction and a layered hypopyon (A) that most patients presented with. Echography obtained at that time confirmed the presence of a dense vitritis with prominent membranes (B). As the inflammation consolidated, several patients developed a dense ring infiltrate (C) which, in Case 8, left a prominent inferior ectasia in the cornea (D). When the view cleared sufficiently to allow for pars plana vitrectomy, the optic nerves were often pale, the vessels sclerosed, and the retina atrophic, as in Case 3 (E, F). Case 7 was the only patient to be regain her pre-injection visual acuity. She presented 3 days after her injection with milder signs and symptoms than the other patients. Her Snellen visual acuity had deteriorated from 20/40 to 20/200, with a hazy vitritis (G) and prominent pupillary fibrin (H).
At 12 months post injection, seven patients (58%) had received an evisceration (2/7) or enucleation (5/7). The eviscerated eyes had not previously been treated with PPV, while the enucleated eyes had all received prior PPV. The average time to evisceration was 47.5 days (range 40–55 days), while the average time to enucleation was 176 days (range 129–215 days).
Among vitrectomized eyes that avoided enucleation (3 patients), the average time from injection to PPV was 17 days, compared to an average injection-to-PPV time of 38 days among the 5 eyes that were eventually enucleated (p=0.036; Mann-Whitney rank test).
Of the five eyes that received silicone oil at the time of PPV, four (80%) were eventually enucleated, while only one (33%) of the three eyes that received PPV without silicone oil required subsequent enucleation; however, this difference did not achieve statistical significance (p = 0.46, Fisher exact test). Similarly, no statistical difference was found in the survival of phakic (3 of 5 eyes required enucleation, 60%) versus pseudophakic (4/7, 57%) eyes (p=0.99, Fisher exact test).
After 12 months, only one patient (8%) regained her pre-injection visual acuity (20/25, patient 7), while seven (58%) were enucleated or eviscerated; two (17%) were hand-motions; and two (17%) were count-fingers at one foot. A summary of the clinical interventions and visual outcomes is provided in Table 1.
Table 1.
Summary of patients with Streptococcus endophthalmitis after intravitreal bevacizumab*
| Case | Baseline diagnosis |
Preinjection Visual Acuity |
Visual Acuity at the Time of Endophthalmitis |
Days to Presentation |
Initial Treatment |
Surgical intervention |
Visual Acuity at 12 months |
Days to PPV |
|---|---|---|---|---|---|---|---|---|
| 1 | AMD | 20/400 | LP | 2 | V,C,D | PPV, Enuc | NLP | 39 |
| 2 | CME | 20/100 | HM | 2 | V,C,D | PPV, Enuc | NLP | 32 |
| 3 | AMD | 20/30 | LP | 2 | V,C,D | PPV | 1/200 | 32 |
| 4 | AMD | 20/400 | LP | 2 | V,C,D | PPV, Enuc | NLP | 39 |
| 5 | AMD | 20/400 | LP | 1 | V,C,D | Evis | NLP | - |
| 6 | AMD | 20/50 | LP | 1 | V,C,D | PPV, Enuc | NLP | 39 |
| 7 | AMD | 20/40 | 20/200 | 3 | V,C,D | 20/25 | - | |
| 8 | CME | CF | LP | 6 | V,C,D | PPV, Enuc | NLP | 42 |
| 9 | VO/NVG | CF | LP | 6 | V,C,D | Evis | NLP | - |
| 10 | AMD | 20/40 | LP | 3 | V,C | HM | - | |
| 11 | AMD | 20/25 | CF | 2 | V, A | PPV | 1/200 | 6 |
| 12 | AMD | 20/25 | HM | 2 | V, A | PPV | HM | 13 |
All culture results were positive for Streptococcus mitis / oralis.
AMD, age-related macular degeneration; CME, cystoid macular edema; VO, vein occlusion; NVG, neovascular glaucoma; NLP, no light perception; LP, light perception; HM, hand motions; CF, count fingers; V, intravitreal vancomycin 1.0 mg/0.1 mL; C, intravitreal ceftazidime 2.25 mg/0.1 mL; A, intravitreal amikacin 0.4 mg/0.1 mL; D, intravitreal dexamethasone 0.4 mg/0.1 mL; PPV, pars plana vitrectomy; Evis, evisceration; Enuc, enucleation
Three patients had persistent-positive cultures for Streptococcus mitis/oralis despite initial treatment with intravitreal and topical vancomycin. Patient 1 had a second tap and inject performed the day after presentation and initial treatment, and this vitreous specimen was culture-positive. The vitreous cassette taken at time of PPV in patient 3, 32 days after bevacizumab injection, grew Streptococcus, as did cultures taken from the evisceration specimen in patient 9, 40 days after injection.
Microbiological evaluation
Traditional culture methods (VITEK 2) identified Streptococcus mitis/oralis in all patient isolates as well seven unused syringes with a probability of 90–99%. Multiple isolates from the S. mitis group were recovered from three patient samples. All isolates were susceptible to levofloxacin, moxifloxacin, gatifloxacin, and vancomycin, but differed in their resistance to ceftriaxone, erythromycin, and penicillin.
DNA sequencing of the groEL gene confirmed all vitreous isolates belonged to the S. mitis group with a consistency of 86–98%. Multilocus sequence analysis of seven streptococcal housekeeping genes performed on nine vitreous specimens and four unused syringes identified four clusters of bacteria, all of which were part of the S. mitis group. Together, these molecular analyses confirm that a common pathogen was present in patient specimens as well as in unused syringes.
FDA investigation of compounding pharmacy
Eleven of the affected patients in this outbreak were members of the same managed care organization (MCO), which contracted with a compounding pharmacy to supply the treating ophthalmologists with pre-filled bevacizumab syringes for each patient. That compounding pharmacy sub-contracted the syringe preparation to a second pharmacy which aliquoted the drug from 4- or 16-mL vials of bevacizumab into individual syringes, and returned the syringes to the initial pharmacy. Each syringe was then labeled for a specific patient, and distributed to the physician office for intravitreal injection. The twelfth patient was a member of a different MCO, but received a bevacizumab syringe prepared by the compounding pharmacy.
While the final reports from health agencies including the FDA and Florida Department of Health have not been published, a ten-page Inspectional Report from the FDA’s site visits to the pharmacy which divided the bevacizumab vials into individual syringes has been completed,17 and an FDA Warning Letter was sent to the implicated pharmacy.18
The FDA investigation reports numerous practices at the compounding pharmacy which may have contributed to this outbreak. These include problems with sterile technique during drug preparation, inconsistent or absent documentation, and insufficient testing and monitoring of equipment to ensure appropriate function.
Problems with sterile technique: During the site visits to the compounding pharmacy, the investigator noted multiple instances in which sterility may have been compromised during drug preparation. These include using non-sterile gloves while handling sterile supplies, using a water-based rather than alcohol-based hand sanitizer, wiping the tops of vials with non-sterile pads immediately prior to puncture, and incorrectly using personal protective equipment such as hairnets.
Inconsistent documentation: The report details specific examples such as empty bottles being re-filled with non-sterile solutions without obliterating the label to prevent its use in sterile procedures, and concludes that the pharmacy lacked the written procedures to assure that the drug products have the identity, strength, quality, and purity they purported or are represented to possess.
Insufficient testing and monitoring of equipment: The investigation noted that routine calibration, maintenance and cleaning of equipment was not performed to assure proper performance, and that environmental monitoring was not performed adequately.
Additionally, the FDA report specifically comments on the preparation and handling of bevacizumab syringes. The report notes that the number of syringes filled and vials used per batch was not always documented on the compounding or repackaging record, leading to discrepancies in documentation. Additionally, the report states that the pharmacy lacked controls to ensure that the safety and quality of compounded drugs would not be affected during transportation and delivery.
Regarding the syringes implicated in this outbreak, on July 5, 2011, the pharmacy prepared 34 syringes from two vials of bevacizumab, one first opened on June 21, and the other opened on July 5. Then, on July 6, the pharmacy prepared 15 additional syringes, using the vial that had been first punctured on July 5.
Discussion
The long-term clinical outcomes in this outbreak have been poor. Only one patient recovered useful vision in the affected eye, perhaps because her vitreous cavity was inoculated with a smaller load of bacteria, though this is speculation. Seven patients required enucleation or evisceration. These poor visual outcomes are consistent with previously reported series of Streptococcal endophthalmitis.2,19
PPV did not improve visual outcomes in this outbreak. Two patients received relatively “early” PPV (at post-injection days 6 and 13) and were able to preserve their eyes, while five of the six eyes that underwent “late” PPV (at days 32 – 42) underwent subsequent enucleation. While this may be interpreted as an indication for rapid PPV in post-injection endophthalmitis, we believe that this likely reflects the disease severity. All patients received PPV as soon as the cornea and anterior chamber permitted visualization of the vitreous cavity. Figure 1 includes a representative case (patient 8) of profound corneal infiltration and ectasia that was not able to be treated with PPV until day 42 when the view cleared; the eye was eventually enucleated.
Despite reports20–23 about the effects of silicone oil as an antimicrobial or globe-salvaging agent, the use of silicone oil did not appear to improve final outcomes in this outbreak. However, as with the interpretation of the timing of PPV, caution should be taken when drawing conclusions from such a limited sample size.
Three patients had positive Streptococcal cultures despite having received appropriate intravitreal and topical antibiotics. Histopathological examination of one enucleated eye did reveal evidence of intravitreal abscess formation, which may serve as a sanctuary for bacteria from antibiotic therapy. This corroborates previously reported evidence24 that endophthalmitis may require multiple intravitreal injections to sterilize the vitreous cavity.
Microbiological testing by three methods provide molecular evidence of a common source for this contamination. The S. mitis in this outbreak was sensitive to fourth-generation fluoroquinolones, as well as vancomycin. However, the effect of peri-injection prophylaxis with a fourth-generation fluoroquinolone, whether before, during, or after injection, appears to be minimal in this outbreak.
The FDA and Florida Department of Health investigations of the compounding pharmacy occurred after this outbreak began. Therefore, the findings in this report may not reflect the actual practices on the specific days the syringes were prepared. That said, the FDA report presents many disturbing findings. Clinicians and patients routinely rely on the safe preparation of medicines that require sterile compounding and preparation.
Bevacizumab is distributed as a 4- or 16-mL preservative-free single-use vial. Numerous reports have described the safe preparation of bevacizumab syringes in accordance with United States Pharmacopeia Chapter 797 (USP <797>), including the importance of using proper sterile technique and puncturing vials only once.25–28 In certain situations, single-dose or single-use vials may be repackaged into smaller doses, though USP <797> infection control standards require that this repackaging occur in International Standard for Organization (ISO) Class 5 air quality conditions, and that repackaging occur within 6 hours of initial needle puncture.29 The pharmacy in this outbreak prepared some of the affected syringes using a vial that had been first opened two weeks earlier.
Proper documentation both at the compounding pharmacy and at the physician’s office is also key to minimizing the impact of any potential contamination. Confusing and inconsistent documentation, such as that reported by the FDA at the implicated pharmacy in this outbreak, makes tracking down possibly contaminated syringes—and the patients affected by them—difficult for physicians and public health officials alike.
This and other outbreaks remind clinicians of the importance of careful selection when choosing a compounding pharmacy for the preparation of drugs for intraocular use. Currently, the American Academy of Ophthalmology recommends selecting a pharmacy that adheres to USP <797> standards and is certified by the Pharmacy Compounding Accreditation Board (PCAB).30 PCAB surveyors provide on-site inspections of pharmacies to assure that they comply with quality standards.
In summary, 12 patients were affected by a virulent strain of S. mitis/oralis endophthalmitis after receiving an intravitreal injection of bevacizumab prepared by a single compounding pharmacy. Microbiological testing confirmed a common source for the contamination, and an FDA investigation of the compounding pharmacy revealed disturbing findings regarding the safe preparation of medications used routinely in patient care. Visual results have been poor after one year. Perhaps this outbreak and others like it will lead to further improvements in the safe production of compounded and prepared medicines, and help prevent such an outbreak from occurring again in the future.
Acknowledgements
The Authors wish to acknowledge the support of Thomas J. Török, MD, MPH, and Ann Schmitz, DVM, AM, at the Bureau of Epidemiology at the Florida Department of Health, as well as William J. Feuer, MS, for help with statistical calculations.
Financial Support: Financial support was provided by the National Institutes of Health Center P30-EY014801, and an unrestricted grant to the University of Miami from Research to Prevent Blindness, New York, New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This report will be presented as a paper at the 2012 Joint Meeting of the American Academy of Ophthalmology and the Asia-Pacific Academy of Ophthalmology.
Conflicts of Interest: RAG: Emmetrope Ophthalmics. HWF: Alimera, Santen, Pfizer. DM: none. SG: none. RFI: none. PMB: none. The authors have no financial or proprietary interests in the material presented herein.
References
- 1.McCannel CA. Meta-analysis of endophthalmitis following intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 2.Moshfeghi AA, Rosenfeld PJ, Flynn HW, Jr, et al. Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: a six-year experience at a university referral center. Retina. 2011;31:662–668. doi: 10.1097/IAE.0b013e31821067c4. [DOI] [PubMed] [Google Scholar]
- 3.CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen JC, McCannel CA, Mochon AB, Garner OB. Bacterial dispersal associated with speech in the setting of intravitreous injections. Arch Ophthalmol. 2011;129:1551–1554. doi: 10.1001/archophthalmol.2011.227. [DOI] [PubMed] [Google Scholar]
- 5.Schimel AM, Scott IU, Flynn HW., Jr Endophthalmitis after intravitreal injections: should the use of face masks be the standard of care? Arch Ophthalmol. 2011;129:1607–1609. doi: 10.1001/archophthalmol.2011.370. [DOI] [PubMed] [Google Scholar]
- 6.Wykoff CC, Flynn HW, Jr, Rosenfeld PJ. Prophylaxis for endophthalmitis following intravitreal injection: antisepsis and antibiotics. Am J Ophthalmol. 2011;152:717–719. doi: 10.1016/j.ajo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Wykoff CC, Flynn HW., Jr Endophthalmitis after intravitreal injection: prevention and management. Retina. 2011;31:633–635. doi: 10.1097/IAE.0b013e31821504f2. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RA, Flynn HW, Jr, Isom RF, et al. An outbreak of Streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153:204–208. doi: 10.1016/j.ajo.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielden M, Nelson B, Kherani A. Acute intraocular inflammation following intravitreal injection of bevacizumab—-a large cluster of cases [letter online] Acta Ophthalmol. 2011;89:e664–e665. doi: 10.1111/j.1755-3768.2010.02054.x. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Emi K, Ikeda T, et al. Severe intraocular inflammation after intravitreal injection of bevacizumab. Ophthalmology. 2010;117:512–516. doi: 10.1016/j.ophtha.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Xu X, Zhang X. Counterfeit bevacizumab and endophthalmitis [letter] N Engl J Med. 2011;365:378–379. doi: 10.1056/NEJMc1106415. author reply 379. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Woo SJ, Park KH, et al. Serratia marcescens endophthalmitis associated with intravitreal injections of bevacizumab. Eye (Lond) 2010;24:226–232. doi: 10.1038/eye.2009.86. [DOI] [PubMed] [Google Scholar]
- 13.Wilemon T. Hospital gave tainted shots. The Tennessean. 2011 Aug 7;:1B–5B. [Google Scholar]
- 14.Pollack A. The New York Times. 2011. Sep 1, [Accessed September 1, 2011]. Five more reports of Avastin injections causing Blindness. Available at: http://www.nytimes.com/2011/09/02/business/more-reports-of-avastin-causing-blindness.html?_r=0. [Google Scholar]
- 15.Notes from the field: Multistate outbreak of postprocedural fungal endophthalmitis associated with a single compounding pharmacy – United States, March–April 2012. [Accessed: May 23, 2012];MMWR Morb Mortal Wkly Rep. 2012 61:310–311. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6117a5.htm. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Health Alert Network. [Accessed October 17, 2012];Update: Multistate outbreak of fungal meningitis and joint infections associated with contaminated steroid medications. 2012 Oct 17; Available at: http://emergency.cdc.gov/HAN/han00329.asp.
- 17.Food and Drug Administration. [Accessed October 18, 2012];Form FDA 483: Inspectional Observations (for a compounding pharmacy in Florida) 2011 July and September; Available at: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM304256.pdf.
- 18.Public Health Service. Food and Drug Administration, Florida District. [Accessed October 18, 2012];Warning letter FLA-12-37. 2012 Jul 30; Available at: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm317190.htm.
- 19.Miller JM, Scott IU, Flynn HW, Jr, et al. Endophthalmitis caused by Streptococcus pneumoniae. Am J Ophthalmol. 2004;138:231–236. doi: 10.1016/j.ajo.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Chan CC, Holland EJ, Sawyer WI, et al. Boston type 1 keratoprosthesis combined with silicone oil for treatment of hypotony in prephthisical eyes. Cornea. 2011;30:1105–1109. doi: 10.1097/ICO.0b013e318207f3bb. [DOI] [PubMed] [Google Scholar]
- 21.Alexandridis E. Silicone oil tamponade in the management of severe hemorrhagic detachment of the choroid and ciliary body after surgical trauma. Ophthalmologica. 1990;200:189–193. doi: 10.1159/000310105. [DOI] [PubMed] [Google Scholar]
- 22.Skorpik C, Menapace R, Gnad HD, Paroussis P. Silicone oil implantation in penetrating injuries complicated by PVR. Results from 1982 to 1986. Retina. 1989;9:8–14. doi: 10.1097/00006982-198909010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ozdamar A, Aras C, Ozturk R, et al. In vitro antimicrobial activity of silicone oil against endophthalmitis-causing agents. Retina. 1999;19:122–126. doi: 10.1097/00006982-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Shaarawy A, Grand MG, Meredith TA, Ibanez HE. Persistent endophthalmitis after intravitreal antimicrobial therapy. Ophthalmology. 1995;102:382–387. doi: 10.1016/s0161-6420(95)31011-1. [DOI] [PubMed] [Google Scholar]
- 25.United States Pharmacopeia. 27th rev. Rockville, MD: United States Pharmacopeial Convention; 2008. Pharmaceutical compounding—sterile preparations (USP Chapter 797) pp. 1–61. [Google Scholar]
- 26.Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006;26:495–511. doi: 10.1097/01.iae.0000225766.75009.3a. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez S, Rosenfeld PJ, Stewart MW, et al. Avastin doesn't blind people, people blind people. Am J Ophthalmol. 2012;153:196–203. doi: 10.1016/j.ajo.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Stucki C, Sautter AM, Favet J, Bonnabry P. Microbial contamination of syringes during preparation: the direct influence of environmental cleanliness and risk manipulation on end-product quality. Am J Health Syst Pharm. 2009;66:2032–2036. doi: 10.2146/ajhp070681. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Services. Office of Clinical Standards and Quality/Survey & Certification Group. Safe use of single dose/single use medications to prevent healthcare-associated infections. [Accessed October 18, 2012];Memorandum to State Survey Agency Directors. 2012 Jun 15; Available at: https://www.premierinc.com/quality-safety/tools-services/safety/topics/guidelines/downloads/CMS-Policy-Single-Dose-Vials-June-2012.pdf.
- 30.American Academy of Ophthalmology Clinical Statements. [Accessed May 24, 2012];Verifying the source of compounded bevacizumab for intravitreal injections. 2012 Feb; Available at: http://one.aao.org/ce/practiceguidelines/clinicalstatements.aspx.