Abstract
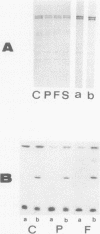
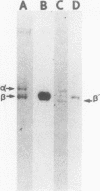
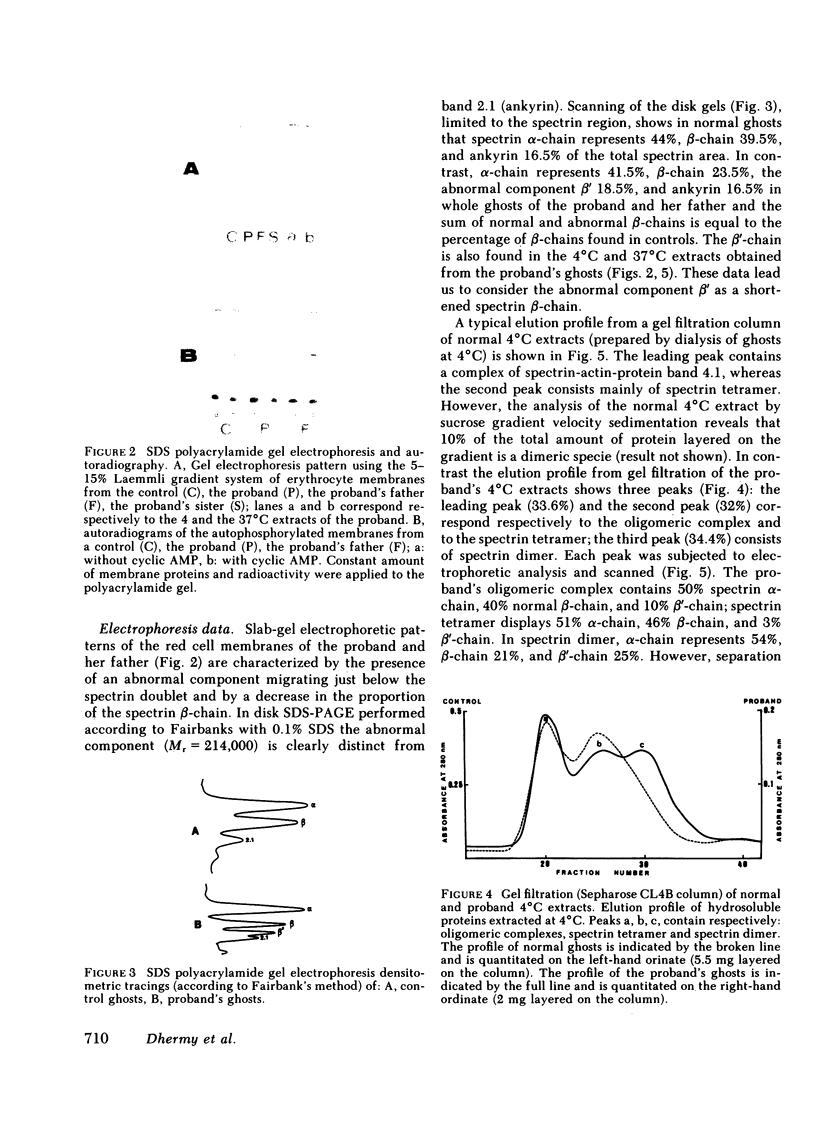
An electrophoretically fast-moving variant of the spectrin beta-chain was discovered in the erythrocyte membranes of a woman and her father who both exhibited elliptocytosis and mild hemolytic anemia. This abnormal beta'-subunit (Mr = 214,000) co-existed with a decreased normal beta-chain and represented about half of the total beta-chains in the membrane. In contrast to the spectrin beta-chain, the beta'-chain was phosphorylated neither in the membrane by endogenous protein kinases nor in solution by pure membrane casein kinase whether or not the spectrin was dephosphorylated by erythrocyte cytosolic spectrin phosphatase. The presence of the beta'-chain was associated with a defective self-association of spectrin dimer to form tetramer as manifested by: (a) an excess of spectrin dimer in the 4 degrees C spectrin crude extract, (b) a defective self-association of the spectrin dimer in the 37 degrees C crude spectrin extracts. Gel electrophoretic analysis of the tetramer and dimer species isolated from the proband's 4 degrees C extract showed that the tetramer contained trace amounts of the beta'-chain, whereas in contrast, a large proportion of beta'-chain was present in the dimer. These results demonstrated the responsibility of the beta'-chain for the defective reassociation of spectrin dimer into tetramer. The study of this abnormal spectrin confirms the participation of spectrin beta-chain in dimer-dimer association and strongly suggests that the phosphorylation sites of the normal beta-chain are located at the end of the molecule involved in the dimer-dimer interactions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Chui D. H., Bennett V. A molecular defect in two families with hemolytic poikilocytic anemia: reduction of high affinity membrane binding sites for ankyrin. J Clin Invest. 1981 Dec;68(6):1566–1576. doi: 10.1172/JCI110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloisio N., Dorléac E., Girot R., Delaunay J. Analysis of the red cell membrane in a family with hereditary elliptocytosis--total or partial of protein 4.1. Hum Genet. 1981;59(1):68–71. doi: 10.1007/BF00278857. [DOI] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Boivin P., Galand C. Compartimentalization of spectrin-phosphorylating enzyme in human erythrocytes. Biochem Biophys Res Commun. 1980 Mar 13;93(1):24–28. doi: 10.1016/s0006-291x(80)80240-3. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Spectrin-actin interaction. Phosphorylated and dephosphorylated spectrin tetramer cross-link F-actin. J Biol Chem. 1979 Sep 10;254(17):8620–8627. [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Tryptic digestion of spectrin in variants of hereditary elliptocytosis. J Clin Invest. 1981 May;67(5):1241–1248. doi: 10.1172/JCI110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. Spectrin-dependent and -independent association of F-actin with the erythrocyte membrane. J Cell Biol. 1980 Aug;86(2):694–698. doi: 10.1083/jcb.86.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feo C. J., Fischer S., Piau J. P., Grange M. J., Tchernia G. Première observation de l'absence d'une protéine de la membrane érythrocytaire (bande 4(1)) dans un cas d'anémie elliptocytaire familiale. Nouv Rev Fr Hematol. 1980;22(4):315–325. [PubMed] [Google Scholar]
- Fowler V., Taylor D. L. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980 May;85(2):361–376. doi: 10.1083/jcb.85.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrow C. E., Jr, Allen J. E., Rasmussen H. Phosphorylation of an endogenous membrane protein by an endogenous, membrane-associated cyclic adenosine 3',5'-monophosphate-dependent protein kinase in human erythrocyte ghosts. J Biol Chem. 1972 Dec 25;247(24):8145–8153. [PubMed] [Google Scholar]
- Harris H. W., Jr, Lux S. E. Structural characterization of the phosphorylation sites of human erythrocyte spectrin. J Biol Chem. 1980 Dec 10;255(23):11512–11520. [PubMed] [Google Scholar]
- Ji T. H., Kiehm D. J., Middaugh C. R. Presence of spectrin tetramer on the erythrocyte membrane. J Biol Chem. 1980 Apr 10;255(7):2990–2993. [PubMed] [Google Scholar]
- Kam Z., Josephs R., Eisenberg H., Gratzer W. B. Structural study of spectrin from human erythrocyte membranes. Biochemistry. 1977 Dec 13;16(25):5568–5572. doi: 10.1021/bi00644a028. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J. T. Defective spectrin dimer-dimer association with hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2072–2076. doi: 10.1073/pnas.79.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V. Visualization of the "membrane skeleton" in human erythrocytes by freeze-etching. Eur J Cell Biol. 1981 Oct;25(2):265–271. [PubMed] [Google Scholar]
- Ralston G., Dunbar J., White M. The temperature-dependent dissociation of spectrin. Biochim Biophys Acta. 1977 Mar 28;491(1):345–348. doi: 10.1016/0005-2795(77)90072-1. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P. DNase-I-dependent dissociation of erythrocyte cytoskeletons. J Cell Biol. 1979 Apr;81(1):266–270. doi: 10.1083/jcb.81.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli M. B., John K. M., Lux S. E. Elliptical erythrocyte membrane skeletons and heat-sensitive spectrin in hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1911–1915. doi: 10.1073/pnas.78.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]