Abstract
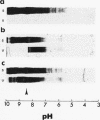
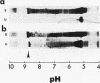
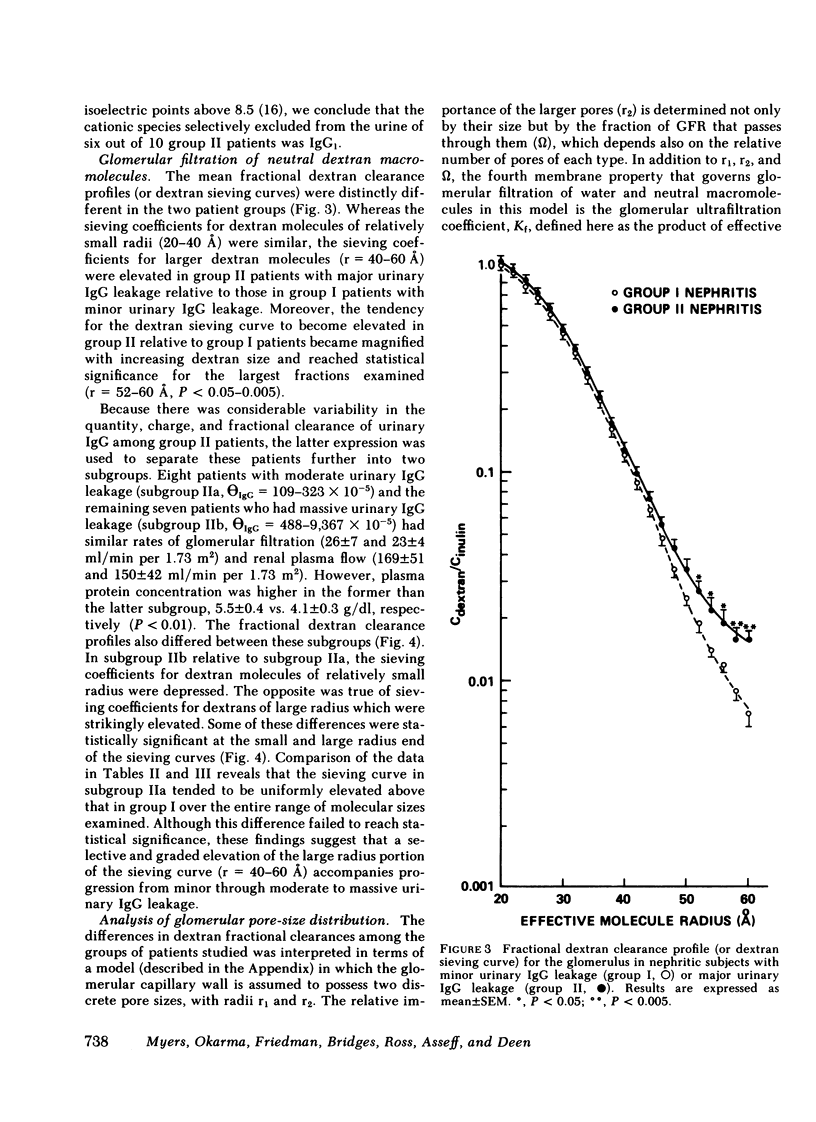
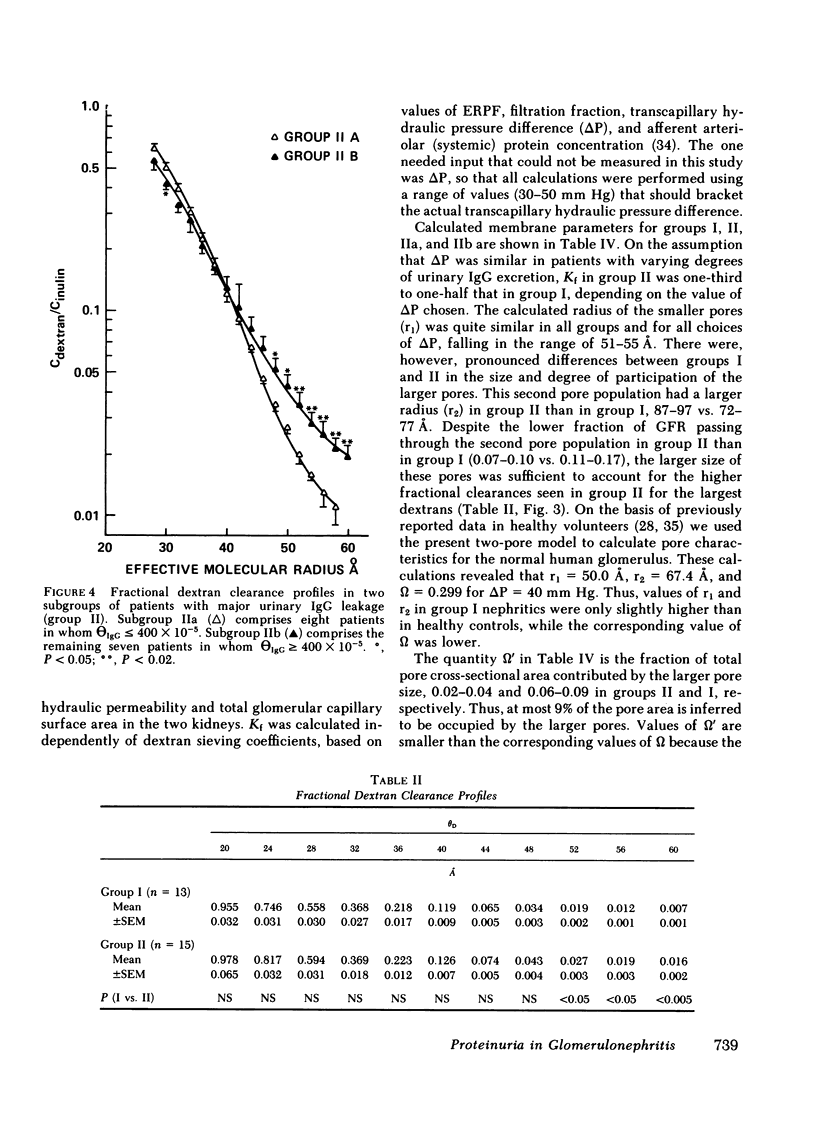
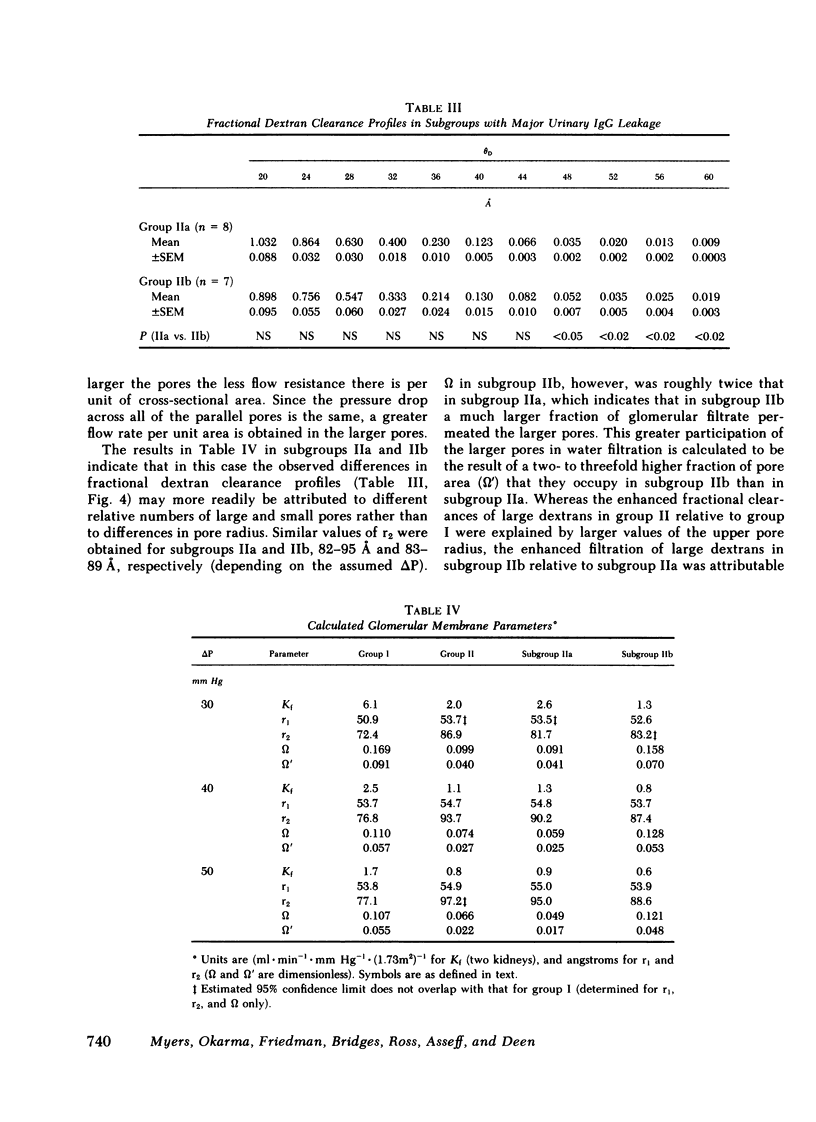
We evaluated glomerular barrier function in 28 patients with glomerulonephritis. Neutral dextrans of graded size were used to characterize the size-selective properties of the barrier. Charge selectivity was characterized by electrofocusing excreted urinary proteins. A fractional IgG clearance (relative to freely permeable inulin), smaller or greater than 100 x 10(-5) was used to distinguish patients with minor (group I, n = 13) and major (group II, n = 15) urinary IgG leakage, respectively. Fractional clearances of smaller dextrans (radii 20-50 A) were similar, but those of larger dextrans (radii 52-60 A) were elevated in group II relative to group I patients. A model of solute transport through a bimodal pore size distribution revealed the values for pore radius in the lower mode to approximate 51-55 A in both group I and group II patients. Pore radius in the upper mode, by contrast, was much larger in group II than in group I patients, approximating 87-97 vs. 72-77 A, respectively. Electrofocusing of urinary protein from group I patients revealed mostly albumin (isoelectric point 5.2). In group II patients, however, immunoglobulin excretion was copious. Moreover, the distribution of anionic, neutral, and cationic species (isoelectric points 5.5-8.5) in urinary and plasma eluates of IgG2 and IgG4 was similar. We conclude that when glomerulonephritis is associated with selective albuminuria, as in group I,, there is an isolated reduction of electrostatic retardation of relatively small anionic proteins. Major urinary IgG leakage (group II), however, appears to result from the development in the glomerular membrane of a subpopulation of enlarged pores that are highly permeable towards proteins of large size and varying charge.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M. Glomerular epithelial alterations resulting from sialic acid surface coat removal. Kidney Int. 1979 Apr;15(4):376–385. doi: 10.1038/ki.1979.49. [DOI] [PubMed] [Google Scholar]
- Bennett C. M., Glassock R. J., Chang R. L., Deen W. M., Robertson C. R., Brenner B. M., Troy J. L., ueki I. R., Rasmussen B. Permselectivity of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using dextran sulfate. J Clin Invest. 1976 May;57(5):1287–1294. doi: 10.1172/JCI108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau E. B., Haas J. E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973 Apr;28(4):477–481. [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Humes H. D., Glassock R. J., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest. 1978 Jan;61(1):72–78. doi: 10.1172/JCI108927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Glomerular permselectivity: barrier function based on discrimination of molecular size and charge. Am J Physiol. 1978 Jun;234(6):F455–F460. doi: 10.1152/ajprenal.1978.234.6.F455. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P., LAUSON H. D., EDER H. A., GREIF R. L., HILLER A. A study on the mechanism of proteinuria in patients with the nephrotic syndrome. J Clin Invest. 1954 Apr;33(4):621–628. doi: 10.1172/JCI102933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. S., Blandford G. The simple assessment of selectivity in heavy proteinuria. Lancet. 1966 Jul 30;2(7457):242–247. doi: 10.1016/s0140-6736(66)92539-6. [DOI] [PubMed] [Google Scholar]
- Carrie B. J., Myers B. D. Proteinuria and functional characteristics of the glomerular barrier in diabetic nephropathy. Kidney Int. 1980 May;17(5):669–676. doi: 10.1038/ki.1980.78. [DOI] [PubMed] [Google Scholar]
- Carrie B. J., Salyer W. R., Myers B. D. Minimal change nephropathy: an electrochemical disorder of the glomerular membrane. Am J Med. 1981 Feb;70(2):262–268. doi: 10.1016/0002-9343(81)90760-9. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Bennett C. M., Glassock R. J., Brenner B. M., Troy J. L., Ueki I. F., Rasmussen B. Permselectivity of of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using neutral dextran. J Clin Invest. 1976 May;57(5):1272–1286. doi: 10.1172/JCI108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Robertson C. R., Deen W. M., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. I. Theoretical considerations. Biophys J. 1975 Sep;15(9):861–886. doi: 10.1016/S0006-3495(75)85862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Bohrer M. P., Brenner B. M. Macromolecule transport across glomerular capillaries: application of pore theory. Kidney Int. 1979 Sep;16(3):353–365. doi: 10.1038/ki.1979.138. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Satvat B. Determinants of the glomerular filtration of proteins. Am J Physiol. 1981 Aug;241(2):F162–F170. doi: 10.1152/ajprenal.1981.241.2.F162. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Satvat B., Jamieson J. M. Theoretical model for glomerular filtration of charged solutes. Am J Physiol. 1980 Feb;238(2):F126–F139. doi: 10.1152/ajprenal.1980.238.2.F126. [DOI] [PubMed] [Google Scholar]
- Eisenbach G. M., Liew J. B., Boylan J. W., Manz N., Muir P. Effect of angiotensin on the filtration of protein in the rat kidney: a micropuncture study. Kidney Int. 1975 Aug;8(2):80–87. doi: 10.1038/ki.1975.83. [DOI] [PubMed] [Google Scholar]
- Fjeldbo W., Stamey T. A. Adapted method for determination of inulin in serum and urine with an AutoAnalyzer. J Lab Clin Med. 1968 Aug;72(2):353–358. [PubMed] [Google Scholar]
- GREGOIRE F., MALMENDIER C., LAMBERT P. P. The mechanism of proteinuria, and a study of the effects of hormonal therapy in the nephrotic syndrome. Am J Med. 1958 Oct;25(4):516–531. doi: 10.1016/0002-9343(58)90041-x. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- HARDWICKE J., SQUIRE J. R. The relationship between plasma albumin concentration and protein excretion in patients with proteinuria. Clin Sci. 1955 Aug;14(3):509–530. [PubMed] [Google Scholar]
- HARVEY R. B., BROTHERS A. J. Renal extraction of para-aminohippurate and creatinine measured by continuous in vivo sampling of arterial and renal-vein blood. Ann N Y Acad Sci. 1962 Oct 31;102:46–54. doi: 10.1111/j.1749-6632.1962.tb13624.x. [DOI] [PubMed] [Google Scholar]
- Harboe M., Fölling I. Recognition of two distinct groups of human IgM and IgA based on different binding to staphylococci. Scand J Immunol. 1974;3(4):471–482. doi: 10.1111/j.1365-3083.1974.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Hardwicke J., White R. H., Williams A. Molecular size of IgG in patients with focal glomerulosclerosis. Clin Nephrol. 1976 Jul;6(1):290–294. [PubMed] [Google Scholar]
- Hourani M. R., Mayor G. H., Greenbaum D. S., Hugget D. O., Patterson M. J. Hepatitis B surface antigen in urine of hemodialysis patients. Kidney Int. 1978 Apr;13(4):324–328. doi: 10.1038/ki.1978.46. [DOI] [PubMed] [Google Scholar]
- Hulme B., Hardwicke J. Human glomerular permeability to macromolecules in health and disease. Clin Sci. 1968 Jun;34(3):515–529. [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Shaffer S. J. Acute reversible proteinuria induced by infusion of the polycation hexadimethrine. Kidney Int. 1981 Jul;20(1):7–17. doi: 10.1038/ki.1981.98. [DOI] [PubMed] [Google Scholar]
- JOACHIM G. R., CAMERON J. S., SCHWARTZ M., BECKER E. L. SELECTIVITY OF PROTEIN EXCRETION IN PATIENTS WITH THE NEPHROTIC SYNDROME. J Clin Invest. 1964 Dec;43:2332–2346. doi: 10.1172/JCI105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Landwehr D. M., Carvalho J. S., Oken D. E. Micropuncture studies of the filtration and absorption of albumin by nephrotic rats. Kidney Int. 1977 Jan;11(1):9–17. doi: 10.1038/ki.1977.2. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mogensen C. E., Sølling K., Vittinghus E. Studies on mechanisms of proteinuria using amino-acid-induced inhibition of tubular reabsorption in normal and diabetic man. Contrib Nephrol. 1981;26:50–65. doi: 10.1159/000396104. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Chui F., Hilberman M., Michaels A. S. Transtubular leakage of glomerular filtrate in human acute renal failure. Am J Physiol. 1979 Oct;237(4):F319–F325. doi: 10.1152/ajprenal.1979.237.4.F319. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Kirschbaum B. B., Landwehr D. M. Micropuncture studies of the mechanisms of normal and pathologic albuminuria. Contrib Nephrol. 1981;24:1–7. doi: 10.1159/000395223. [DOI] [PubMed] [Google Scholar]
- Pessina A. C., Hulme B., Peart W. S. Renin induced proteinuria and the effects of adrenalectomy. II. Morphology in relation to function. Proc R Soc Lond B Biol Sci. 1972 Jan 18;180(1058):61–71. doi: 10.1098/rspb.1972.0005. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Venkatachalam M. A. Glomerular permeability: in vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 1977 Jan;11(1):44–53. doi: 10.1038/ki.1977.6. [DOI] [PubMed] [Google Scholar]
- Robson A. M., Giangiacomo J., Kienstra R. A., Naqvi S. T., Ingelfinger J. R. Normal glomerular permeability and its modification by minimal change nephrotic syndrmone. J Clin Invest. 1974 Nov;54(5):1190–1199. doi: 10.1172/JCI107862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler M. W., Venkatachalam M. A., Cotran R. S. Glomerular epithelium: structural alterations induced by polycations. Science. 1975 Aug 1;189(4200):390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- Tiggeler R. G., Hulme B., Wijdeveld P. G. Effect of indomethacin on glomerular permeability in the nephrotic syndrome. Kidney Int. 1979 Sep;16(3):312–321. doi: 10.1038/ki.1979.133. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Rennke H. G. The structural and molecular basis of glomerular filtration. Circ Res. 1978 Sep;43(3):337–347. doi: 10.1161/01.res.43.3.337. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Winetz J. A., Robertson C. R., Golbetz H. V., Carrie B. J., Salyer W. R., Myers B. D. The nature of the glomerular injury in minimal change and focal sclerosing glomerulopathies. Am J Kidney Dis. 1981 Sep;1(2):91–98. doi: 10.1016/s0272-6386(81)80035-2. [DOI] [PubMed] [Google Scholar]