Abstract
Background
Experimental studies have demonstrated that unstable repolarization dynamics is a risk factor of arrhythmia. We have recently developed an algorithm to detect QT interval (QTI) instability from the clinical ECG. In this study we developed a clinical arrhythmia risk stratification index based on the detection of QTI instability.
Methods
Intracardiac electrocardiograms were recorded at rest in 114 patients with implanted ICDs. Patients were followed up until appropriate ICD therapy or death occurred, whichever came first. Each recording was divided into 1-min episodes (minECGs); the instability in QTI dynamics, if any, of each minECG was detected with our algorithm. An arrhythmia risk index termed QTI instability index (QTII) was defined as the number of minECGs with unstable QTI dynamics normalized by the number of minECGs with premature activations (PA). The performance of QTII in arrhythmia risk stratification was examined with survival analysis, and was compared with other risk indices, such as mean RR interval (RRI), the standard deviation of RRI and QTI, and the frequency of PA. We hypothesized that the index QTII, which account for multiple risk factors and their interdependence, perform better than indices quantifying individual arrhythmia risk factors in the stratification of arrhythmia risk.
Results
The results of survival analysis show that QTII outperformed all other studied indices in arrhythmia risk stratification and was the only independent indicator of arrhythmia propensity in a multivariate survival model.
Conclusion
QTII is a promising arrhythmia risk stratification index.
Keywords: arrhythmia, implantable cardioverter-defibrillator, QT interval electrocardiography, risk stratification, sudden death
Cardiovascular disease remains the number one killer in the United States. 1 Research has reported that about half of the total deaths from cardiovascular diseases resulted from sudden cardiac death (SCD), typically caused by arrhythmia. 2-4 The most effective anti-arrhythmia therapy is the implantable cardioverter-defibrillator (ICD). 5-8 However, the prophylactic implantation of ICDs has been questioned because of its high cost-effectiveness ratio.9-11 A reliable arrhythmia risk stratification approach would reduce this cost-effectiveness ratio by identifying patients who would benefit from ICD implantation, thus optimizing ICD prophylactic implantation and reducing mortality due to arrhythmia.
Much effort has been devoted to stratifying patients’ propensity for arrhythmias and SCD using indices that quantify different arrhythmia risk factors. For example, the heart rate variability index (HRV) quantifies heart rate dynamics, which is known to contribute to arrhythmia likelihood,12, 13 while the ventricular premature beats index (VPB) quantifies the presence of frequent ventricular premature activations (PAs), also implicated in arrhythmia propensity.14, 15 However, the performance of these indices has not been consistent across various studies, with some studies reporting good performance of the indices,12-17 while others documenting failures.18-20 A possible explanation is that each of these indices assesses a single arrhythmia risk factor, while propensity for arrhythmia has been found to be a function of multiple risk factors, among which heart rate dynamics, the presence of frequent PAs, and QTI dynamics. 21, 22 If this is the case, an index that quantifies multiple arrhythmia risk factors simultaneously and accounts for their interdependence has a better chance to correctly stratify arrhythmia risk.
Such indices are already in existence. The QT variability index (QTVI) quantifies the dynamics of both heart rate and QTI;23 accounting for their interdependence; several studies have reported that QTVI successfully stratified arrhythmia risk.24, 25 Heart rate turbulence (HRT), an index that stratifies the risk of mortality and arrhythmia, is based on the relationship between PA occurrence and heart rate dynamics, and has shown promising results.26
In a recent study, we developed a novel methodology27 for detecting QT interval (QTI) dynamics instability from the ECG. The algorithm defines QTI dynamics as a function of both its preceding R-R interval (RRI) and activation history and assesses the stability of this function. Because the effect of short-term memory is included in the algorithm,27 the latter uses directly the clinical ECG to analyze the instability in QTI dynamics. In contrast to QTI dynamic instability assessment using the restitution relation,28, 29 our methodology does not require a specific pacing protocol, and is thus noninvasive. Using this novel methodology, we found that unstable QTI dynamics preceded the onset of ventricular tachycardia in patients with cardiac disease.30 The results identified the presence of instability in QTI dynamics as an indicator of arrhythmia risk. Furthermore, we found that a positive correlation existed between PA frequency and QTI instability in the studied patient population.30
In the present study, we build on these recent finding to develop a new arrhythmia risk stratification index. We term the index QTI instability index (QTII). It assesses the likelihood of arrhythmia by quantifying the interdependence of two risk factors, namely the instability in QTI dynamics and the occurrence of PAs. We then apply the new index to ECG recordings from patients with implanted ICDs to determine whether it successfully stratifies the risk of arrhythmia. In addition, we test the hypothesis that an index that accounts for more than one risk factor and the possible interdependence between those performs better in risk stratification of arrhythmia than risk indices quantifying individual arrhythmia risk factors.
METHODS
A detailed description of all methodology, including study population, data collection, and end points; ECG signal processing; methods for detecting instability in QTI dynamics from the EGM; definition of the new arrhythmia risk index QTII; and data analysis can be found in the Online Supplement.
RESULTS
Clinical demographics
The clinical characteristics of all enrolled patients were compared between event and no-event groups (Table 1), as well as between patients with and without large (Q3) QTII (Table 2). We found that patients with large QTII value have a significantly larger percentage of AF occurrences. No other significant differences in clinical characteristics were observed in these comparisons.
Table 1.
The comparisons of clinical characteristics between patients with and without VT/VF events.
| Clinical Characteristic | Event (27) | No-event (87) | P |
|---|---|---|---|
| Age (mean/sd) | 61.1±15.1 | 64.9±13 | 0.24 |
| Male, (%) | 87.5 | 75.4 | 0.22 |
| Ischemic CM with MI history, (%) | 69.6 | 68.2 | 0.9 |
| LVEF±SD, % | 33.0±11.7 | 33.9±12.3 | 0.76 |
| NYHA class I, (%) | 34.8 | 21.2 | 0.76 |
| NYHA class II, (%) | 34.8 | 43.9 | |
| NYHA class III, (%) | 18.2 | 17.4 | |
| Diabetes mellitus, (%) | 47.8 | 28.8 | 0.1 |
| Hypertension, (%) | 78.3 | 77.3 | 0.9 |
| CABG, (%) | 47.8 | 37.9 | 0.41 |
| PTCA, (%) | 30.4 | 28.8 | 0.88 |
| beta-blockers, (%) | 81.5 | 84.9 | 0.49 |
| Atria Fibrillation (%) | 17.4 | 18.2 | 0.93 |
| Class III antiarrhythmic medication, (%) | 30.4 | 24.2 | 0.56 |
CM: ischemic cardiomyopathy. MI: myocardial infarction. LVEF: left ventricular ejection fraction. NYHA: New York Heart Association. CABG: Coronary artery bypass grafting. PTCA: Percutaneous transluminal coronary angioplasty.
Table 2.
The comparisons of clinical characteristics between patients with and without large QTII (> QTII Q3) value.
| Clinical Characteristic | QTII < QTII Q3 (78) |
QTII >= QTII Q3 (36) |
p |
|---|---|---|---|
| Age (mean/sd) | 63.8±14.3 | 64.3±12.4 | 0.43 |
| Male, (%) | 73.8 | 87.5 | 0.13 |
| Ischemic CM with MI history, (%) | 67.2 | 71.0 | 0.72 |
| LVEF±SD, % | 34.5±12.4 | 32.1±11.5 | 0.82 |
| NYHA class I, (%) | 25.9 | 22.6 | 0.19 |
| NYHA class II, (%) | 34.5 | 54.8 | |
| NYHA class III, (%) | 19.0 | 16.1 | |
| Diabetes mellitus, (%) | 29.3 | 41.9 | 0.23 |
| Hypertension, (%) | 74.1 | 83.9 | 0.3 |
| CABG, (%) | 37.9 | 45.2 | 0.51 |
| PTCA, (%) | 27.6 | 32.3 | 0.64 |
| beta-blockers, (%) | 81.8 | 88.9 | 0.56 |
| Atria Fibrillation (%) | 10.3 | 32.3 | 0.018 |
| Class III antiarrhythmic medication, (%) | 24.1 | 29.0 | 0.62 |
CM: ischemic cardiomyopathy. MI: myocardial infarction. LVEF: left ventricular ejection fraction. NYHA: New York Heart Association. CABG: Coronary artery bypass grafting. PTCA: Percutaneous transluminal coronary angioplasty.
Receiver operating characteristic (ROC) curve
The results of the ROC tests show that, among all studied indices, QTII was the only significant index of arrhythmia risk. The areas under the ROC curves of QTII, RRImean, and fPA were 0.61±0.06 (p=0.04), 0.5±0.06 (p=0.49), and 0.51±0.064 (p = 0.44), respectively.
Survival analysis
The results of Kaplan-Meier analysis (Table 3) demonstrate that QTII (Figure 2, p=0.01) had a better performance in the risk stratification for arrhythmia compared with the other indices. Kaplan-Meier analysis also demonstrated that QTII (p=0.002) outperformed the other indices in the survival analysis for arrhythmia/death. The results of MC1 (Table 3) showed that, among all studied indices, QTII was the only independent index of arrhythmia risk. The hazard ratio of QTII in MC1 was 3.68 (p=0.006). MC1 modeling also demonstrated that QTII (p=0.003) and QTstd (p=0.02) were significant indices of arrhythmia/death risk.
Table 3.
The results of survival analyses (Kaplan-Meier and multivariate Cox model MC1) with respect to the primary endpoint event.
| Kaplan-Meier | MC1 | ||
|---|---|---|---|
| P | HR | P | |
| RRImean(29) | 0.94 | 1.00 | 0.99 |
| RRIstd(62) | 0.82 | 1.42 | 0.45 |
| fPA(29) | 0.72 | 0.70 | 0.57 |
| QTIstd(29) | 0.33 | 0.41 | 0.13 |
| QTII(36) | 0.01 | 3.68 | 0.006 |
The number in the parenthesis following each risk index is the number of patients above Q3.
Figure 2.
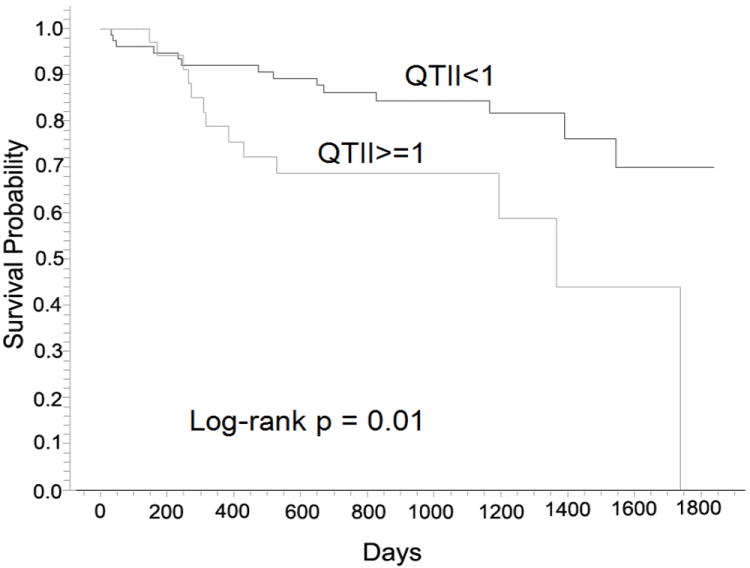
Arrhythmia-free survival probability curves for QTII>=1 and QTII<1 resulting from Kaplan-Meier analysis.
The performance of QTII in risk stratification for arrhythmia was further examined with MC2, adjusted for the demographic indices DM, LVEF, and sex. QTstd was also included in this analysis because it had the smallest p value (p=0.12) resulting from MC1 among all indices other than QTII. Among all demographic indices, DM was the only significant indicator of arrhythmia (p=0.02), as identified with Kaplan-Meier analysis. The results of MC2 (Table 4) show that although QTII had better performance when compared against the other indices included in MC2, none of the indices in MC2 was an independent indicator of arrhythmia risk.
Table 4.
The results of multivariate Cox model MC2 with respect to the primary endpoint event.
| With CLASS III AA | Without CLASS III AA | |||
|---|---|---|---|---|
| Hazard ratio (95% C.I.) | P | Hazard ratio (95% C.I.) | p | |
| DM | 2.29 (0.96, 5.43) | 0.06 | 2.06 (0.72, 5.88) | 0.18 |
| LVEF | 1.51 (0.63, 3.61) | 0.35 | 2.47 (0.84, 7.28) | 0.1 |
| QTII | 2.48 (0.99, 6.19) | >0.05 | 4.32 (1.37, 13.6) | 0.01 |
| QTstd | 0.56 (0.18, 1.83) | 0.35 | 0.22 (0.05, 1.1) | 0.07 |
| sex | 0.67 (0.19, 2.42) | 0.54 | 0.65 (0.14, 3.02) | 0.58 |
CLASS III AA: Class III antiarrhythmic medication. DM: Diabetes mellitus. LVEF: left ventricular ejection fraction.
It has been reported that the performance of indices for risk stratification of arrhythmia could be weaker for patients who use CLASS III AA.24 Therefore, we hypothesized that CLASS III AA might have a similar effect on QTII in this study. To exclude this effect, we repeated MC2 for patients who did not have CLASS III AA. The results (Table 4) show that QTII was the only independent indicator of arrhythmia in these patients. The hazard ratio of QTII was 4.32 (p=0.01). Table 4 also demonstrates that, among all other indices in this MC2, LVEF was associated with the highest hazard ratios. The p-value of QTstd was very close to 0.05, and the hazard ratio was 0.22. The hazard ratios of fPA, RRImean, QTstd, and QTII resulting from MC1 and MC2 (before and after the exclusion of CLASS III AA patients) are presented in Figure 3.
Figure 3.
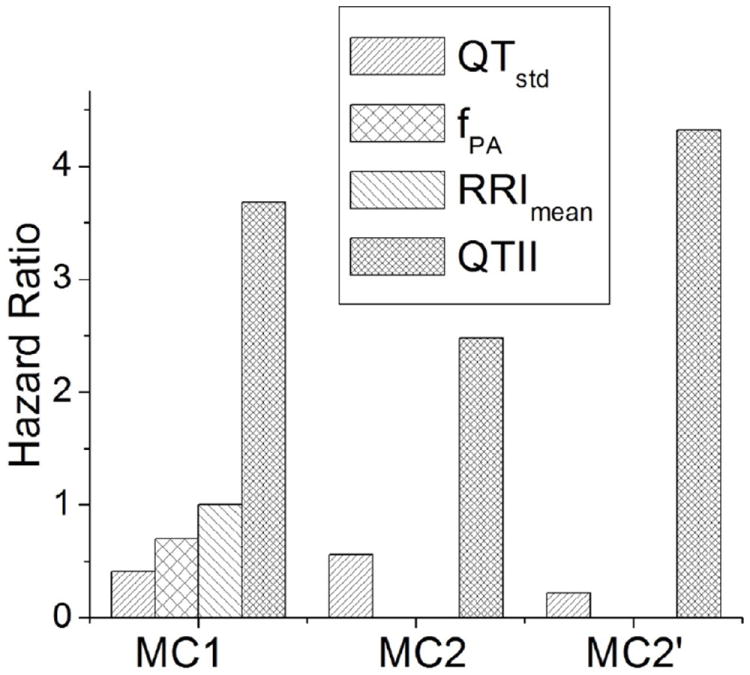
Hazard ratios for fPA, QTstd, RRImean, and QTII in relation to arrhythmia events resulting from the univariate Cox model, multivariate Cox for all patients (MC2), and multivariate Cox model for Class III antiarrhythmic medication (CLASS III AA)-free patients (MC2’).
QTII performance
The comparison of QTII values between event and no-event groups (Table 5) shows that QTII was larger in the event group that had arrhythmia than in the no-event group, but the difference was insignificant (p=0.12). The results also show that QTII was significantly larger (p=0.04) in the event group that had arrhythmia/death, compared against the no-event group. We also compared the values of QTstd between event and no-event groups; the results (Table 5) indicate that QTstd was smaller in the event groups (arrhythmia (p=0.21) and arrhythmia/death (p=0.16)), but the differences were insignificant.
Table 5.
Comparisons of QTstd and QTII between event and no-event groups with respect to the primary (arrhythmia) or secondary (arrhythmia/death) endpoint events.
| Arrhythmia | Arrhythmia/death | |||||
|---|---|---|---|---|---|---|
| Mean/Median | p | Mean/Median | p | |||
| Event | NoEvent | Event | NoEvent | |||
| QTstd (ms) | 4.6 (3.5-6.8) | 5.5 (3.9-8.6) | 0.21 | 4.64 (3.5-6.9) | 5.5 (3.9-9.1) | 0.16 |
| QTII | 0.71±0.36 | 0.6±0.34 | 0.12 | 0.71±0.35 | 0.57±0.33 | 0.04 |
The value of QTII was also cross-tabulated with each demographic index to examine whether QTII was affected by these indices. The results show that QTII was significantly (p=0.04) larger in AF patients (0.81) than in patients without AF (0.61). To examine whether the risk stratification performance of QTII is affected by the presence of AF, we repeated the Kaplan-Meier analysis and MC1 after all AF patients were excluded. The Kaplan-Meier analysis results show that QTII was a significant indicator of both arrhythmia events (p=0.025) and arrhythmia/death (p=0.034) in this case. The results of MC1 demonstrate that QTII is an independent indicator of arrhythmia (hazard ratio=3.97, p=0.007) and arrhythmia/death (hazard ratio=2.74, p=0.02) in AF-free patients. No significant difference in QTII was found in other demographic groups, suggesting that QTII is independent of these demographics.
The effect of CLASS III AA on QTII performance
As presented in Table 4, the performance of QTII in risk stratification for arrhythmia was found to be weaker when CLASS III AA patients were included. To better understand the relationship between QTII and CLASS III AA, we compared the mean QTII between CLASS III AA patients with and without arrhythmia as well as with and without arrhythmia/death. We found that QTII was significantly higher (p = 0.03) in CLASS III AA patients with arrhythmia/death (p=0.03). This difference was not observed in CLASS III AA patients with arrhythmia. This result is consistent with the distribution of QTII in the entire studied population as presented above (Table 5). We performed Kaplan-Meier analysis of CLASS III AA patients, and found that QTII cannot stratify arrhythmia risk in these patients (p=0.08). Kaplan-Meier analysis also showed that CLASS III AA insignificantly increased the arrhythmia-free survival in the studied population.
DISCUSSION
In this study, we proposed a new arrhythmia risk stratification index termed QTII, which accounts for two interdependent risk factors, QTI instability and the occurrence of PAs. We applied QTII to recordings collected from patients with implanted ICDs to stratify their risk of arrhythmia and all-cause death. The major findings of the study are: 1) QTII can be reliably used as a risk index of both arrhythmia and arrhythmia/death; 2) QTII, which quantifies two interdependent arrhythmia risk factors, outperformed, in the risk stratification of arrhythmia, the studied indices quantifying individual risk factors; and 3) the risk stratification performance of QTII with respect to arrhythmia-free survival was affected by CLASS III AA. These findings have important implications regarding the prognostic utility of QTII.
QTII and arrhythmia risk
The results of this study demonstrated that QTII is a promising new arrhythmia risk index. The mechanistic underpinnings by which QTII stratifies arrhythmia risk are discussed below.
QTII represents the probability that QTI dynamics becomes unstable when PA presents, as defined by Equation s1 in online supplement. Instability in QTI dynamics is a manifestation of ventricular repolarization instability in the ECG signal. Experimental results have demonstrated that unstable repolarization dynamics is unmasked by PAs; unstable repolarization dynamics increases repolarization heterogeneity in cardiac tissue,31, 32 which has been found to be proarrhythmic.29, 32, 33 These findings suggest that arrhythmia risk can be stratified by detecting the instability in repolarization dynamics. However, it has been very difficult to assess the degree of stability of the latter from clinical recordings, and thus arrhythmia risk stratification based on the assessment of repolarization instability has had a limited utility. 21, 22 In our previous study,27 we reported a novel algorithm that successfully detected the manifestation of repolarization instability in the clinical ECG recording; we, also observed a positive correlation between instability in QTI dynamics and PA frequency in patients with high risk of arrhythmia. However, it remained unknown whether patients with low arrhythmia risk could also exhibit unstable QTI dynamics and a positive correlation between QTI instability and PA frequency.
In this study, we used QTII to quantify the relationship between QTI dynamic instability and PA occurrence in patients with ICD. The results demonstrate that unstable QTI dynamics exists in both high and low arrhythmia risk patients, as evidenced by the non-zero mean values of QTII in both groups (Table 5). Kaplan-Meier analysis showed that the risk of arrhythmia in patients with unstable QTI dynamics is not significantly different from that in patients without unstable QTI dynamics (p=0.82). The results also show that the risk of arrhythmia is significantly higher in patients with larger QTII value (QTII>=1) compared to those with smaller QTII value (QTII<1), as demonstrated by Figures 2 and 3. These findings indicate that it is QTII (the probability that QTI dynamics becomes unstable when PA presents), but not QTI instability itself, that stratifies arrhythmia risk.
QTII vs. indices that quantify single arrhythmia risk factors
In this study, we hypothesize that an index that quantifies multiple arrhythmia risk factors and their interdependence performs better in risk stratification of arrhythmia than indices quantifying individual risk factors. The performance of QTII was compared to that of indices RRImean, RRIstd, QTIstd, and fPA, each quantifying a single risk factor. The ROC results of this study demonstrate that QTII was the only significant predictor of arrhythmia. Univariate survival analyses demonstrated that QTII was the only significant index of arrhythmia-free survival. In a multivariate survival model of arrhythmia (MC1), QTII was the only independent index. After excluding patients who received CLASS III AA, and after adjusting for LVEF, DM, and sex, QTII remained the only independent index of arrhythmia risk. Univariate survival analysis also found that QTII was a significant index (p<0.05) of risk for the combined endpoint of arrhythmia and all-cause death. These results demonstrate that QTII outperformed RRImean, RRIstd, QTIstd, and fPA, and thus confirmed our hypothesis.
Compared to other well-known VF risk indicators, a unique feature of QTII is that it is capable of accessing VF risk from short ECG recordings with high frequency of PAs. These recordings are excluded from the calculation of VF risk factors such as RMSSD, QTVI, Heart Rate Turbulence, and T-wave alternans. This unique feature makes QTII a powerful VF indicator that is easy to implement in clinical practice.
QTII and CLASS III AA
In this study, we found that the performance of QTII in stratifying arrhythmia risk was worse in patients with CLASS III AA. Our results demonstrate that the mean QTII values in the event and no-event groups of CLASS III AA patients were similar to those in the entire studied population (Table 5), indicating that QTII was not significantly affected by CLASS III AA. Kaplan-Meier analysis also demonstrated that the arrhythmia-free survival probability was insignificantly higher in CLASS III AA patients than in other patients, which indicates that CLASS III AA slightly improves arrhythmia-free survival. These findings suggest that the mechanisms by which CLASS III AA improves arrhythmia-free survival are different from those by which QTII stratifies arrhythmia risk. However, the sample size of CLASS III AA patients was relatively small to determine the actual relationship between QTII, CLASS III AA, and arrhythmia susceptibility. Studies with larger sample sizes are needed to address this issue.
Limitations
A limitation of the new arrhythmia risk stratification index QTII is that it cannot be applied to PA-free ECGs. The occurrence of PAs is a critical component of QTII. It unmasks the instability in QTI dynamics, as established previously.30 For this reason, 6 PA-free patients out of a total of 120 patients were excluded from this study.
The value of QTII is determined by the number of minECGs that have PAs (NPA). However, the PAs in different minECGs may differ from each other in frequency and the degree of prematurity. Additional research will be needed to determine whether the performance of QTII in arrhythmia risk stratification could be improved by including these factors into the index calculation.
The unequal length of ECG recordings used in this study could be a limitation in the calculation of the HRV parameters.
Finally, QTII accounts for the temporal variation in QTI, but does not account for the spatial variation in repolarization, which is a known risk factor of arrhythmia. The latter risk has been assessed by detecting the presence of T-wave alternans, widely considered to be an indicator of arrhythmia propensity. It is possible that the performance of QTII could be further improved if the spatial variation in repolarization could be accounted for in the calculation of QTII.
Supplementary Material
Figure 1.
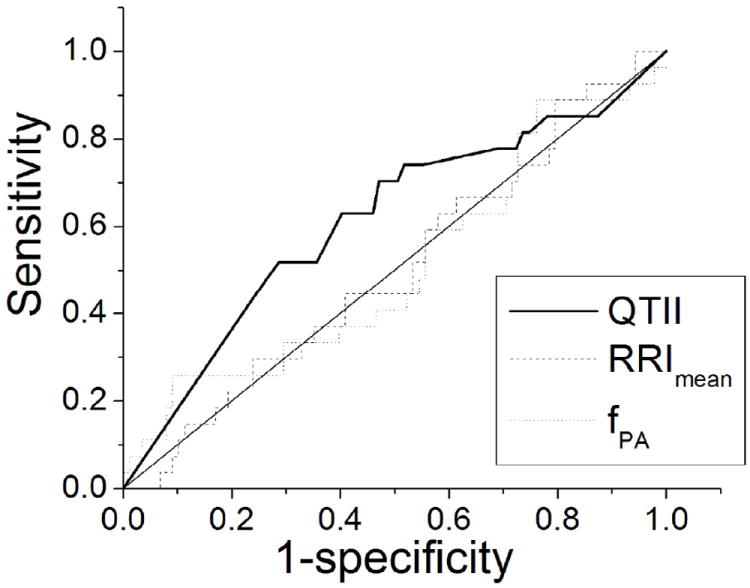
Receiver operating characteristic (ROC) curves of QTII, RRImean, and fPA.
Acknowledgments
FUNDING RESOURCES
This study is supported by NIH grant R01HL103428 to Dr. Trayanova. This study was also supported by Medtronic, Inc, as an investigator-initiated research project awarded to Dr. Tereshchenko and Dr. Berger.
Footnotes
DISCLOSURES
Dr. Trayanova is a partial owner of CardioSolv LLC. CardioSolv LLC was not involved in this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart Disease and Stroke Statistics 2010 Update. A Report From the American Heart Association. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Huikuri H, Castellanos A, Myerburg R. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Muller D, Agrawal R, Arntz H. How sudden is sudden cardiac death? Circulation. 2006;114:1146–1150. doi: 10.1161/CIRCULATIONAHA.106.616318. [DOI] [PubMed] [Google Scholar]
- 4.Luna ABd, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117(1):151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML ftMAD. Implantation Trial II Investigators Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Connolly S, Hallstrom A, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies Antiarrhythmics vs Implantable Defibrillator study Cardiac Arrest Study Hamburg Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 7.Ezekowitz JA, Armstrong PW, McAlister FA. Implantable Cardioverter Defibrillators in Primary and Secondary Prevention: A Systematic Review of Randomized, Controlled Trials. Ann Intern Med. 2003;138:445–452. doi: 10.7326/0003-4819-138-6-200303180-00007. [DOI] [PubMed] [Google Scholar]
- 8.Parkes J, Bryant J, R M. Implantable cardioverter-defibrillators in arrhythmias:a rapid and systematic review of effectiveness. Heart. 2002;87:438–442. doi: 10.1136/heart.87.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders GD, Hlatky MA, Owens DK. Cost-Effectiveness of Implantable Cardioverter–Defibrillators. N Engl J Med. 2005;353(14):1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 10.Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemicdilated cardiomyopathy and asymptomaticnonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41(10):1707–1712. doi: 10.1016/s0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 11.Owens DK, Sanders GD, Harris RA, et al. Cost-Effectiveness of Implantable Cardioverter Defibrillators Relative to Amiodarone for Prevention of Sudden Cardiac Death. Ann Intern Med. 1997;126(1):1–12. doi: 10.7326/0003-4819-126-1-199701010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kleiger RE, Miller JP, Jr, B JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 13.Rovere MTL, Pinna GD, Hohnloser SH, et al. Investigators obotATaRAMIA. Baroreflex Sensitivity and Heart Rate Variability in the Identification of Patients at Risk for Life-Threatening Arrhythmias. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 14.Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44:130–142. doi: 10.1161/01.cir.44.1.130. [DOI] [PubMed] [Google Scholar]
- 15.Rabkin S, Mathewson F, Tate R. Relationship of ventricular ectopy in men without apparent heart disease to occurrence of ischemic heart disease and suddent death. Am Heart J. 1981;101:135–142. doi: 10.1016/0002-8703(81)90655-4. [DOI] [PubMed] [Google Scholar]
- 16.Lanza G, Guido V, Galeazzi M, Mustilli M, Natali R, Ierardi C. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol. 1998;82:1323–1328. doi: 10.1016/s0002-9149(98)00635-3. [DOI] [PubMed] [Google Scholar]
- 17.Schulze R, Strauss H, Pitt B. Sudden death in the year following myocardial infarction: Relation to ventricular premature contractions in the late hospital phase and left ventricular ejection fraction. Am J Cardiol. 1977;62:192–199. doi: 10.1016/0002-9343(77)90314-x. [DOI] [PubMed] [Google Scholar]
- 18.Grimm W, Herzum I, Müller H-H, Christ M. Value of Heart Rate Variability to Predict Ventricular Arrhythmias in Recipients of Prophylactic Defibrillators with Idiopathic Dilated Cardiomyopathy. PACE. 2003;26 doi: 10.1046/j.1460-9592.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 19.Grimm W, Christ M, Bach J, Muller H, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy:results of the Marburg cardiomyopathy study. Circulation. 2003;108:2883–2891. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy H, Whitlock J, Sprague M, Kennedy L, Buckingham T, Goldberg R. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193–197. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour RF, Riccio ML, Locati EH, Maison-Blanche P, Coumel P, Schwartz PJ. Time- and rate-dependent alterations of the QT interval precede the onset of torsade de pointes in patients with acquired QT prolongation. J Am Coll Cardiol. 1997;30:209–217. doi: 10.1016/s0735-1097(97)00105-8. [DOI] [PubMed] [Google Scholar]
- 22.Fossa AA, Wisialowski T, Crimin K, et al. Analyses of dynamic beat-to-beat QT-TQ interval (ECG Restitution) changes in humans under normal sinus rhythm and prior to an event of torsades de pointes during QT prolongation caused by sotalol. Ann Noninvasive Electrocardiol. 2007;12:338–348. doi: 10.1111/j.1542-474X.2007.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96(5):1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 24.Tereshchenko LG, Fetics BJ, Domitrovich PP, Lindsay BD, Berger RD. Prediction of ventricular tachyarrhythmias by intracardiac repolarization variability analysis. Circ Arrhythm Electrophysiol. 2009;2:276–284. doi: 10.1161/CIRCEP.108.829440. [DOI] [PubMed] [Google Scholar]
- 25.Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-Beat Repolarization Lability Identifies Patients at Risk for Sudden Cardiac Death. J Cardiovasc Electrophysiol. 1998;9:899–908. doi: 10.1111/j.1540-8167.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Trayanova NA. A Novel Methodology for Assessing the Bounded-Input Bounded-Output Instability in QT Interval Dynamics: Application to Clinical ECG with Ventricular Tachycardia. IEEE Trans Biomed Eng. 2012;59:2111–2117. doi: 10.1109/TBME.2011.2170837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol. 1968;25:191–196. doi: 10.1152/jappl.1968.25.2.191. [DOI] [PubMed] [Google Scholar]
- 29.Koller ML, Riccio ML, Gilmour RF. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Hu Y, Berger RD, Fetics BJ, Trayanova NA. Unstable QT interval dynamics precedes VT onset in patients with acute myocardial infarction: A novel approach to detect instability in QT interval dynamics from clinical ECG. Circ Arrhythm Electrophysiol. 2011;4:858–866. doi: 10.1161/CIRCEP.110.961763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Fenton FH, Gray RA. Head-tail interactions in numerical simulations of reentry in a ring of cardiac tissue. Heart Rhythm. 2005;2:1038–1046. doi: 10.1016/j.hrthm.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 33.Garfinkel A, Kim Y, Voroshilovsky O, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


