Abstract
Aims
Activation of the angiotensin II type 1 (AT1) receptor has been shown to mediate the structural and electrical remodelling of the atrial myocardium associated with atrial fibrillation. We hypothesized that AT1 genotypic variation is associated with atrial fibrillation or diseases predisposing to atrial fibrillation, such as hypertension, heart failure, ischaemic heart disease and myocardial infarction, in the general population.
Methods
We resequenced the AT1 gene in 760 individuals with atrial fibrillation and identified two nonsynonymous variants (I103T and A244S), which were subsequently genotyped in the prospective Copenhagen City Heart Study (n = 10 603) and the prospective Copenhagen General Population Study (n = 60 647).
Results
Risk of atrial fibrillation for heterozygotes for AT1 genetic variants A244S and I103T/A244S vs. noncarriers was increased by 2.7-fold (95% confidence interval 1.5- to 5.1-fold) and 2.6-fold (95% confidence interval 1.6- to 4.2-fold), respectively, for men.
Conclusions
Heterozygosity for the nonsynonymous AT1 genetic variants A244S and I103T/A244S was associated with increased risk of atrial fibrillation in men. The AT1 recptor might be a target for the pharmaceutical industry. This finding needs to be validated in independent studies.
Keywords: AT1 gene, atrial fibrillation, epidemiology, genetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
A meta-analysis from 2010 demonstrated substantial benefits from inhibition of the renin–angiotensin system in both primary and secondary prevention of atrial fibrillation, suggesting that the angiotensin II type I (AT1) receptor plays a central role in the pathogenesis of atrial fibrillation. However, there was substantial heterogeneity among trials. Thus, the question of whether the AT1 receptor plays a central role in the pathogenesis of atrial fibrillation still remains unsolved.
WHAT THIS STUDY ADDS
This study reveals that heterozygosity for AT1 A244S and I103T/A244S is associated with risk of atrial fibrillation in men. The finding supports the notion of the AT1 receptor playing a role in the pathogenesis of atrial fibrillation, and the AT1 receptor might thus be a target for the pharmaceutical industry.
Introduction
The angiotensin II type 1 (AT1) receptor 1 is the primary effecter of the renin–angiotensin system (RAS), and it serves as a key regulator of cardiovascular physiology, acting to maintain salt and fluid homeostasis, as well as blood pressure 2. Activation of the AT1 receptor also induces inflammation, cell proliferation and fibrosis, all processes that are involved in cardiovascular disease. Activation of the AT1 receptor has been shown to mediate the structural and electrical remodelling of the atrial myocardium associated with atrial fibrillation (AF) 3, 4. Atrial fibrillation is the most common cardiac arrhythmia and is increasing in prevalence as the population ages.
A meta-analysis from 2010 demonstrated substantial benefits from inhibition of the RAS by angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in both primary and secondary prevention of atrial fibrillation 5, suggesting that the AT1 receptor plays a central role in the pathogenesis of atrial fibrillation. However, there was substantial heterogeneity among trials, and the recent European Society of Cardiology guidelines on atrial fibrillation management state that angiotensin receptor blocker therapy is not recommended as a first-line treatment for atrial fibrillation, that is, if not indicated for other reasons 6. Thus, the question of whether the AT1 receptor plays a central role in the pathogenesis of AF still remains unresolved.
We therefore tested the following hypotheses: AT1 genotypic variation is associated with atrial fibrillation in the general population; and AT1 genotypic variation is associated with known risk factors for atrial fibrillation, such as hypertension, heart failure, ischaemic heart disease and myocardial infarction, in the general population. To test these hypotheses, we selected 760 individuals with atrial fibrillation from the Copenhagen City Heart Study and resequenced the AT1 gene in each individual. Two nonsynonymous, potentially functional genetic variants, I103T and A244S, identified during this screening were subsequently genotyped in all individuals in the Copenhagen City Heart Study (n = 10 603) and in the Copenhagen General Population Study (n = 60 647).
Here we show that heterozygosity for AT1 A244S and I103T/A244S is associated with risk of atrial fibrillation in men. This suggests that the AT1 receptor may play a role in the pathogenesis of atrial fibrillation and might be a target for the pharmaceutical industry.
Methods
Study populations
The Copenhagen City Heart Study is a prospective study of the Danish general population initiated in 1976–1978 with follow-up examinations in 1981–1983, 1991–1994 and 2001–2003 7. Individuals were selected based on the national Danish Civil Registration System to reflect the Danish population aged 20–100 years. Participants in the present study were those who participated in the 1991–1994 and/or 2001–2003 examinations and gave blood for DNA extraction (n = 10 603).
The atrial fibrillation cohort consisted of 760 individuals with atrial fibrillation identified in the Copenhagen City Heart Study. We emphasized inclusion of the youngest part of the total atrial fibrillation cohort of 960 individuals, because a genetic component of a multifactorial disease like atrial fibrillation might have a greater impact on younger individuals not yet suffering from other diseases predisposing to atrial fibrillation.
A diagnosis of atrial fibrillation was defined as irregular and unco-ordinated atrial electrical activity on a surface electrocardiogram and, in patients with intact atrioventricular conduction, the presence of an irregular ventricular response 8. Atrial fibrillation diagnosed at one of the four study examinations from electrocardiographic recordings was confirmed by two independent reviewers 9. The remaining subjects who had atrial fibrillation events were drawn from the national Danish Patient Registry (World Health Organization; International Classification of Diseases, 8th edition, codes 427.93 and 427.94; and 10th edition, codes I48.0–I48.9) from 1976 to 2011. From 1994 to 2011, this registry also includes information from emergency wards. Of all atrial fibrillation events, 74% were identified through the national Danish Patient Registry alone, 26% through the national Danish Patient Registry and at one of the examinations, while none was diagnosed solely at an examination. This means that each individual diagnosed with a new-onset event of atrial fibrillation at one of the four study examinations was afterwards referred to a hospital and thus registered in the national Danish Patient Registry. The national Danish Patient Registry captures 100% of diseases diagnosed at all hospitals in Denmark 10, 11.
Information on a diagnosis of hypertension, heart failure, ischaemic heart disease and myocardial infarction (WHO; International Classification of Diseases, 8th edition, codes 401–404, 427.09–427.11, 410–414 and 410 until the end of 1993; and thereafter based on the 10th edition, codes I10–I15, I50–I50.9, I20–I25 and I21–I22) was collected until May 2011 by reviewing all hospital admissions and diagnoses entered in the national Danish Patient Registry. Hypertension was systolic blood pressure ≥ 140 mmHg (≥135 mmHg for diabetics) and/or a diastolic blood pressure ≥ 90 mmHg (≥85 mmHg for diabetics). Heart failure was congestive and left ventricular heart failure. The definitions of congestive heart failure and left ventricular heart failure were in accordance with the international diagnostic criteria at the time of diagnosis 12. A diagnosis of myocardial infarction required the presence of characteristic chest pain, elevated cardiac enzymes and/or electrocardiographic changes indicative of myocardial infarction, following changing diagnostic criteria over time 13. Ischaemic heart disease was defined as myocardial infarction and/or characteristic symptoms of angina pectoris. Information on mortality was obtained from the national Danish Civil Registration System.
The Copenhagen General Population Study is a prospective study initiated in 2003, with ongoing inclusion 7. Participants were ascertained exactly as in the Copenhagen City Heart Study. At the time of genotyping, 60 647 participants had been included. Diagnoses of atrial fibrillation, hypertension, heart failure, ischaemic heart disease, myocardial infarction and death were ascertained as in the Copenhagen City Heart Study, except that individual electrocardiographic recordings were not performed at the examination.
In the present study, individuals participating in the 1991–1994 and/or 2001–2003 examinations of the Copenhagen City Heart Study or the Copenhagen General Population Study were included, and the examination at which DNA had been extracted was considered baseline. Individuals with an incident end-point at baseline were excluded from analyses. Due to this, 1633 individuals with incident atrial fibrillation, 3892 with incident hypertension, 834 with incident heart failure, 3848 with incident ischaemic heart disease and 1652 individuals with incident myocardial infarction were excluded from analyses. Median follow-up time was 4.2 years (range 0.003–19.6 years).
All participants were white and of Danish descent. No individuals appeared in both populations, and follow-up was 100% complete, that is, we did not loose track of even a single individual. Studies were approved by Herlev Hospital, Copenhagen University Hospital and Danish ethical committees (KF-100.2039/91 and H-KF-01-144/01) and were conducted according to the Declaration of Helsinki. Informed consent was obtained from participants.
Resequencing and genotyping
Activation of the AT1 receptor induces growth responses 1 and has been shown to mediate the structural and electrical remodelling of the atrial myocardium associated with atrial fibrillation 3, 4. Exons and the flanking 50 bp (splice donor and splice acceptor regions) of the AT1 gene were resequenced in 760 individuals with atrial fibrillation using a LightScanner, which is a post-PCR high-resolution melting system for initial mutation screening. Finally, single nucleotide polymorphism/mutation-positive DNA fragments, detected by the LightScanner, were sequenced by Sanger sequencing on an Applied Biosystems 3730 system. The ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems Inc., Foster, CA, USA) was used to genotype for the two nonsynonymous mutations in the AT1 gene, I103T and A244S, in the entire Copenhagen City Heart Study and Copenhagen General Population Study (n = 71 250). Primers and probes are given in Supplementary Table S1.
Biochemical analyses
Plasma C-reactive protein was measured by a high-sensitivity turbidimetry assay (Dako, Glostrup, Denmark). Plasma fibrinogen, creatinine, total cholesterol and high-density lipoprotein cholesterol were measured using standard hospital assays (Boehringer Mannheim, Konelab or ILS Laboratories Scandinavia). All analyses were assessed daily for precision and monthly for accuracy through an external Scandinavian quality control programme.
Other covariates
Body mass index was calculated as measured bodyweight (in kilograms) divided by measured height (in metres squared). Diabetes mellitus was assessed as a self-reported disease, treatment with antidiabetic medication and/or a nonfasting plasma glucose ≥ 11 mmol l−1. Information on a diagnosis of hyperthyroidism and ischaemic cerebrovascular disease was obtained from the national Danish Patient Registry (WHO International Classification of Diseases, 8th edition, code 242.0-99 and 432–435; and 10th edition, code E05.0-9 and I63–I64, G45). Use of statin was self-reported. Alcohol consumption was self-reported in units per week (one unit = 12 g) and heavy drinking defined as more than 14 and 21 units week–1 for women and men, respectively. Smokers were current smokers. Less that 1.0% of the covariates for the entire cohort were missing.
Statistical analyses
Data were analysed using Stata 10.1 (StataCorp, College Station, TX, USA). Two-sided probability values of <0.05 were considered significant. To compare evolutionary conservation with functional effects of the genetic variants, we aligned the human AT1 amino acid sequence with the orthologous sequences from the chimpanzee, gorilla, rat and chicken. The effect of nonsynonymous variants on protein function was predicted using the computer-based algorithms SIFT 14 and PANTHER 15.
The relationship between AT1 genotype and risk of atrial fibrillation was studied in strata of covariates using Cox regression models with age as the time scale. Adjustment was for age and gender. Interaction was tested for by using two-factor interaction terms and a likelihood ratio test in the Cox regression model.
The association of AT1 genotype with plasma C-reactive protein, fibrinogen, creatinine levels, heart rate and diastolic and systolic blood pressures was analysed using unpaired t-test for two group comparisons.
We analysed the relationship between AT1 genotype and risk of atrial fibrillation, hypertension, heart failure, ischaemic heart disease, myocardial infarction and early death using Cox regression models with age as the time scale and left truncation (delayed entry). For Cox proportional hazards regression analyses, the assumption of proportional hazards was assessed graphically by plotting the logarithm (cumulative incidence) for each end-point as a function of age; no violation of the assumption was detected. Adjustment was for age and gender.
Results
Characteristics of participants from the general population, the Copenhagen City Heart Study and the Copenhagen General Population Study combined are shown in Table 1 as a function of AT1 genotype. The minor allele frequency was 0.26% for the I103T mutation and 0.13% for the A244S mutation in the atrial fibrillation cohort. One individual was a compound heterozygous carrier of both mutations.
Table 1.
Baseline characteristics of participants from the general population studies, the Copenhagen City Heart Study and the Copenhagen General Population Study by AT1 genotype
| Characteristic | Noncarriers | Heterozygotes | |
|---|---|---|---|
| I103T | A244S | ||
| Number of individuals | 70 795 | 158 | 297 |
| Women (%) | 55 | 59 | 57 |
| Age (years) | 57 (46–67) | 57 (47–67) | 56 (45–67) |
| Body mass index (kg m−2) | 26 (23–28) | 27 (23–30) | 26 (23–28) |
| C-Reactive protein (mg l−1) | 2.8 (1.1–2.6) | 2.4 (1.2–2.5) | 3.2 (1.1–2.8) |
| Fibrinogen (g l−1) | 3.8 (3.1–4.3) | 3.8 (3.3–4.3) | 3.8 (3.2–4.4) |
| Total cholesterol (mmol l−1) | 5.7 (4.9–6.4) | 5.6 (4.7–6.4) | 5.6 (4.8–6.3) |
| Hypertension (%) | 12 | 20 | 10 |
| Ischaemic heart disease (%) | 10 | 10 | 9 |
| Heart failure (%) | 4.2 | 3.2 | 4.7 |
| Diabetes mellitus (%) | 4.0 | 7.0 | 3.7 |
| Hyperthyroidism (%) | 1.7 | 0.6 | 2.4 |
| Statin use (%) | 8.7 | 12.1 | 11.8 |
| Heavy drinkers (%) | 2.7 | 2.6 | 0.7 |
| Current smoking (%) | 25 | 23 | 22 |
Values are from baseline at the 1991–1994 examination or 2000–2003 examination of the Copenhagen City Heart Study or from the Copenhagen General Population Study initiated in 2003. Continuous values are summarized as means and interquartile range. Categorical values are summarized in as percentages.
Genetic variation in the AT1 gene
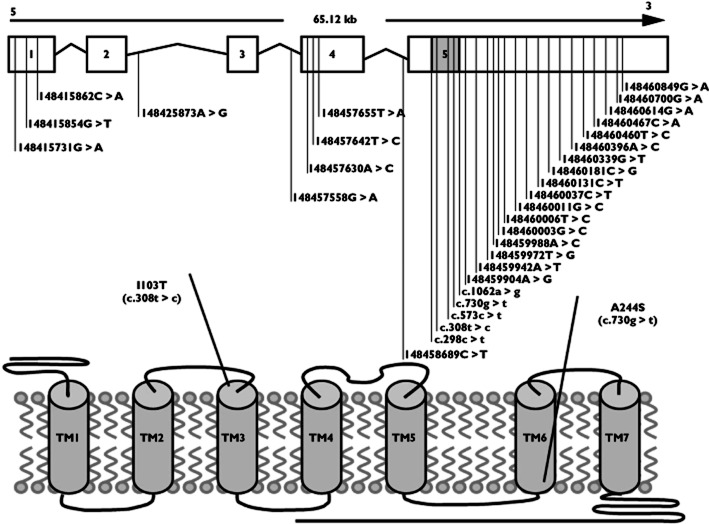
The AT1 gene comprises five exons. Only a small part of exon five accounts for the coding sequence of the gene (Figure 1, top). The gene product is a seven transmembrane receptor (Figure 1, bottom).
Figure 1.
Topological model of the AT1 gene and the AT1 protein. Upper panel shows the relative position of genetic variations identified during resequencing of exons 1–5. Open boxes indicate untranslated exons. Shaded box indicates the coding region of exon 5. Lines indicate introns. Lower panel shows the relative position of the two nonsynonymous variants selected for genotyping in the Copenhagen City Heart Study and the Copenhagen General Population Study
During resequencing of the exons in the AT1 gene in 760 individuals with atrial fibrillation, a total of 31 genetic variants were identified (Figure 1). In the coding region, two variants were known nonsynonymous (I103T and A244S), one was unknown synonymous (L100L) and two were known synonymous mutations (L191L and P354P). In noncoding exons, 12 variants were novel and 12 were previously described. Our resequencing of the exons also identified two intronic mutations, one novel and one previously described.
Evolutionary conservation and predicted effect of genetic variants
The amino acid positions of both the nonsynonymous (I103T and A244S) and the synonymous variants (L100L, L191L and P354P) are highly conserved among mammals and bird. In silico prediction of nonsynonymous variants on protein function, using SIFT 14 and PANTHER 15, predicted A244S to be highly deleterious by both programs and I103T to be highly deleterious by PANTHER, but not by SIFT.
Risk of atrial fibrillation in strata
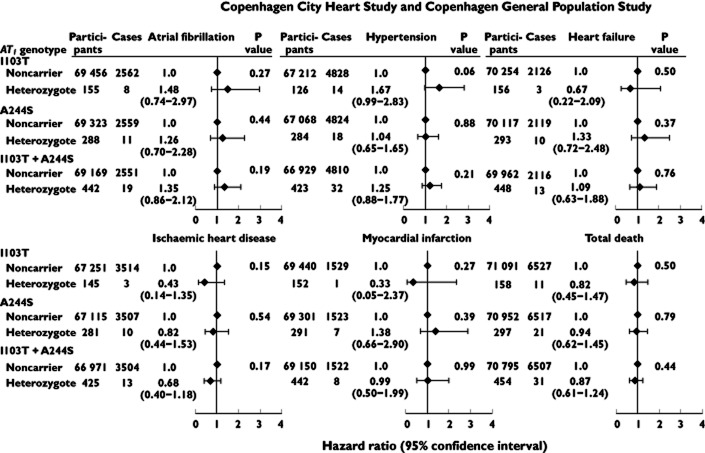
Studying AT1 genotype and risk of atrial fibrillation in strata of different covariates (gender, age, body mass index, hypertension, heart failure, ischaemic heart disease and ischaemic cerebrovascular disease) and by study revealed that the association between A244S and I103T/A244S heterozygosity and risk of atrial fibrillation appeared to be strongest among men, with a 2.7-fold (95% confidence interval 1.5- to 5.1-fold) and a 2.6-fold (95% confidence interval 1.6- to 4.2-fold) increased risk of atrial fibrillation compared with being a noncarrier (P value for interaction 0.0005 and 0.003; Figure 2).
Figure 2.
Risk of atrial fibrillation as a function of AT1 I103T, A244S and I103T/A244S genotype, in strata of covariates. Hazard ratios (HR s) are adjusted for age and gender. Interaction was tested for by using two-factor interaction terms and a likelihood ratio test in the Cox regression model. Individuals with atrial fibrillation before genotyping were excluded
Association between AT1 genotype, plasma markers and blood pressure
Activation of the AT1 receptor induces inflammation; therefore, we studied plasma levels of inflammatory markers, such as C-reactive protein and fibrinogen, as a function of AT1 genotype. No associations were found. The AT1 receptor is expressed in the heart, kidneys and vascular smooth muscle cells; therefore, we also studied the level of creatinine, heart rate and diastolic and systolic blood pressure as a function of AT1 genotype, but found no associations between these factors either (Figure 3).
Figure 3.
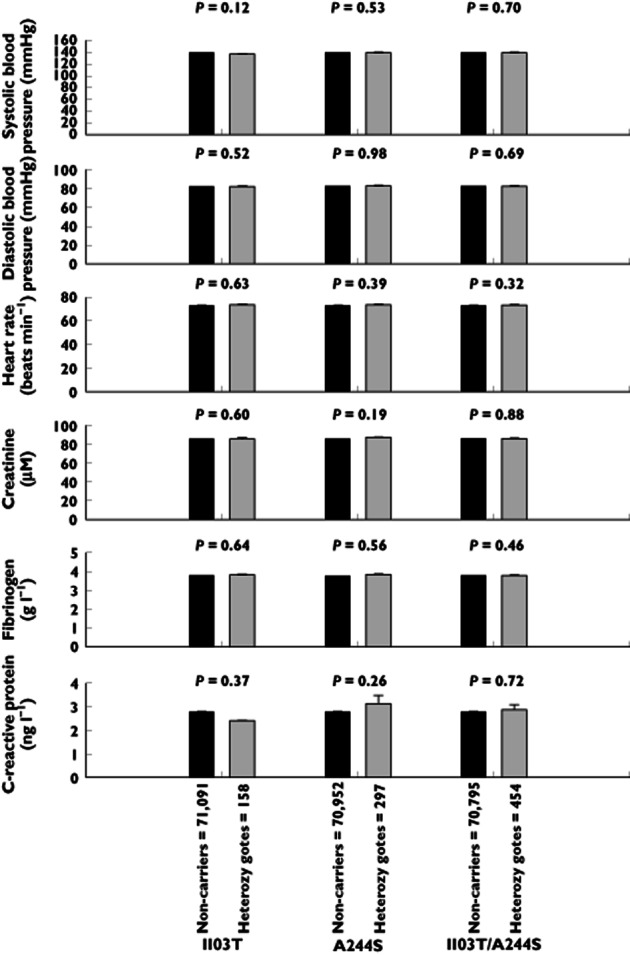
Plasma C-reactive protein, fibrinogen and creatinine levels, heart rate and diastolic and systolic blood pressure as a function of AT1 I103T, A244S and I103T/A244S genotype
AT1 genotype and risk of heart disease and early death
In AT1 I103T or A244S heterozygous individuals, risk of atrial fibrillation was 1.35 (95% confidence interval 0.86–2.12), risk of hypertension 1.25 (95% confidence interval 0.88–1.77), risk of heart failure 1.09 (95% confidence interval 0.63–1.88), risk of ischaemic heart disease 0.68 (95% confidence interval 0.40–1.18), risk of myocardial infarction 0.99 (95% confidence interval 0.50–1.99) and risk of early death 0.87 (95% confidence interval 0.61–1.24; Figure 4).
Figure 4.
Age- and gender-adjusted hazard ratios for atrial fibrillation, hypertension, heart failure, ischaemic heart disease, myocardial infarction and early death as a function of AT1 I103T, A244S and I103T/A244S genotype
Discussion
The main finding of this study is that heterozygosity for AT1 A244T and I103T/A244S is associated with risk of atrial fibrillation in men. The mechanism behind this finding deserves further investigation.
This association supports the hypothesis that the AT1 receptor may play a role in the pathogenesis of atrial fibrillation. A possible explanation could be that these mutations may change the receptor response and induce increased inflammation, fibrosis and expression of ion channels and gap junctions, thereby leading to the structural and electrical remodelling of the atrial myocardium that is known to be associated with atrial fibrillation 3, 4. The association of genotype with risk of atrial fibrillation was observed in men. This could be due to a higher penetrance of atrial fibrillation in men 9. However, this is merely speculation, and further functional studies are needed, as is independent confirmation of our finding.
At first glance, one could gain the impression that the AT1 genotype may even be protective in women. However, since the 95% confidence interval overlaps 1.0 for I103T, A244S and I103T/A244S in heterozygous women, we interpret this effect as a chance finding. Likewise, one could gain the impression that the I103T and I103T/A244S mutations have the largest effect in younger individuals. This is also biologically plausible, because genetic mutations may have a greater impact in young individuals who have not yet developed other diseases predisposing to atrial fibrillation. However, the significant interaction term between I103T and age (P value for interaction 0.04) could also be due to play of chance (Bonferroni corrected P values for significance 0.05/8 = 0.006); furthermore, no interaction was found between AT1 I103T/A244S and age (P value for interaction 0.128; Figure 2).
Epigenetics and other genetic loci may also be important for the risk of developing atrial fibrillation. Previous studies have suggested that increased drive on the RAS in general is associated with increased risk of atrial fibrillation 16–19, and several studies have found an association between variations in genes encoding key proteins in the RAS, such as angiotensinogen (AGT) and angiotensin-converting-enzyme (ACE), and increased risk of atrial fibrillation 20–22. However, a meta-analysis from 2011 suggested that there is insufficient evidence to demonstrate a significant association between a known insertion/deletion variant in the ACE gene and the risk of AF 23. An association between genetic variation in the AT1 gene, which is also a key gene in the RAS, and the risk of atrial fibrillation has only been studied for the common A1166C (rs5186) variant, which has been found to be associated with increased risk of atrial fibrillation 24. In the present study, we have expanded the search for genetic variation in the AT1 gene important for risk of atrial fibrillation by resequencing the AT1 gene in individuals with atrial fibrillation and found two previously undescribed nonsynonymous genetic variations, I103T and A244S, with respect to atrial fibrillation.
Structurally, the AT1 receptor belongs to family A of the seven transmembrane G-protein-coupled receptors 25. The seven transmembrane G-protein-coupled receptors represent the largest family of membrane proteins in the human genome and the richest source of targets for the pharmaceutical industry 26.
Studies exploring the molecular mechanisms of AT1 receptor activation have shown that separation of cytoplasmic parts of transmembrane domains TM3 and TM6 is involved in the seven transmembrane receptor activation 27–30. The role of this separation is not fully understood, but it has been suggested that mutations may disrupt constraining intramolecular interactions, thereby leading to agonist-independent movements and thus receptor activation 29. The fact that most activating mutations are located in the transmembrane elements, with a significant cluster in TM3 31, is regarded as a confirmation of the importance of transmembrane domain movement for receptor activation 32. The I103T and A244S variants that we studied are located in TM3 and TM6, respectively (Figure 1). The circumstantial evidence of the location of the variants in close proximity to potential functional sites (S107F, F110C, N111S and L112F/H in TM3, and I245T in TM6) 32, in addition to the association of heterozygosity for the I103T/A244S variants combined with atrial fibrillation in men, may suggest that I103T and A244S might be activating variants; however, such speculations need to be tested directly in functional studies. Evidence against gain-of-function properties of I103T and A244S include the finding that AT1 receptors have a very low basal activity and appear to be difficult to activate constitutively by a single mutation 32.
Even though the AT1 receptor is the primary effecter of the RAS and serves as a key regulator of cardiovascular physiology, acting to maintain salt and fluid homeostasis, as well as blood pressure, we did not find an association between AT1 genotype and hypertension when estimated using follow-up from study entry into the Copenhagen City Heart Study (initiated in 1991) or Copenhagen General Population Study (initiated in 2003; Figure 4). However, given that an individual carries the same genotype throughout life, we experimentally changed study entry to the day of birth. This revealed an association between I103T heterozygosity and risk of hypertension (Supplementary Figure S1), with a median follow-up time of 63.2 years (range 16.4–104.7 years).
Limitations of the study include that the study population consisted of whites only, and results may, owing to the large variation in mutation spectrum between ethnicities, not apply to other ethnicities. Individuals could only participate in the study if they survived to the time of recruitment and blood sampling in 1991–1994 or 2003 and onward, thus excluding individuals who died before these examinations.
Ascertainment and classification of atrial fibrillation is a potential limitation of the present study. Atrial fibrillation can be asymptomatic and thus may have occurred in some individuals without having been diagnosed. The atrial fibrillation diagnosis may also have been incorrectly coded in the national Danish Patient Registry. Finally, some individuals may not have had atrial fibrillation at one of the follow-up examinations, if they had paroxysmal atrial fibrillation. Nondifferential misclassification of a diagnosis of atrial fibrillation would result in an underestimation of the risk and thus in more conservative estimates for the association of AT1 genotype with risk of atrial fibrillation than observed in the present study.
Given that a fraction of events of atrial fibrillation, hypertension, heart failure, ischaemic heart disease and myocardial infarction are lethal events, we cannot completely exclude the possibility that such potential survival bias might have reduced our estimates to less significant levels. Other potential limitations of this study include, as always, selection bias and misclassification of AT1 genotype, atrial fibrillation, hypertension, heart failure, ischaemic heart disease and myocardial infarction. However, the study populations used were selected using the national Danish Civil Registration system, drawing two random samples from the Danish adult general population without knowledge of AT1 genotype, atrial fibrillation, hypertension, heart failure, ischaemic heart disease and myocardial infarction, largely excluding important selection bias. Misclassification of genotype is unlikely in the present study, because overall call rates were more than 99.9% as a result of repeated reruns.
The main finding of this study is that heterozygosity for AT1 A244S and I103T/A244S is associated with risk of atrial fibrillation in men. This suggests that the AT1 receptor may play a role in the pathogenesis of atrial fibrillation and might be a target for the pharmaceutical industry. The mechanism behind this finding deserves further investigation. Also, this finding needs to be validated in independent studies.
Acknowledgments
We are indebted to staff and participants of the Copenhagen City Heart Study and the Copenhagen General Population Study for their important contributions. This work was supported by The Danish Agency for Science, Technology and Innovation, The Danish Medical Research Council; Chief Physician Johan Boserup and Lise Boserup's Foundation; The Lundbeck Foundation; Aase and Ejnar Danielsen's Foundation; and the Danish Heart Foundation, all of which are public or nonprofit organizations with no right to approve or disapprove of the manuscript.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare: M.B. had support from The Danish Agency for Science, Technology and Innovation, and S.C.W.M. and B.G.N. had support from Chief Physician Johan Boserup and Lise Boserup's Foundation, The Lundbeck Foundation, Aase and Ejnar Danielsen's Foundation and the Danish Heart Foundation for the submitted work; none of the authors had relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1
Risk of atrial fibrillation, hypertension, heart failure, ischaemic heart disease, myocardial infarction and early death as a function of AT1 I103T, A244S and 103T/A244S genotype.
Follow-up since day of birth
Table S1
Sequences for primers and probes
References
- 1.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 3.Lau YF, Yiu KH, Siu CW, Tse HF. Hypertension and atrial fibrillation: epidemiology, pathophysiology and therapeutic implications. J Hum Hypertens. 2011;26:563–569. doi: 10.1038/jhh.2011.105. [DOI] [PubMed] [Google Scholar]
- 4.Tsai CT, Lai LP, Lin JL, Chiang FT, Hwang JJ, Ritchie MD, Moore JH, Hsu KL, Tseng CD, Liau CS, Tseng YZ. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–1646. doi: 10.1161/01.CIR.0000124487.36586.26. [DOI] [PubMed] [Google Scholar]
- 5.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Goette A. The vanishing story of angiotensin II receptor blockers in the treatment of atrial fibrillation. Europace. 2011;13:451–452. doi: 10.1093/europace/eur045. [DOI] [PubMed] [Google Scholar]
- 7.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Friberg J, Scharling H, Gadsboll N, Jensen GB. Sex-specific increase in the prevalence of atrial fibrillation (The Copenhagen City Heart Study) Am J Cardiol. 2003;92:1419–1423. doi: 10.1016/j.amjcard.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Jespersen CG, Borre M, Norgaard M. Validity of the recorded codes of gonadotropin-releasing hormone agonist treatment and orchiectomies in the Danish National Patient Registry. Clin Epidemiol. 2012;4:145–149. doi: 10.2147/CLEP.S32313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull. 1999;46:354–357. [PubMed] [Google Scholar]
- 12.Finucane TE. NICE guideline for management of chronic heart failure in adults. Ann Intern Med. 2012;156:69–70. doi: 10.7326/0003-4819-156-1-201201030-00023. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas PD, Kejariwal A, Campbell MJ, Mi H, Diemer K, Guo N, Ladunga I, Ulitsky-Lazareva B, Muruganujan A, Rabkin S, Vandergriff JA, Doremieux O. PANTHER: a browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucleic Acids Res. 2003;31:334–341. doi: 10.1093/nar/gkg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 17.Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 18.Goette A, Arndt M, Rocken C, Spiess A, Staack T, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000;101:2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- 19.Roberts R. Mechanisms of disease: genetic mechanisms of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2006;3:276–282. doi: 10.1038/ncpcardio0509. [DOI] [PubMed] [Google Scholar]
- 20.Ravn LS, Benn M, Nordestgaard BG, Sethi AA, Agerholm-Larsen B, Jensen GB, Tybjaerg-Hansen A. Angiotensinogen and ACE gene polymorphisms and risk of atrial fibrillation in the general population. Pharmacogenet Genomics. 2008;18:525–533. doi: 10.1097/FPC.0b013e3282fce3bd. [DOI] [PubMed] [Google Scholar]
- 21.Topal NP, Ozben B, Hancer VS, Tanrikulu AM, Diz-Kucukkaya R, Fak AS, Basaran Y, Yesildag O. Polymorphisms of the angiotensin-converting enzyme and angiotensinogen gene in patients with atrial fibrillation. J Renin Angiotensin Aldosterone Syst. 2011;12:549–556. doi: 10.1177/1470320311399605. [DOI] [PubMed] [Google Scholar]
- 22.Wang QS, Li YG, Chen XD, Yu JF, Wang J, Sun J, Lu SB, Jin L, Wang XF. Angiotensinogen polymorphisms and acquired atrial fibrillation in Chinese. J Electrocardiol. 2010;43:373–377. doi: 10.1016/j.jelectrocard.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Korantzopoulos P, Xu G, Shehata M, Li D, Wang X, Li G. Association between angiotensin-converting enzyme insertion/deletion gene polymorphism and atrial fibrillation: a meta-analysis. Europace. 2011;13:346–354. doi: 10.1093/europace/euq407. [DOI] [PubMed] [Google Scholar]
- 24.Belenkov YN, Privalova EV, Kaplunova VY, Stambol'skii DV, Fomin AA. [Analysis of morpho-functional parameters of the heart and polymorphisms of Renin-Angiotensin-aldosterone system genes in patients with different variants of the course of hypertrophic cardiomyopathy] Kardiologiia. 2010;50:27–34. [PubMed] [Google Scholar]
- 25.Aplin M, Bonde MM, Hansen JL. Molecular determinants of angiotensin II type 1 receptor functional selectivity. J Mol Cell Cardiol. 2009;46:15–24. doi: 10.1016/j.yjmcc.2008.09.123. [DOI] [PubMed] [Google Scholar]
- 26.Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta. 2007;1768:794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol. 2001;60:1–19. [PubMed] [Google Scholar]
- 28.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 29.Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK. Agonists induce conformational changes in transmembrane domains III and VI of the beta2 adrenoceptor. EMBO J. 1997;16:6737–6747. doi: 10.1093/emboj/16.22.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh SP, Zvyaga TA, Lichtarge O, Sakmar TP, Bourne HR. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature. 1996;383:347–350. doi: 10.1038/383347a0. [DOI] [PubMed] [Google Scholar]
- 31.Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 32.Parnot C, Bardin S, Miserey-Lenkei S, Guedin D, Corvol P, Clauser E. Systematic identification of mutations that constitutively activate the angiotensin II type 1A receptor by screening a randomly mutated cDNA library with an original pharmacological bioassay. Proc Natl Acad Sci U S A. 2000;97:7615–7620. doi: 10.1073/pnas.110142297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.