Abstract
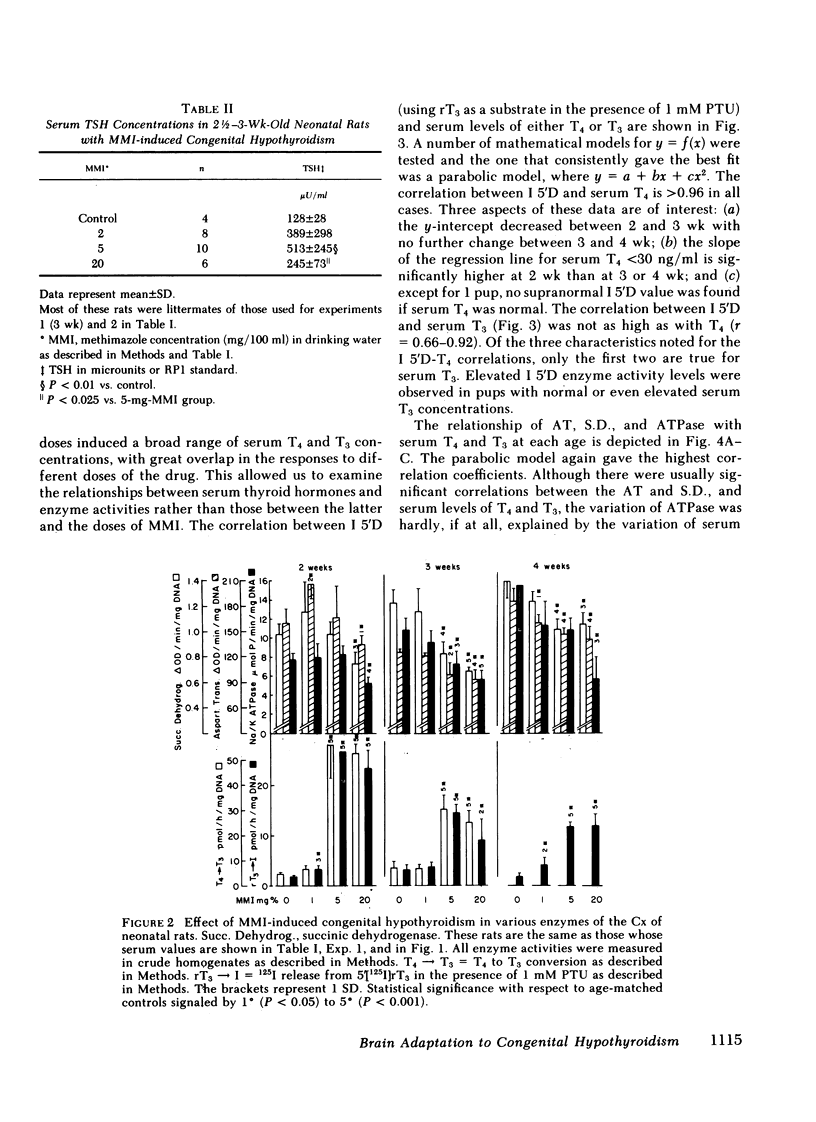
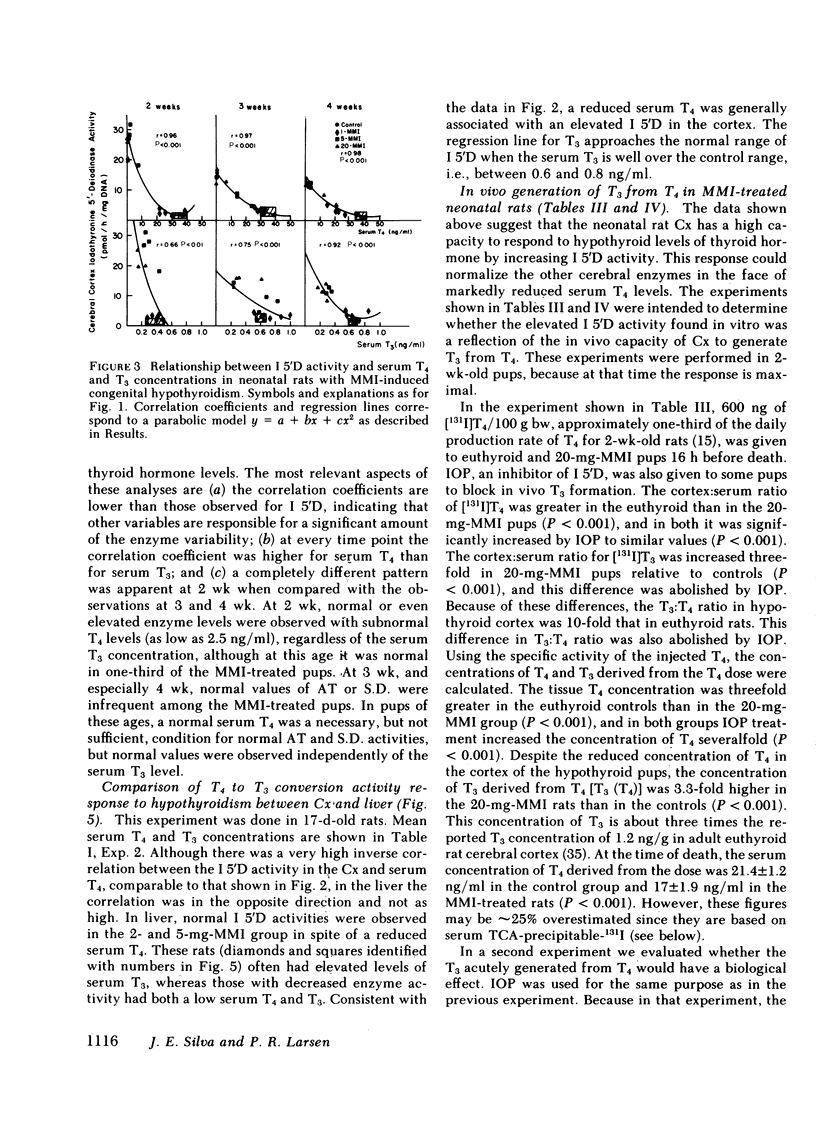
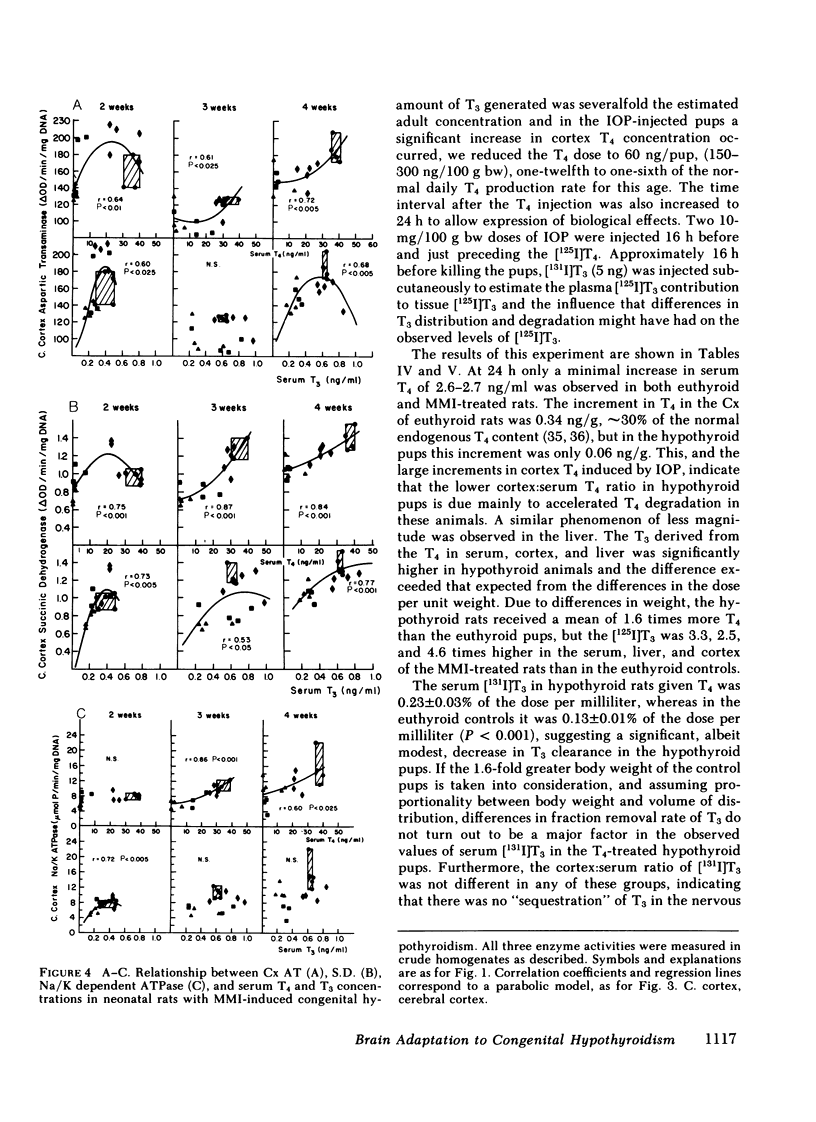
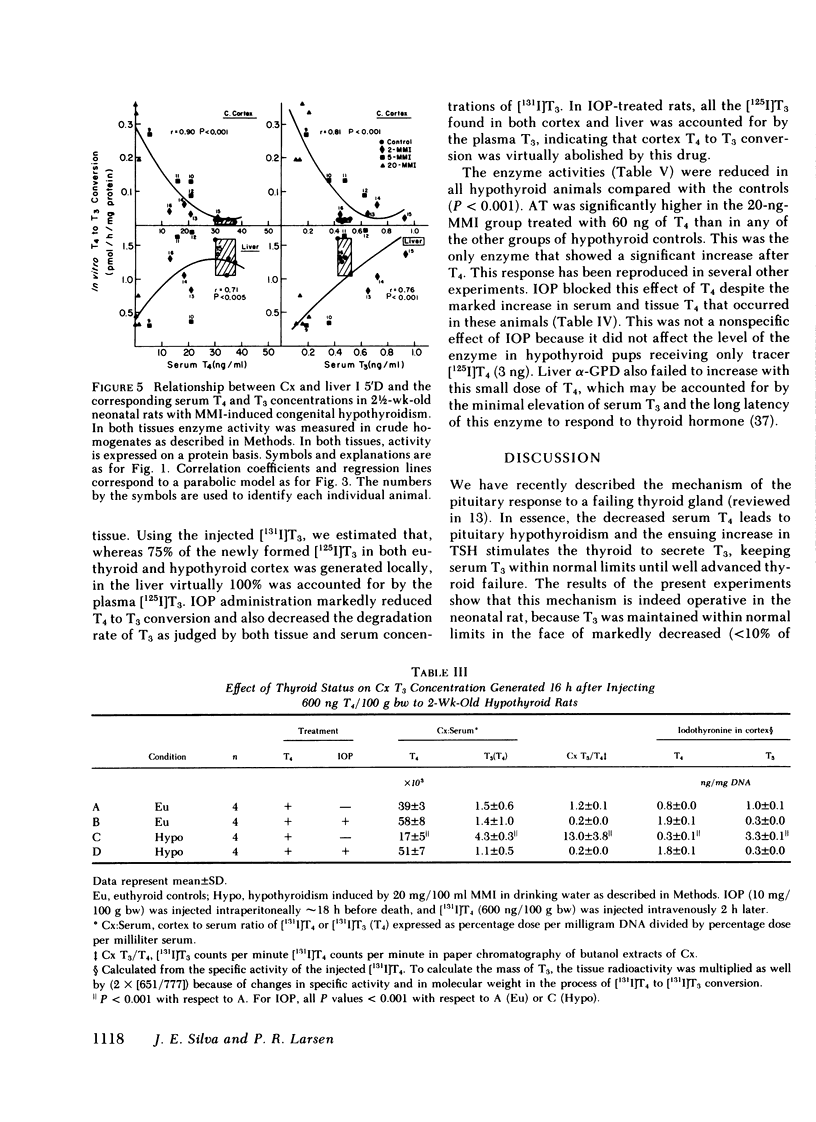
Recent studies have shown that ∼75% of the nuclear 3,5,3′-triiodothyronine (T3) present in adult rat cerebral cortex (Cx) derives from 5′-deiodination of thyroxine (T4) within this tissue. The activity of iodothyronine 5′-deiodinase (I 5′D), the enzyme catalyzing T4 to T3 conversion, increases rapidly after thyroidectomy, suggesting that this could be a compensatory response to hypothyroxinemia. To evaluate this possibility during the period of central nervous system maturation, we studied several thyroid hormone-responsive enzymes (aspartic transaminase [AT], succinic dehydrogenase [S.D.], and Na/K ATPase) in the Cx of 2-, 3-, and 4-wk-old rats. The rats were made congenitally hypothyroid by placing 1, 2, 5, and 20 mg methimazole (MMI) in 100 ml of the mothers' drinking water from day 16 of gestation throughout the nursing period and to the litters after weaning. In addition, serum thyroid hormones, I 5′D, and, in some experiments, in vivo T4 to T3 conversion in Cx were measured in the same pups. Serum T4 concentrations varied from <1 to 40 ng/ml and were generally inversely related to maternal MMI dose. Serum T3 was less affected by MMI than was T4. At 2 wk, decreases in AT, S.D., and ATPase were present in the 20-mg-MMI group but not in the 5-mg-MMI pups despite low serum T4 (<10 ng/ml) in the latter. At 3 and 4 wk, both 5- and 20-mg-MMI groups had significant reductions in these cortical enzymes despite a normal serum T3 in the 5-mg-MMI rats. Cortical I 5′D activity was 10-fold the control value in 5- and 20-mg-MMI animals at 2 wk but increased only three- to fivefold at 3 and 4 wk. I 5′D correlated inversely with serum T4 (r ≥ 0.96) at all ages, but the less marked elevation of this enzyme in 3- and 4-wk-old pups was not accompanied by an increase in serum T4. Serum T3 increased or remained the same between 2 and 3 wk. These results suggested that the 10-fold increase in I 5′D at 2 wk protected the 5-mg-MMI group from tissue hypothyroidism, but that the three- to fivefold increase at 3 and 4 wk could not. Injection of ∼250 ng T4/100 g body weight to 2-wk-old, 20-mg-MMI pups (one-sixth the normal T4 production rate) led to both a 1.8-ng/g cortical tissue increment in cortical T3 and a significant increase in AT at 24 h, compared with a 0.38-ng/g cortical tissue T3 increment and no change in AT in euthyroid controls. The larger increment in T3 of the 20-mg-MMI pups was due in great part to increased fractional T4 to T3 conversion. Although the latter resulted in greater serum T3 concentrations, three-fourths of the newly formed T3 in the cortex was generated in situ, and it was blocked by iopanoic acid as was the increase in AT. We conclude that 70-80% of the T3 in the Cx of the neonatal rat is produced locally. Serum T4 appears to serve both as a precursor for T3 and as a critical signal for increases in cortical I 5′D. The increased I 5′D can result in normal or near-normal cerebrocortical T3 concentrations despite marked reductions in serum T4. This mechanism seems to be particularly effective around 2 wk of age when many thyroid-hormone-dependent maturational changes occur in the rat Cx.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Cocks J. A., Balázs R., Johnson A. L., Eayrs J. T. Effect of thyroid hormone on the biochemical maturation of rat brain: conversion of glucose-carbon into amino acids. J Neurochem. 1970 Aug;17(8):1275–1285. doi: 10.1111/j.1471-4159.1970.tb03376.x. [DOI] [PubMed] [Google Scholar]
- Crantz F. R., Larsen P. R. Rapid thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat cerebral cortex and cerebellum. J Clin Invest. 1980 Apr;65(4):935–938. doi: 10.1172/JCI109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crantz F. R., Silva J. E., Larsen P. R. An analysis of the sources and quantity of 3,5,3'-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982 Feb;110(2):367–375. doi: 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- EAYRS J. T. The cerebral cortex of normal and hypothyroid rats. Acta Anat (Basel) 1955;25(2-4):160–183. doi: 10.1159/000141068. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Sack J., Chopra I. J. Ontogenesis of hypothalamic--pituitary--thyroid function and metabolism in man, sheep, and rat. Recent Prog Horm Res. 1976;33:59–116. doi: 10.1016/b978-0-12-571133-3.50010-6. [DOI] [PubMed] [Google Scholar]
- García Argiz C. A., Pasquini J. M., Kaplún B., Gómez C. J. Hormonal regulation of brain development. II. Effect of neonatal thyroidectomy on succinate dehydrogenase and other enzymes in developing cerebral cortex and cerebellum of the rat. Brain Res. 1967 Dec;6(4):635–646. doi: 10.1016/0006-8993(67)90121-7. [DOI] [PubMed] [Google Scholar]
- Gardner R. S. A sensitive colorimetric assay for mitochondrial alpha-glycerophosphate dehydrogenase. Anal Biochem. 1974 May;59(1):272–276. doi: 10.1016/0003-2697(74)90033-5. [DOI] [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Kaplan M. M., McCann U. D., Yaskoski K. A., Larsen P. R., Leonard J. L. Anatomical distribution of phenolic and tyrosyl ring iodothyronine deiodinases in the nervous system of normal and hypothyroid rats. Endocrinology. 1981 Aug;109(2):397–402. doi: 10.1210/endo-109-2-397. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Effects of congenital hypothyroidism and partial and complete food deprivation on phenolic and tyrosyl ring iodothyronine deiodination in rat brain. Endocrinology. 1982 Mar;110(3):761–767. doi: 10.1210/endo-110-3-761. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest. 1981 Apr;67(4):1208–1214. doi: 10.1172/JCI110136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980 Sep;66(3):551–562. doi: 10.1172/JCI109887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. H., Foley T. P., Jr, Larsen P. R., Agustin A. V., Hopwood N. J. Neonatal thyroid function in congenital hypothyroidism. J Pediatr. 1976 Oct;89(4):545–549. doi: 10.1016/s0022-3476(76)80383-6. [DOI] [PubMed] [Google Scholar]
- Klein A. H., Meltzer S., Kenny F. M. Improved prognosis in congenital hypothyroidism treated before age three months. J Pediatr. 1972 Nov;81(5):912–915. doi: 10.1016/s0022-3476(72)80542-0. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Larsen P. R. Direct immunoassay of triiodothyronine in human serum. J Clin Invest. 1972 Aug;51(8):1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Dockalova J., Sipula D., Wu F. M. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973 Aug;37(2):177–182. doi: 10.1210/jcem-37-2-177. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Kaplan M. M., Visser T. J., Silva J. E., Larsen P. R. Cerebral cortex responds rapidly to thyroid hormones. Science. 1981 Oct 30;214(4520):571–573. doi: 10.1126/science.7291997. [DOI] [PubMed] [Google Scholar]
- Lo C. S., Edelman I. S. Effect of triiodothyronine on the synthesis and degradation of renal cortical (Na+ + k+)-adenosine triphosphatase. J Biol Chem. 1976 Dec 25;251(24):7834–7840. [PubMed] [Google Scholar]
- NACHLAS M. M., MARGULIES S. I., SELIGMAN A. M. A colorimetric method for the estimation of succinic dehydrogenase activity. J Biol Chem. 1960 Feb;235:499–503. [PubMed] [Google Scholar]
- Obregon M. J., Mallol J., Escobar del Rey F., Morreale de Escobar G. Presence of L-thyroxine and 3,5,3'-triiodo-L-thyronine in tissues from thyroidectomized rats. Endocrinology. 1981 Sep;109(3):908–913. doi: 10.1210/endo-109-3-908. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Morreale de Escobar G., Escobar del Rey F. Concentrations of triiodo-L-thyronine in the plasma and tissues of normal rats, as determined by radioimmunoassay: comparison with results obtained by an isotopic equilibrium technique. Endocrinology. 1978 Dec;103(6):2145–2153. doi: 10.1210/endo-103-6-2145. [DOI] [PubMed] [Google Scholar]
- Okamura K., Taurog A., Krulich L. Hypothyroidism in severely iodine-deficient rats. Endocrinology. 1981 Aug;109(2):464–468. doi: 10.1210/endo-109-2-464. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Silva E., Schwartz H. L., Surks M. I. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by L-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest. 1977 Mar;59(3):517–527. doi: 10.1172/JCI108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini J. M., Kaplún B., García Argiz A., Gómez C. J. Hormonal regulation of brain development. I. The effect of neonatal thyroidectomy upon nucleic acids, protein and two enzymes in developing cerebral cortex and cerebellum of the rat. Brain Res. 1967 Dec;6(4):621–634. doi: 10.1016/0006-8993(67)90120-5. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., RUCKER D. L. Latency and solubilization of the mitochondrial aspartate transminase of rat cerebral cortex. Biochim Biophys Acta. 1963 Mar 12;67:504–507. doi: 10.1016/0006-3002(63)91856-0. [DOI] [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Peripheral metabolism of homologous thyrotropin in euthyroid and hypothyroid rats: acute effects of thyrotropin-releasing hormone, triiodothyronine, and thyroxine. Endocrinology. 1978 Jun;102(6):1783–1796. doi: 10.1210/endo-102-6-1783. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L., Crantz F. R., Larsen P. R. Evidence for two tissue-specific pathways for in vivo thyroxine 5'-deiodination in the rat. J Clin Invest. 1982 May;69(5):1176–1184. doi: 10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Silva S. Interrelationships among serum thyroxine, triiodothyronine, reverse triiodothyronine, and thyroid-stimulating hormone in iodine-deficient pregnant women and their offspring: effects of iodine supplementation. J Clin Endocrinol Metab. 1981 Apr;52(4):671–677. doi: 10.1210/jcem-52-4-671. [DOI] [PubMed] [Google Scholar]
- Thilly C. H., Delange F., Lagasse R., Bourdoux P., Ramioul L., Berquist H., Ermans A. M. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab. 1978 Aug;47(2):354–360. doi: 10.1210/jcem-47-2-354. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Different pathways of iodothyronine 5'-deiodination in rat cerebral cortex. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1297–1304. doi: 10.1016/0006-291x(81)91588-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. J., Izumi M., Larsen P. R. Isolation of labeled triiodothyronine from serum using affinity chromatography: application to the extimation of the peripheral T4 to T3 conversion in rats. Metabolism. 1978 Mar;27(3):303–313. doi: 10.1016/0026-0495(78)90110-5. [DOI] [PubMed] [Google Scholar]


