Abstract
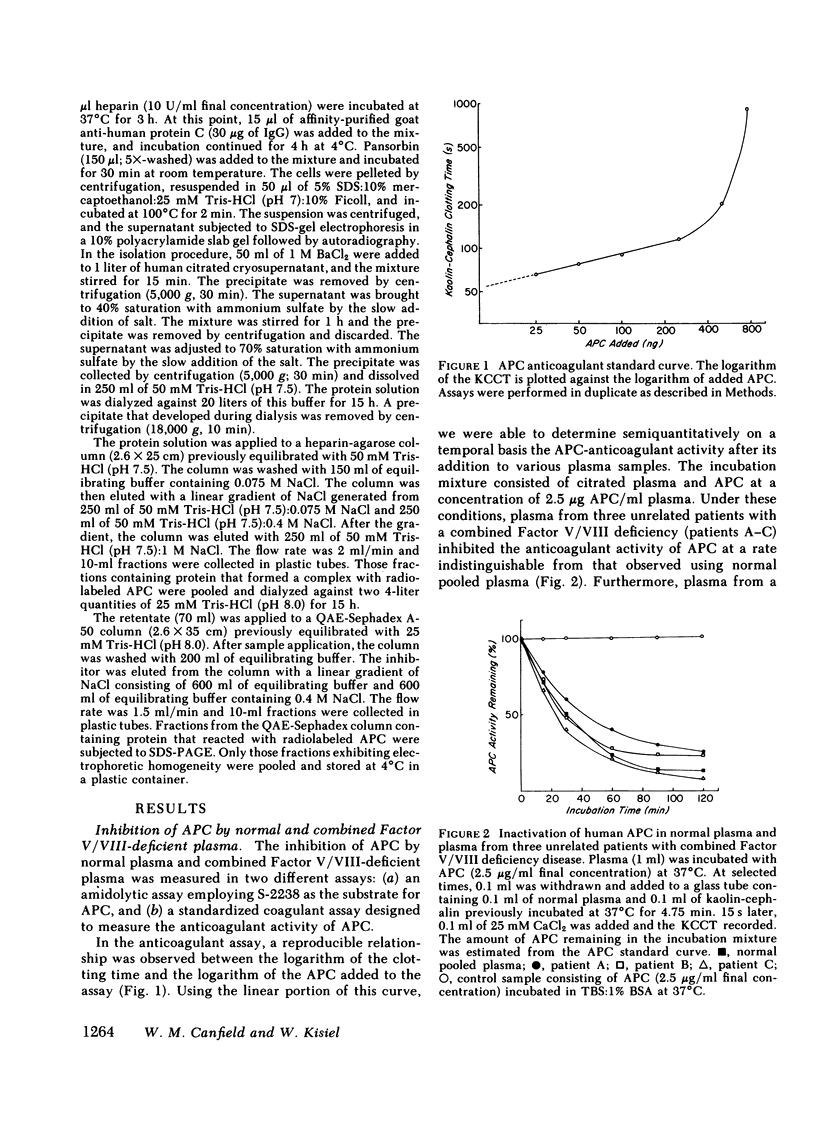
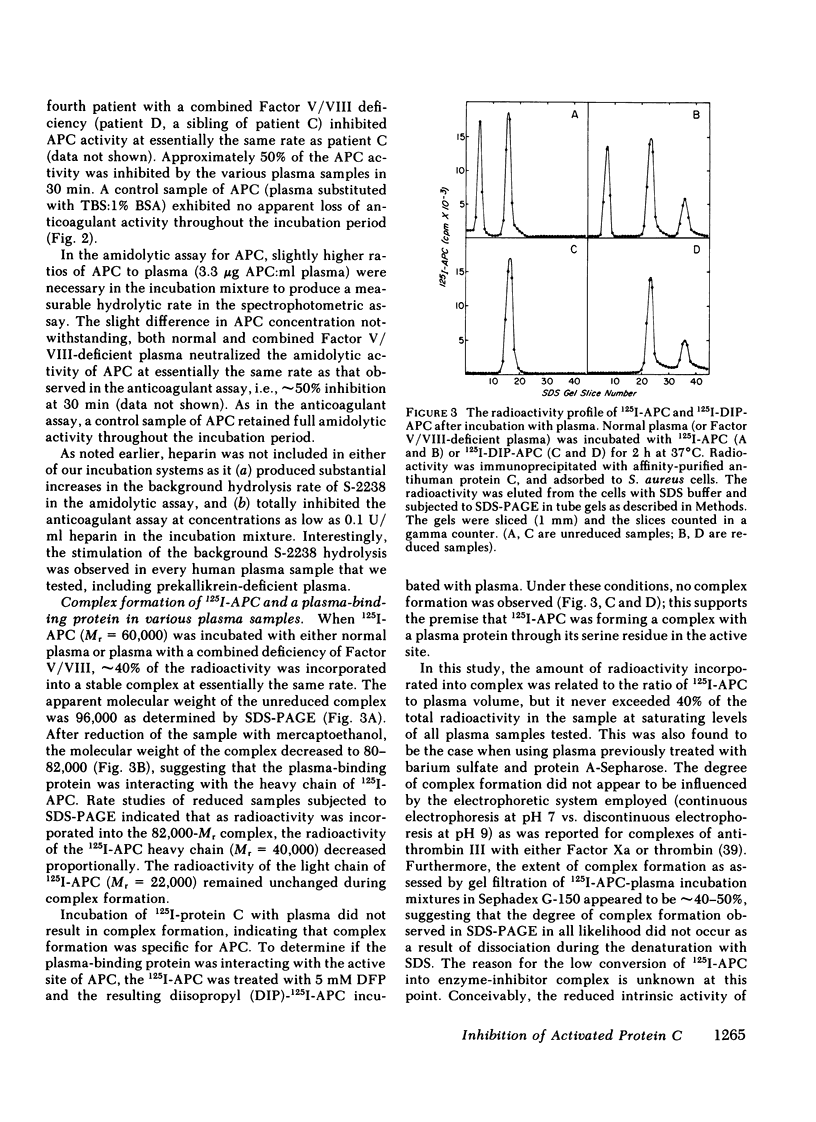
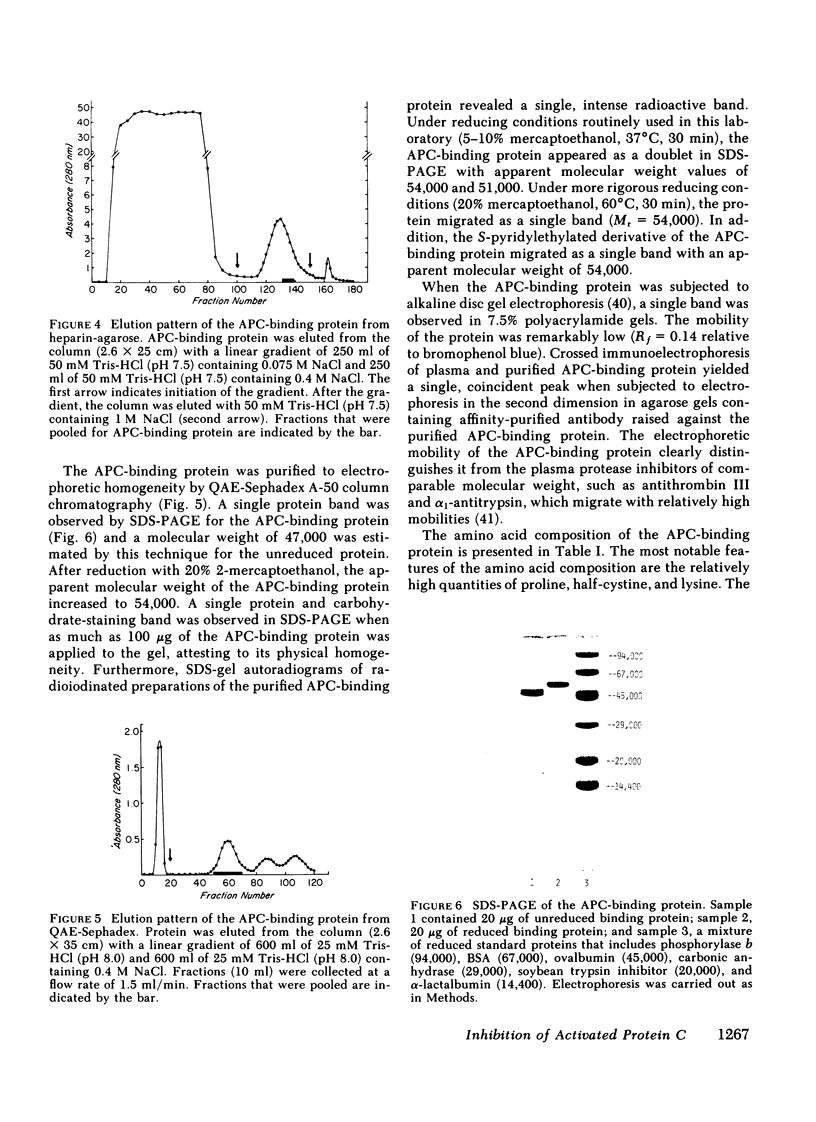
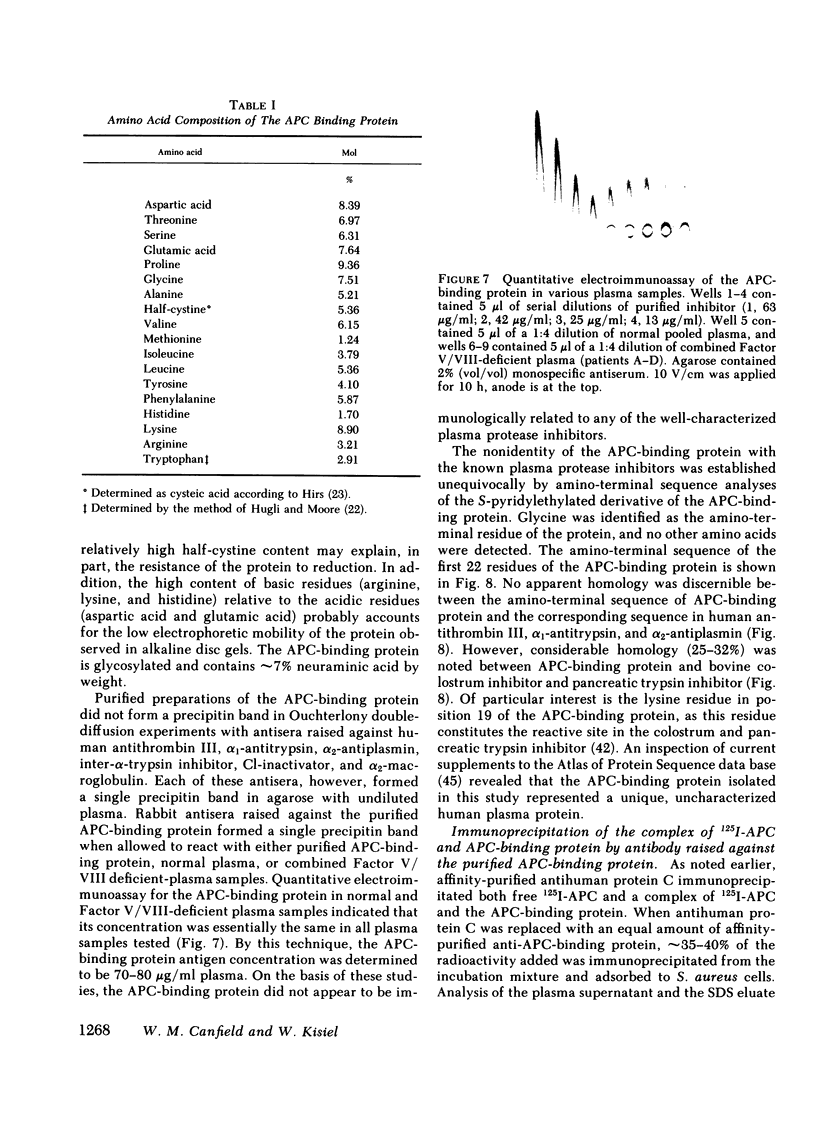
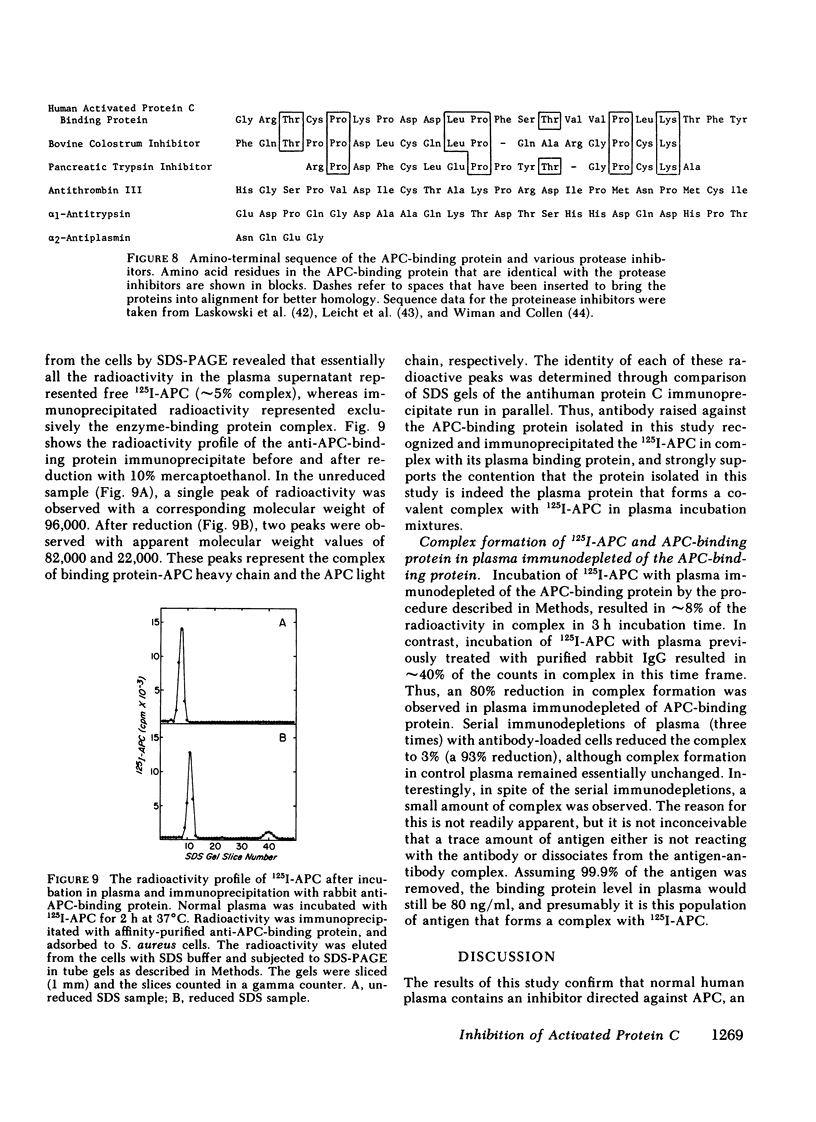
Human activated protein C (APC) is a plasma serine protease that possesses amidolytic and anticoagulant activity. The rate at which the amidolytic and anticoagulant activity of APC was neutralized in normal plasma was essentially identical to that observed in plasma obtained from four individuals with combined Factor V/VIII deficiency disease. Incubation of radioiodinated APC with either normal human plasma or the combined Factor V/VIII-deficient plasmas resulted in the formation of a stable complex (Mr = 96,000) of the enzyme and a plasma protein as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Pretreatment of the radiolabeled APC with diisopropyl fluorophosphate prevented the formation of the enzyme-protein complex. On the basis of its ability to form a complex with radiolabeled APC, the APC-binding protein was purified to homogeneity from normal human plasma by ammonium sulfate fractionation, heparin-agarose chromatography, and QAE-Sephadex A-50 chromatography. The APC-binding protein (Mr = 54,000) is a glycoprotein, and possesses an amino-terminal sequence of Gly-Arg-Thr-Cys-Pro-Lys-Pro-Asp. The amino-terminal sequence of the APC-binding protein exhibited considerable homology with bovine colostrum inhibitor and pancreatic trypsin inhibitor, but no apparent sequence homology with the plasma serine protease inhibitors. Affinity-purified antibody against APC-binding protein immunoprecipitated a complex of radiolabeled APC and native APC-binding protein from normal human plasma. Complex formation was virtually eliminated in plasma immunodepleted of the APC-binding protein. Quantitative electroimmunoassay indicated essentially equal levels of APC-binding protein antigen in normal plasma compared with plasma from four patients with combined Factor V/VIII deficiency disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL W. N., ALTON H. G. A brain extract as a substitute for platelet suspensions in the thromboplastin generation test. Nature. 1954 Nov 6;174(4436):880–881. doi: 10.1038/174880a0. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Generation of fibrinolytic activity by infusion of activated protein C into dogs. J Clin Invest. 1981 Nov;68(5):1221–1228. doi: 10.1172/JCI110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damus P. S., Rosenberg R. D. Antithrombin-heparin cofactor. Methods Enzymol. 1976;45:653–669. doi: 10.1016/s0076-6879(76)45056-5. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Rosselin G., Hoa D. H., Cote T. E., Laburthe M. Structural requirements for glucagon receptor binding and activation of adenylate cyclase in liver. Study of chemically modified forms of the hormone, including N alpha-trinitrophenyl glucagon, an antagonist. J Biol Chem. 1981 Feb 10;256(3):1128–1132. [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Ganrot P. O. Crossed immunoelectrophoresis. Scand J Clin Lab Invest Suppl. 1972;124:39–47. doi: 10.3109/00365517209102749. [DOI] [PubMed] [Google Scholar]
- Gordon L. K. A reliable method for repetitively bleeding rabbits from the central artery of the ear. J Immunol Methods. 1981;44(2):241–245. doi: 10.1016/0022-1759(81)90352-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Evatt B., Zimmerman T. S., Kleiss A. J., Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981 Nov;68(5):1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg L., Borge L., Ljung R., Nilsson I. M. Measurement of antihaemophilic factor A antigen (VII:CAg) with a solid phase immunoradiometric method based on homologous non-haemophilic antibodies. Scand J Haematol. 1979 Jul;23(1):17–24. doi: 10.1111/j.1600-0609.1979.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Moore S. Determination of the tryptophan content of proteins by ion exchange chromatography of alkaline hydrolysates. J Biol Chem. 1972 May 10;247(9):2828–2834. [PubMed] [Google Scholar]
- Hultin M. B., Eyster M. E. Combined factor V-VIII deficiency: a case report with studies of factor V and VIII activation by thrombin. Blood. 1981 Nov;58(5):983–985. [PubMed] [Google Scholar]
- Jesty J. Dissociation of complexes and their derivatives formed during inhibition of bovine thrombin and activated factor X by antithrombin III. J Biol Chem. 1979 Feb 25;254(4):1044–1049. [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Davie E. W. Isolation and characterization of bovine factor VII. Biochemistry. 1975 Nov 4;14(22):4928–4934. doi: 10.1021/bi00693a023. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Ericsson L. H., Davie E. W. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979 Sep;64(3):761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Kurachi K., Hermodson M. A. Formation and dissociation of the covalent complexes between trypsin and two homologous inhibitors, alpha 1-antitrypsin and antithrombin III. Eur J Biochem. 1980 Apr;105(3):545–552. doi: 10.1111/j.1432-1033.1980.tb04531.x. [DOI] [PubMed] [Google Scholar]
- Marlar R. A., Griffin J. H. Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J Clin Invest. 1980 Nov;66(5):1186–1189. doi: 10.1172/JCI109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesheim M. E., Canfield W. M., Kisiel W., Mann K. G. Studies of the capacity of factor Xa to protect factor Va from inactivation by activated protein C. J Biol Chem. 1982 Feb 10;257(3):1443–1447. [PubMed] [Google Scholar]
- O'Keefe E., Vennett V. Use of immunoglobulin-loaded protein A-bearing staphylococci as a primary solid phase immunoadsorbent in radioimmunoassay. J Biol Chem. 1980 Jan 25;255(2):561–568. [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1981 Jun 10;256(11):5532–5535. [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Soff G. A., Levin J. Familial multiple coagulation factor deficiencies. I. Review of the literature: Differentiation of single hereditary disorders associated with multiple factor deficiencies from coincidental concurrence of single factor deficiency states. Semin Thromb Hemost. 1981 Fall;7(2):112–148. doi: 10.1055/s-2007-1005073. [DOI] [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Blank M. K. Detection of a new heparin-dependent inhibitor of thrombin in human plasma. J Clin Invest. 1981 Sep;68(3):589–596. doi: 10.1172/JCI110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tracy P. B., Eide L. L., Bowie E. J., Mann K. G. Radioimmunoassay of factor V in human plasma and platelets. Blood. 1982 Jul;60(1):59–63. [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Walker F. J. Regulation of bovine activated protein C by protein S: the role of the cofactor protein in species specificity. Thromb Res. 1981 May 1;22(3):321–327. doi: 10.1016/0049-3848(81)90125-0. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- Weeke B. Carbamylated human immunoglobulins tested by electrophoresis in agarose and antibody containing agarose. Scand J Clin Lab Invest. 1968;21(4):351–354. doi: 10.3109/00365516809077006. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. Purification and characterization of human antiplasmin, the fast-acting plasmin inhibitor in plasma. Eur J Biochem. 1977 Aug 15;78(1):19–26. doi: 10.1111/j.1432-1033.1977.tb11709.x. [DOI] [PubMed] [Google Scholar]