Abstract
Chronic stress or glucocorticoid exposure simplifies hippocampal CA3 apical dendritic arbors in male rats. In contrast to males, chronic stress either reduces CA3 basal branching or exerts no observable morphological effects in gonadally intact female rats. Under conditions that females display stress-induced CA3 dendritic retraction, such as following ovariectomy, chronic exposure to 17β-estradiol or cholesterol can negate these changes. Whether glucocorticoids produce CA3 dendritic retraction in ovariectomized females and whether neuroprotection from 17β-estradiol or cholesterol is sex-specific remains unknown. The current study examined the effects of chronic glucocorticoid exposure, in conjunction with 17β-estradiol or cholesterol administration, on hippocampal CA3 dendritic complexity. Adult male and female Sprague-Dawley rats were gonadectomized and implanted with 25% 17β-estradiol in cholesterol, 100% cholesterol, or blank Silastic capsules. Rats were then assigned to either a 21-day corticosterone (CORT) drink (400µg/mL CORT, 2.4% ethanol in tap water) or tap water (Tap, 2.4% ethanol in tap water) treatment. Brains were processed for Golgi staining, and hippocampal CA3 dendritic architecture was quantified. Results showed 21-day CORT administration reduced hippocampal CA3 apical dendritic branch points, CA3 apical dendritic length, body weight gain, and adrenal weights compared to male and female control counterparts. Furthermore, male and female rats implanted with Silastic capsules containing cholesterol or 25% 17β-estradiol in cholesterol were protected from CORT-induced CA3 apical dendritic branch reduction. No effects were observed in the CA3 basal dendritic arbors. The present results demonstrate that CORT produces hippocampal CA3 dendritic retraction in gonadectomized male and female rats and that cholesterol and 25% 17β-estradiol in cholesterol prevent this dendritic simplification.
Keywords: Sex Differences, Neuroprotection, Glucocorticoids, Stress, Golgi, Morphology
1. Introduction
The stress response involves the release of glucocorticoids (GCs), which interact with receptors in the brain, especially those located in limbic structures such as the hippocampus (McEwen et al., 1968, McEwen et al., 1969). It is widely documented that GCs influence hippocampal neurochemistry (Luine et al. 1993, Bush et al. 2003, Schubert et al. 2008), GC receptor expression (Kitraki et al., 2004, Wright et al., 2006), neuronal excitability (Pfaff et al., 1971; Kerr et al., 1989; Beck et al., 1994), and hippocampal-dependent behaviors such as spatial ability (Oitzl & de Kloet, 1992, de Quervain et al. 1998, McLay et al. 1998, Conrad et al. 1999, Coburn-Litvak et al. 2003, Dumas et al. 2010, Schwabe et al. 2010). Moreover, the hippocampus shows marked morphological alterations following chronic stress when GC elevations persist or when exogenous GCs are chronically administered. For example, chronic stress or chronic administration of corticosterone (CORT), the predominate GC in rodents, produces hippocampal CA3 dendritic retraction (Woolley et al. 1990, Watanabe et al. 1992a, Magariños et al. 1995a; Sousa et al., 2000; Conrad et al., 2007), reduces neuropil volume in the hippocampus (Sousa et al. 1998a & 1998b, Tata et al., 2006), and even produces dendritic retraction in other hippocampal areas when stress or elevated GCs persist (for review, see Conrad 2006). Consequently, the hippocampus is highly sensitive to stress and GCs.
Past studies demonstrate several mechanisms by which chronic stress and GCs alter hippocampal CA3 dendritic morphology. GCs appear necessary because interfering with the synthesis of GCs by administration of cyanoketone prevents stress-induced dendritic retraction (Magariños et al, 1995b). Excitatory input is essential because lesions of the entorhinal cortex, a main excitatory input into the hippocampus and CA3 region, or interference of excitatory amino acid release block stress-induced hippocampal CA3 dendritic retraction (Sunanda et al., 1997, Watanabe et al., 1992b: respectively). These studies provide supporting evidence that GCs and CA3 afferent connections contribute to diminished hippocampal dendritic complexity following chronic GC exposure.
The aforementioned studies commonly used male subjects, and subsequent reports showed that chronic stress also caused robust hippocampal CA3 dendritic retraction in females, but with some important differences than observed in males. For example, one of the first studies investigating chronic stress and sex differences on hippocampal dendritic complexity found that chronic stress produced CA3 dendritic retraction in the apical region of males and in the basal region of females (Galea et al, 1997). Since then, ovarian hormones were discovered to protect against stress-induced CA3 dendritic retraction in females, as ovariectomized rats exhibited robust hippocampal CA3 apical and basal dendritic retraction following chronic stress (McLaughlin et al., 2005, 2010; Conrad et al., 2012). Subsequently, estradiol- or cholesterol-filled Silastic implants were observed to protect against stress-induced CA3 dendritic retraction (McLaughlin et al., 2010). These studies reveal that ovarian hormones and/or their precursors may be important in chronic stress influences on hippocampal CA3 dendritic complexity. Consequently, an important follow-up question is whether estradiol and/or cholesterol may also protect against CA3 dendritic retraction in males. In addition, research has yet to investigate whether GCs produce hippocampal CA3 dendritic retraction in females, with some recent evidence since the start of this study that chronic CORT administered postpartum reduces CA3 basal dendritic complexity (Workman et al., 2013). It is possible that exogenous administration of GCs will impact female hippocampal architecture differently than exposure to chronic stress, given that restraint and CORT injections could produce differences in hippocampal GABA receptor subunit expression, which could impact neuronal plasticity (Lussier et al., 2013). Thus, the present study aimed to determine whether estradiol protects against GC-induced dendritic retraction in males and whether GCs produce dendritic retraction in females. We hypothesized that chronically elevated GC levels would produce hippocampal CA3 dendritic retraction in both males and females and that 17β-estradiol and cholesterol administration would block this dendritic remodeling.
2. Experimental Procedures
All procedures conformed to federal and institutional guidelines set forth by the Arizona State University Institutional Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, National Research Council, Institute of Laboratory Animal Resources on Life Science, Washington, D.C., 1996).
2.1 Subjects
Sprague-Dawley rats (72 male and 72 female) were purchased from Charles River Laboratories (Hartford, CT) and kept at Arizona State University housing facilities. All rats were pair-housed with a same-sex littermate in a colony room containing both female and male rats in the same treatment conditions (e.g., untreated controls were housed together and separate from the CORT-treated group). Lights were set on a reverse 12:12 hr light cycle (lights off at 6:00 am), and the rats had free access to food and water throughout the experiment.
2.2 Surgery
One week after arrival, all rats were gonadectomized and implanted with a Silastic capsule containing 25% 17β-estradiol (Steraloids, Inc., Newport, RI), 100% cholesterol (ICN Biomedicals, Inc., Aurora, OH), or filled with glue (X; referred to as “blank”). Previous studies have used either implants filled with nothing (glue, Kudwa et al., 2009; Heideman et al., 2010; Wood and Rice 2013;) or implants filled with the vehicle base (cholesterol, Evuarherhe et al., 2009; Bohacek and Daniel, 2010; Wang et al., 2011), but not both. Given our recent finding that there may be differences in these two types of “control” conditions (McLaughlin et al., 2010), we implemented both types of implants. Surgeries were performed under aseptic sterile conditions. Rats were anesthetized with a ketamine cocktail (1ml/kg, i.p., 70 mg/kg ketamine, 6 mg/kg xylazine, 10 mg/kg acepromazine, in 0.9% sodium chloride). Silastic implants were then inserted just below the nape of the neck with a 10-gauge precision trochar (Innovative Research of America, Sarasota, FL). For males, the scrotum was treated with Betadine surgical scrub. The scrotum and underlying tunica were then incised and the cauda epididymis, caput epididymis, vas deferens, and testes were extracted. The vas deferens was clamped bilaterally and the testes were removed before the vas deferens was reinserted back into the scrotal sac. The surgical site was then closed using sterilized suture thread. For females, bilateral ovariectomies were performed as described previously (McLaughlin et al., 2005, 2010; Conrad et al., 2012). Briefly, the abdomen was treated with Betadine surgical scrub, and an incision (1 cm) was made ventrally along the pelvic region. The uterine horns were identified, the oviduct was secured with dissolvable suture thread, and the ovaries were excised and removed. The abdominal muscles were sutured shut and the skin was bound with wound clips, which were removed approximately seven to ten days following surgery.
2.3 Treatment Conditions and Procedure
CORT drinking water was made by dissolving CORT (MP Biomedicals, LLC, Solon, OH) in 100% ethanol to produce a concentration of 400 µg/mL of CORT dissolved in 2.4% ethanol with the remaining volume being tap water. Past studies have found this concentration of CORT in the drinking water to produce hippocampal CA3 dendritic retraction, disrupt adrenal weights and body weight gain, and elevate circulating levels of CORT (Magariños et al., 1998; Magariños et al., 1999; Conrad et al., 2004). A previous report using this CORT dose and drink protocol found serum CORT levels to reach 20 to 29 µg/dl two hours into the light phase of the light cycle, and these CORT levels maintained elevations six hours later (14 to 29 µg/dl) (Magariños et al., 1998). Rats receiving the “control water” were given 2.4% ethanol dissolved in tap water. For simplicity, the drink for rats in the control group will be referenced as “tap” water even though it contained 2.4% ethanol and food coloring. In order to delineate treatment conditions and to reduce the risk of experimenter error, red food coloring was added to CORT-treated water, and green food coloring was added to tap water. These manipulations have previously been performed in our lab (Conrad et al., 2004; Conrad et al., 2007) as well as other labs (Waters and McCormick 2011; Overk et al., 2012). All rats received their respective drink treatment for 21 days; drinks were freshly made every two to three days, and water bottles were monitored daily.
One week after surgery, male and female rats were separately divided into six experimental conditions according to CORT treatment and Silastic implant, which resulted in the following groups: tap water and blank Silastic implant (Tap-X), tap water and cholesterol Silastic implant (Tap-Chol), tap water and 17β-estradiol Silastic implant (Tap-E), CORT-treated water and blank Silastic implant (CORT-X), CORT-treated water and cholesterol Silastic implant (CORT-Chol), and CORT treated water and 17β-estradiol Silastic implants (CORT-E). Each treatment condition started with 12 males and 12 females; however, due to methodological factors and Golgi staining restrictions, final analyses included fewer animals per group (see below).
2.4 Staining and Histology
The day after the last Tap/CORT water treatment (day 22), rats were euthanized with a lethal dose of sodium pentobarbital (100 mg/kg, i.p.). When rats failed to respond to foot or tail pinch, they were rapidly decapitated, unperfused brains were removed, and trunk blood was collected to assay for 17β-estradiol levels. Brains were processed for Golgi staining according to the FD Rapid GolgiStain™ kit (FD NeuroTechnologies, Baltimore, MD, USA). Briefly, the brains were immersed in the impregnation solution and kept in the dark for three weeks. They were then transferred to a final solution and stored at 4°C for another week. Brains were then quick frozen in 2-methylbutane, kept chilled with dry ice, and cut into coronal sections (100 µm) using a cryostat (Microtome HM 500 OM cryostat, kept at −22 to −25 °C). Sections were placed on subbed slides, blotted with bibulous blotting paper (Fisher Scientific International Inc., USA), and allowed to dry in a dark location prior to staining. Then, slides were rinsed in distilled water, placed in the developing solution for 10 minutes, rinsed in distilled water, dehydrated in ascending series of ethanol, and then cleared in neoclear, a xylene substitute (Harleco®, Gibbstown, NJ). Finally, slides were coverslipped with Permount Mounting Media (Fisher Scientific International Inc.) and left to dry in a dark dry place for one to two weeks.
The following criteria were used for neuronal selection and quantification: fully stained/impregnated cell body and dendritic branches, intact cell body and dendrites (no pieces or truncated branch sections), and the cell body was positioned in the hippocampal CA3 region. Selected neurons were traced using a light microscope (Olympus BX51 at 320×) and an attached camera Lucida. An individual who was blind to experimental conditions traced neurons, classified cells (short-shaft: SS and long-shaft: LS), and analyzed branch properties. Dendritic branch points were counted for both apical and basal CA3 neurons, and dendritic length was calculated using a Scale Master II digital plan measuring system (Calculated Industries, Carson City, NV, USA) linked by a PC interface to a Dell computer. After quantification, SS and LS neuronal values were averaged together to obtain one measure of dendritic complexity for each rat. For a rat to be included in the final analysis, the brains must have contained at minimum three successfully stained neurons from either the SS or LS category and at least two from the other category (minimum of 5 neurons/animal). Given these criteria, the final number of rats with sufficiently stained CA3 neurons ranged from six to ten rats/group. Intra-rater reliability was 96.0% ± 3.4% and inter-rater reliability was 97.5% ± 4.7%.
2.5 17β-estradiol Assay
A solid phase radioimmunoassay kit using 125I-labeled estradiol (Siemens Medical Solutions Diagnostics, Los Angeles, CA) determined serum estradiol levels. A subset of the rats from the original 144 animals was analyzed (assay completed by Dr. Laurence Demers’ Core Endocrine Laboratory at Penn State College of Medicine). Rats with 17β-estradiol serum levels greater than two standard deviations from the mean were removed from analyses. Thus, one rat was removed from the female group administered CORT in the drinking water and implanted with Cholesterol implants (Female-CORT-Chol) and one rat was removed from the female group administered tap water and implanted with 17β-estradiol (Female-Tap-E). The final number of rats per group ranged from 5−6 for this assay.
2.6 Statistics
The statistical software package, SPSS (version 19), and a Macintosh computer (OS X 10.6.8) were used to analyze the data. Analyses of variance (ANOVAs) were followed by Fisher’s LSD posthoc tests when p< 0.05. Levene’s test for homogeneity of variance was used to ensure that the ANOVA assumption of homogeneity between groups was not violated.
3. Results
3.1. CA3 apical dendritic arbors: Effects of CORT, cholesterol, and 17β–estradiol
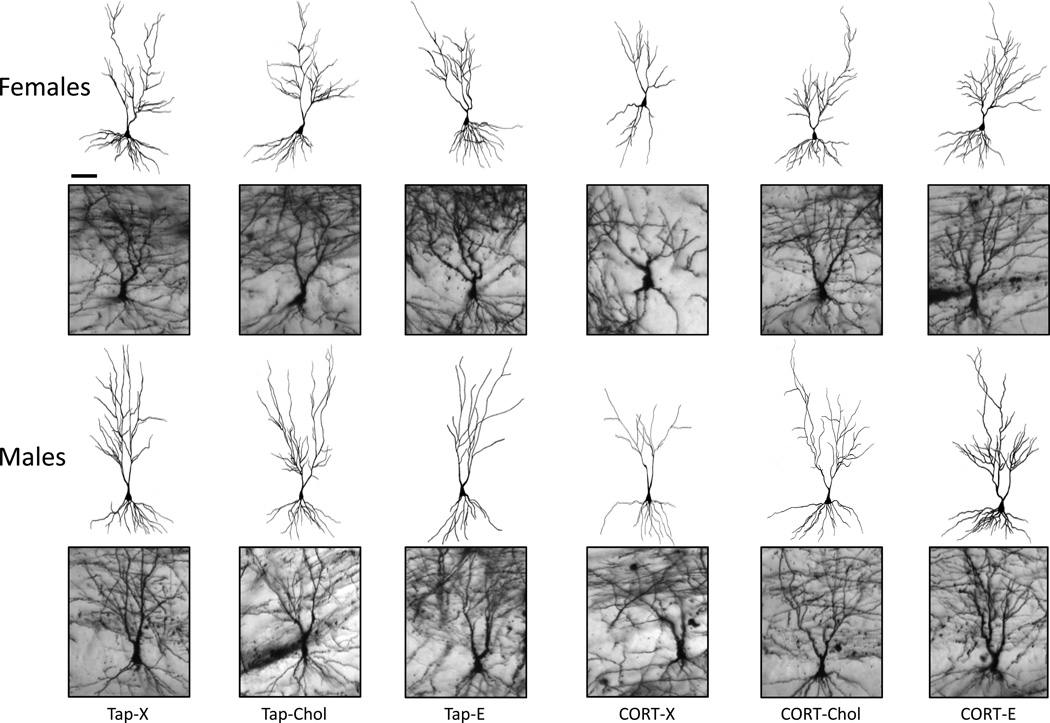
Representative neuronal images and tracings from each treatment are shown (Fig. 1). CA3 neurons showed marked dendritic branching in the apical region, which was truncated following chronic CORT treatment in both females (top row) and males (bottom row, Fig. 1, compare CORT-X to VEH-X). Cholesterol or 17β-estradiol filled Silastic implants prevented the apical dendritic retraction produced by chronic CORT ingestion (Fig 1, compare CORT-X with CORT-Chol or CORT-E).
Figure 1.
Effects of chronic CORT exposure on CA3 dendritic complexity. Camera Lucida tracings of CA3 neurons are represented above corresponding Golgi-stained cells. CORT reduced CA3 apical dendritic branch points in both females and males, while cholesterol and 25% 17β-estradiol in cholesterol prevented these effects. Basal dendritic arbors were unaffected by drink treatment. Tap water treatment and blank Silastic implants (Tap-X), tap water treatment and cholesterol-filled implants (Tap-Chol), tap water treatment and 17β-estradiol-filled implants (Tap-E), CORT drink treatment and blank implants (CORT-X), CORT drink treatment and cholesterol-filled implants (CORT-Chol), CORT drink treatment and 17β-estradiol-filled implants (CORT-E). Scale bars = 100 µm.
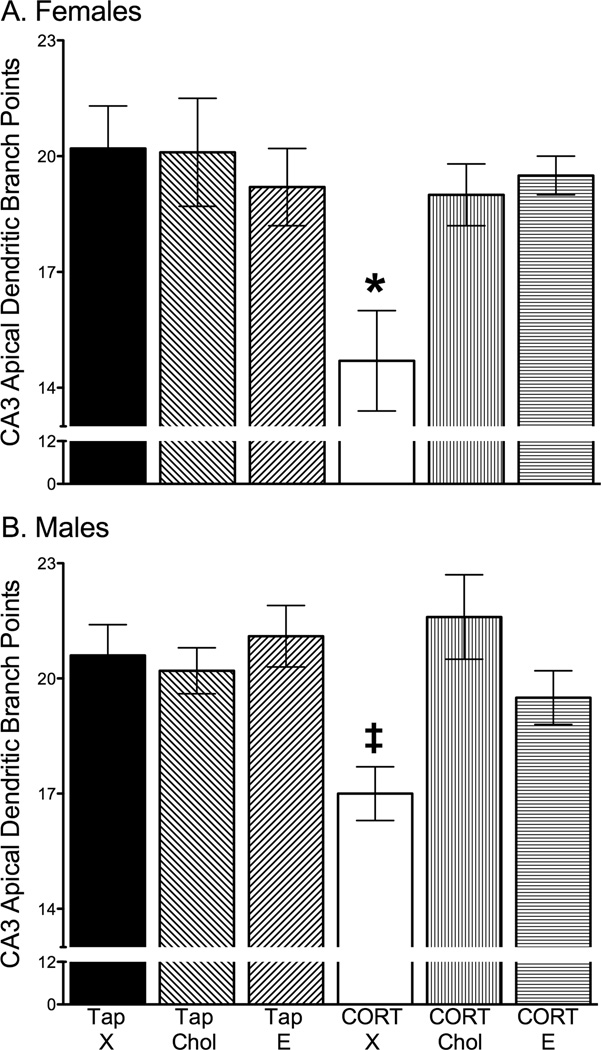
17β-estradiol and cholesterol prevented CORT-induced reductions in CA3 apical dendritic branch points in both females and males. A 2 × 2 × 3 ANOVA with sex (female, male), drink (Tap, CORT) and Silastic implant (X, Chol, E) as independent variables and apical dendritic branch points as the dependent variable revealed significant main effects of sex (F1,89 = 4.74, p = 0.03), drink (F1,89 = 8.99, p = 0.004) and Silastic implant (F2,89 = 5.27, p = 0.007), and a significant interaction between drink and Silastic implant (F2,89 = 6.66, p = 0.002) with no other significant effects. Posthoc tests probing the significant drink and Silastic implant interaction showed that exposure to CORT drinking water significantly decreased CA3 apical dendritic branch points, which was prevented by cholesterol or 17β-estradiol Silastic implants (p < 0.04, collapsed across sex due to a lack of a sex interaction, Fig. 2).
Figure 2.
Effects of chronic CORT exposure and cholesterol or 25% 17β-estradiol in cholesterol treatment on hippocampal CA3 dendritic branch points. A) Effects from females showing that CORT-treated rats with blank implants (CORT-X) exhibited diminished hippocampal CA3 dendritic complexity compared to all other groups, as marked by a reduction in apical branch points. Cholesterol and 25% 17β-estradiol in cholesterol Silastic implants prevented the simplification of CA3 apical dendritic branch points following CORT treatment. B) CA3 apical dendritic branch points in males showing that CORT drink induced a reduction in CA3 apical dendritic branch points and this CORT-induced effect was prevented by cholesterol and 25% 17β-estradiol in cholesterol Silastic implants. * p ≤ 0.05 compared to all other groups. Tap water treatment and blank Silastic implants (Tap-X), tap water treatment and cholesterol-filled implants (Tap-Chol), tap water treatment and 17β-estradiol-filled implants (Tap-E), CORT treatment and blank implants (CORT-X), CORT treatment and cholesterol-filled implants (CORT-Chol), CORT treatment and 17β-estradiol-filled implants (CORT-E).
An additional analysis was used to determine whether the CORT and Silastic implant interaction on CA3 apical branch points was separately maintained for females and males. For females, a significant main effect of drink (F1,48 = 5.61, p = 0.02) and an interaction between drink and Silastic implant (F2,48 =3.85, p = 0.03) was found for apical dendritic branch points. Posthoc tests showed that females provided with CORT drink and implanted with blank capsules (CORT-X) expressed significantly reduced branch point numbers compared to all other female conditions (p < 0.009, Fig. 2A). Importantly, cholesterol (p = 0.009) or 17β-estradiol (p = 0.006) implants prevented CA3 apical dendritic retraction in CORT-treated female rats (Fig. 2A). Likewise, for males, a 2 × 3 ANOVA revealed significant main effects for drink (F1,41 = 3.94, p = 0.05) and Silastic implant (F2,41 = 3.98, p =0.03), and a significant interaction between drink and Silastic implant (F2, 41 = 4.90, p = 0.01). Males implanted with blank Silastic implants and given CORT drinking water (CORT-X) displayed significantly fewer apical branch points than did all other male treatment groups (p = 0.03). As found with the female data, cholesterol (p = 0.001) or 17β-estradiol (p = 0.03) prevented CA3 apical dendritic retraction in male rats given CORT (Fig. 2B).
For CA3 apical dendritic branch length, a 2 × 2 × 3 ANOVA revealed significant main effects of sex (F1,89 = 7.53, p = 0.007), drink (F1,89 = 5.38, p = 0.02) and Silastic implant content (F2,89 = 5.638, p = 0.005), with no significant interactions. Males expressed greater CA3 apical dendritic length than did females (p < 0.05). The significant main effect for CORT suggested that all rats given CORT drink expressed shorter CA3 apical dendritic branch length than all rats given tap water, regardless of sex or Silastic implant (p < 0.05). However, this effect appeared to be driven predominately by the shorter branch length of the CORT-X group (in females and males). Given that past studies found CORT to produce CA3 dendritic retraction in the apical length of male neurons (Woolley et al., 1990; Sousa et al., 2000), planned comparisons were performed for CORT-X and Tap-X in males; however, we did not observe statistically significant effects. Finally, the post-hoc analyses for the significant effect of Silastic implant showed that cholesterol prevented the CORT-induced CA3 dendritic length reductions (females, p = 0.02 and males, p = 0.05), with a pattern observed for CORT-E that did not reach statistical significance (Table 1).
Table 1.
Summary of CA3 apical and basal dendritic length and basal dendritic branch points
| Sex | Female | Male | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drink | Tap | CORT | Tap | CORT | ||||||||
| Implant | X | Chol | E | X | Chol | E | X | Chol | E | X | Chol | E |
| Group | Tap-X | Tap-Chol | Tap-E | CORT-X | CORT-Chol | CORT-E | Tap-X | Tap-Chol | Tap-E | CORT-X | CORT-Chol | CORT-E |
| Apical length (µm)§ + | 1911.3 ± 118.7 | 2021.9 ± 99.2 | 1878.9 ± 83.7 | 1569.8 ± 145.0 | 1939.0 Δ ± 85.2 | 1821.5 ± 69.3 | 1945.6 ± 100.4 | 2136.5 ± 77.4 | 2163.4 ± 130.0 | 1839.3 ± 91.2 | 2131.9 Δ ± 88.3 | 1915.5 ± 91.6 |
| Basal branch points | 17.7 ± 0.7 | 17.9 ± 0.8 | 16.4 ± 0.6 | 15.8 ± 0.9 | 16.5 ± 0.6 | 18.1 ± 1.1 | 16.3 ± 0.7 | 17 ± 1.3 | 19 ± 0.7 | 16.6 ± 1.1 | 17.9 ± 1.5 | 16.1 ± 0.7 |
| Basal length (µm) | 1711.5 ± 71.4 | 1694.1 ± 67.6 | 1523.7 ± 70.3 | 1446.3 ± 114.2 | 1562.5 ± 73.3 | 1618.2 ± 93.9 | 1547.8 ± 117.5 | 1552 ± 88.6 | 1741.8 ± 76.8 | 1652.8 ± 102.0 | 1708.8 ± 88.8 | 1599.7 ± 72.4 |
Data are represented as mean ± S.E.M.
p < 0.05 significant main effect of Sex, Male versus Female for CA3 Apical dendritic length.
p < 0.05 significant main effect of Drink, CORT versus Tap water for CA3 Apical dendritic length.
p < 0.05 compared to CORT-X.
3.2. CA3 basal dendritic arbors: Effects of CORT, cholesterol, and 17β–estradiol
For CA3 basal branch points and length, an analysis of sex, drink, and Silastic implant revealed a significant three-way interaction (basal branch points, F2,89 = 5.05, p = 0.008; and basal length, F2,89 = 3.61, p = 0.03) with no other significant effects. When posthoc tests probed these interactions, no significant effects of sex or treatment groups were found. Notably, females given CORT water and implanted with blank implants (CORT-X) tended to display fewer basal branch points and shorter basal branch lengths than did the other female groups, although these effects were not statistically significant (Table 1).
3.3. Effects of CORT, 17β–estradiol, and cholesterol on physiological measures
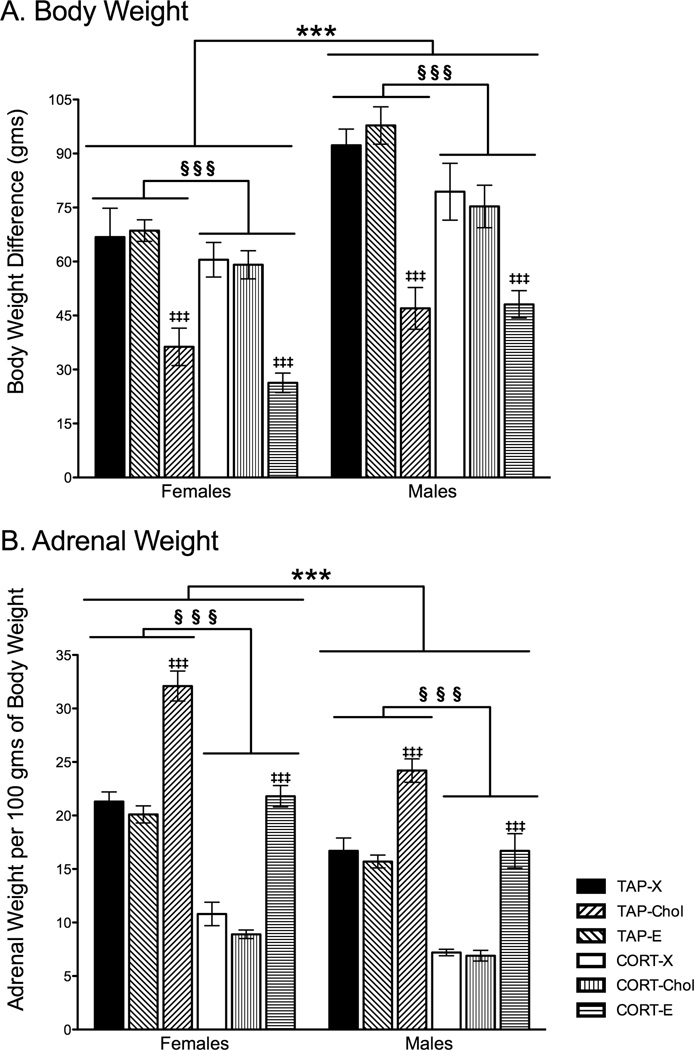
CORT treatment and 17β-estradiol-filled implants significantly altered body weight. A 2 × 2 × 3 ANOVA revealed significant main effects of sex (F1, 127 = 44.667, p < 0.0001), drink (F1,127 = 10.734, p < 0.001), and Silastic implant (F2,127 = 60.237, p < 0.0001) on body weight gain, with no significant interactions. Females gained less weight than did males, yet the effects of CORT drink and Silastic implant were similar between the sexes. CORT-treated rats gained less weight than did tap water-treated rats (p < 0.001), and this weight change occurred regardless of sex and Silastic implant contents (Fig. 3A). Moreover, 17β-estradiol reduced body weight gain in both females and males regardless of CORT or tap water administration (p < 0.001 for both blank and Chol treatments). Rats implanted with cholesterol or blank Silastic implants had similar body weight gain (p > 0.9).
Figure 3.
Effects of prolonged exposure to CORT, cholesterol, and 17β-estradiol on body weight gain and adrenal weights. A) Although females gained significantly less weight than did males, as would be expected, CORT significantly reduced body weight gain in both sexes compared to tap water-treated rats, and weight was further attenuated by 17β-estradiol. B) Adrenal weights are represented as a ratio of adrenal weight per 100 grams of body weight. Rats that underwent chronic CORT exposure had significantly diminished adrenal weights regardless of sex. Rats implanted with 17β-estradiol capsules had significantly enlarged adrenals compared to their blank and cholesterol-implanted same-sex counterparts. Furthermore, females had significantly larger adrenal weights per 100 grams of body weight than compared to males. *** p< 0.001 Significant main effect of Sex. §§§ p<0.001 Significant main effect of CORT compared to tap water treatment. ‡‡‡ p<0.001 Significant main effect of 17β-estradiol Silastic implant. Tap water treatment and blank Silastic implants (Tap-X), tap water treatment and cholesterol-filled implants (Tap-Chol), tap water treatment and 17β-estradiol-filled implants (Tap-E), CORT drink treatment and blank implants (CORT-X), CORT drink treatment and cholesterol-filled implants (CORT-Chol), CORT drink treatment and 17β-estradiol-filled implants (CORT-E).
For adrenal weights, sex, CORT, and Silastic implants produced similar outcomes as found with body weight gain (Fig. 3B). Analyses revealed significant main effects of sex (F1,127 = 65.935, p < 0.001), drink treatment (F1,127 = 290.951, p < 0.001), and Silastic implant (F2,127 = 146.871, p < 0.001), as well as a significant interaction between sex and Silastic implant (F2,127 = 2.96, p = 0.05) on adrenal weights corrected for body weight. Females showed greater adrenal weight per body weight unit than did males, which was expected given the smaller size of female rats. CORT treatment reduced the ratio of adrenal to body weight in both females and males relative to tap water-treated rats (Fig. 3B). For Silastic implants, 17β-estradiol increased adrenal weight to body weight ratio compared to both blank and cholesterol Silastic implants (p < 0.001 for both blank and Chol). The posthoc tests probing the sex by Silastic implant interaction revealed that females implanted with 17β-estradiol (Tap-E and CORT-E combined) showed significantly larger adrenal to body weight ratio compared to all other groups (p < 0.05), and that males implanted with estradiol showed larger adrenal to body weight ratio (Tap-E and CORT-E combined) compared to the remaining groups (p < 0.05 for Female-X, Female-Chol, Male-X, Male-Chol). The remaining groups were statistically similar to each other.
3.4. Effects of Silastic implant content on serum 17β-estradiol levels
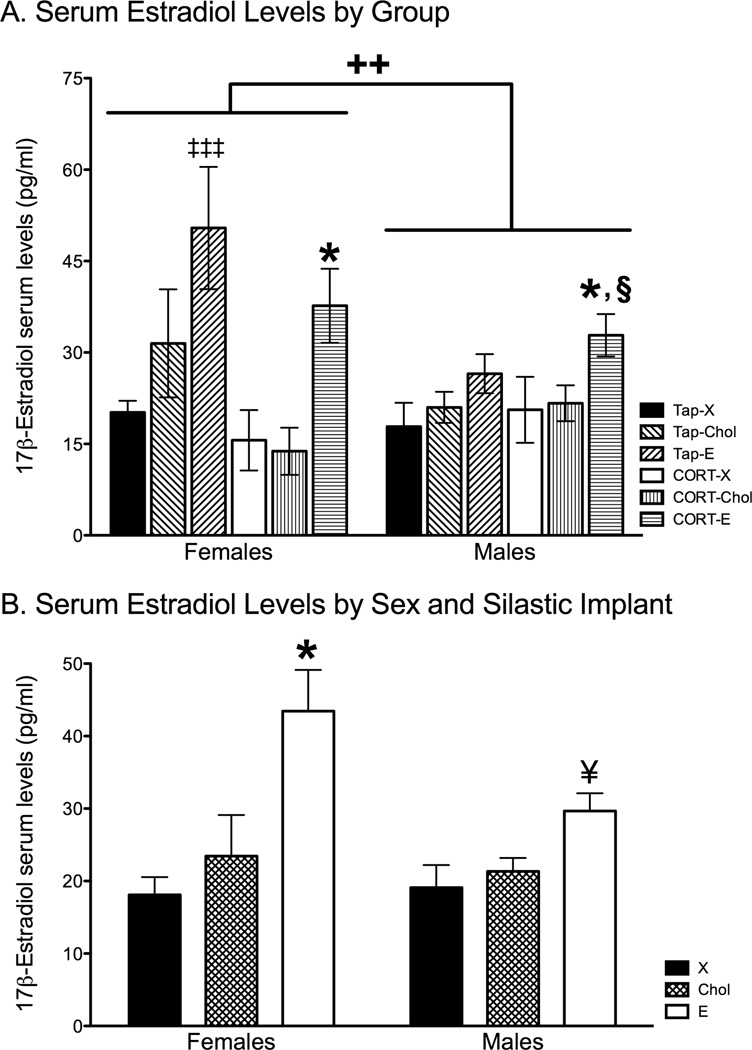
Silastic implant content significantly affected serum 17β-estradiol levels, as demonstrated by a 2 × 2 × 3 ANOVA showing a significant main effect of implant (F2, 56 = 13.96, p < 0.0001) on 17β-estradiol serum levels. Posthoc analyses showed that 17β-estradiol-filled capsules significantly elevated 17β-estradiol serum levels compared blank (p < 0.001) or cholesterol (p < 0.001) filled capsules (Fig. 4A). While both female and male rats implanted with cholesterol-filled capsules had elevated serum levels of 17β-estradiol, these levels did not differ from female and male rats implanted with blank-Silastic capsules. The ANOVA also revealed a significant interaction between sex and drink treatment (F1,56 = 5.90, p = 0.02), such that females given tap drinking water had significantly elevated estradiol levels compared to males given tap water (p = 0.03) and females given CORT water (p = 0.05). The remaining groups were statistically similar. The ANOVA also revealed a significant interaction between sex and Silastic implant (F2,56 = 3.07, p = 0.05). Females implanted with 17β-estradiol capsules showed statistically elevated serum 17β-estradiol than the remaining groups (p ≤ 0.01, Female-X, Female-Chol, Male-X, Male-Chol, and Male-E). Males implanted with 17β-estradiol capsules also showed significantly higher serum 17β-estradiol levels than males implanted with blank capsules (p < 0.05, Fig. 4B). There were no other significant results.
Figure 4.
Effects of chronic CORT treatment and cholesterol and 17β-estradiol administration on 17β-estradiol serum levels. A) Female rats implanted with 17β-estradiol implants and given tap water (Tap-E) displayed significantly elevated 17β-estradiol serum levels compared to all other groups, except females treated with 17β-estradiol and CORT water (CORT-E). Females treated with 17β-estradiol and CORT water showed significantly higher 17β-estradiol serum levels than did CORT-treated females implanted with blank or cholesterol capsules. Males exposed to CORT and implanted with 17β-estradiol implants demonstrated significantly elevated serum levels of 17β-estradiol compared to males implanted with blank implants and given tap water (Tap-X), and females given CORT drink treatment and implanted with blank- and cholesterol-filled Silastic implants (CORT-X and CORT-Chol). B) Contents of implants significantly affected serum 17β-estradiol levels. Females implanted with 17β-estradiol-filled Silastic implants displayed higher serum levels of 17β-estradiol compared to female and male rats implanted with blank- and cholesterol-filled Silastic implants (X and Chol). Moreover, male rats implanted with 17β-estradiol showed significantly higher 17β-estradiol serum levels than did males with blank- and cholesterol-filled Silastic implants. ‡‡‡ p<0.001 compared to all other groups except females given CORT drink and implanted with 17β-estradiol implants. ++ p < 0.02 compare females to males. * p < 0.05 compared to females given CORT drink and blank (females CORT-X) or Cholesterol implants (females CORT-Chol) § p < 0.05 compared to males given tap water and blank Silastic implants (males Tap-X). † p < 0.01 compared to females and males implanted with blank- or cholesterol-filled Silastic implants and males implanted with 17β-estradiol Silastic implants. ¥ p < 0.05 compared to males implanted with blank Silastic implants. Tap water treatment and blank Silastic implants (Tap-X), tap water treatment and cholesterol-filled implants (Tap-Chol), tap water treatment and 17β-estradiol-filled implants (Tap-E), CORT drink treatment and blank implants (CORT-X), CORT drink treatment and cholesterol-filled implants (CORT-Chol), CORT drink treatment and 17β-estradiol-filled implants (CORT-E).
4. Discussion
In this study, we investigated the effects of prolonged CORT exposure on hippocampal CA3 dendritic architecture in gonadectomized male and female rats given 17β-estradiol-, cholesterol-, or blank-filled Silastic capsules. Our results revealed that CORT was associated with hippocampal CA3 apical dendritic retraction in male and female rats. Moreover, 25% 17β-estradiol in cholesterol and cholesterol alone implants prevented this CORT-induced reduction of CA3 apical dendrites in both sexes. These findings are the first to demonstrate that 25% 17β-estradiol in cholesterol and cholesterol alone protected against CORT-induced hippocampal dendritic remodeling in males and that CORT produced hippocampal CA3 apical dendritic retraction in females.
During the stress response, the release of CORT, neurotransmitters, and neuropeptides are altered and any one of these alterations can potentially influence neuronal integrity. In our study, CORT was administered in drinking water to chronically elevate circulating GC levels without the concomitant changes in neurochemicals that are released during a stress response. This CORT drinking water regimen (400 µg/ml) was previously compared to a separate cohort of rats that underwent restraint (Magariños et al., 1998). They reported that CORT drink produced circulating serum CORT levels similar to the restraint group near the end of the restraint period, ranging from 14 to 29 µg/dl (for the CORT drink condition) and 16 to 21 µg/dl (for the restraint condition). Levels prior to the start of restraint, however, differed as restrained rats exhibited basal serum CORT levels (1 to 2 µg/dl), whereas the CORT-drink treated rats maintained high serum CORT levels (20 to 29 µg/dl). Consequently, CORT drink and restraint increased serum CORT levels, but CORT drink might extend the duration of serum CORT elevations. A potential limitation of the current study is how much CORT was actually ingested by the rats. It is possible that CORT treated rats in the current study drank less than did the tap water treated group as one study observed that less CORT drink was consumed by CORT treated rats compared to a control drink group given tap water (Waters and McCormick, 2011). While the amount of CORT drink ingested might vary in our study, the ramifications of prolonged exposure to this concentration of CORT in the drinking water manifested physiological effects consistent with effective GC treatment, such as reduced body weight gain (Watanabe et al. 1992a; Sousa et al. 1998a & 1998b; Magariños et al., 1998; Conrad et al., 1999; Coburn-Litvak et al., 2003; Conrad et al., 2004; Tata et al., 2006; Conrad et al., 2007; Schubert et al., 2008; Brummelte et al., 2010) and altered adrenal weights (Magariños et al., 1998; Tata et al., 2006). In addition, 17β-estradiol further modified body weights and adrenal weights without negating the effect of CORT on physiological parameters per se, as rats exposed to both CORT and 17β-estradiol showed the lowest body weight gain and the largest adrenal/body weight ratio. Consequently, physiological changes confirmed effective CORT administration.
The effects of CORT on hippocampal CA3 dendritic morphology in males evident in this study have some similarities and differences with the literature. We found that CORT drink provided to male rats reduced CA3 apical dendritic branch number compared to male rats given tap water. While we also found a significant main effect for CORT drink to reduce CA3 apical branch length (for both females and males and across Silastic implant condition), planned comparisons between CORT-X and Tap-X in males did not reveal statistical differences. Our results support the finding that CORT reduces apical dendritic complexity of hippocampal CA3 neurons in male subjects (Woolley et al., 1990; Magariños et al., 1998; Sousa et al., 2000; Conrad et al., 2007). However, the degree of dendritic remodeling varies across studies. For example, one of these reports found CORT reduced CA3 dendritic complexity in both apical branch points and length (Woolley et al., 1990), another found CORT only reduced CA3 apical dendritic length (Sousa et al., 2000), while the other two studies found CORT effects only in apical branch points (Magariños et al., 1998; Conrad et al., 2007). Our current study aligns with the latter reports, and we speculate that the differences as to whether CORT produces effects on both dendritic measures may reflect the amount and/or duration of exposure to CORT. These discrepancies may be relevant because the amount of CORT ingested could vary across individual rats, which may contribute to some of the variability in morphological outcomes. Moreover, as the duration of CORT exposure in the drinking water is extended from three to five weeks, CORT effects are observed in both CA3 apical dendritic branch points and length from the same laboratory (please compare Magariños et al., 1998 with Margariños et al., 1999). Taken together, our current findings support previous research showing that CORT reduced male hippocampal CA3 architecture and these changes, whether in branch points or length, are reliably shown in the apical region.
How CORT influences CA3 dendritic complexity in females differs with reports that manipulated chronic stress. From our laboratory, chronic stress produced CA3 apical and basal dendritic retraction in ovariectomized female rats (McLaughlin et al., 2005, 2010; Conrad et al., 2012), and this dendritic pruning was prevented with Silastic implants of cholesterol or estradiol (McLaughlin et al., 2010). These findings appear to contrast with a report that found chronic stress to produce CA3 dendritic retraction in the basal region, as measured by branch points without altering basal dendritic length (Galea et al., 1997). One difference is the former studies used ovariectomized female rats, whereas the latter study used gonadally intact female rats. Consequently, the ovarian hormones could have attenuated the stress-induced CA3 dendritic retraction, as revealed by one metric (branch numbers) being reduced as opposed to seeing effects in both measures by Galea et al, (1997). Another variable is the number of cell types used in the analysis. In our assessment, the number of long-shafted and short-shafted cells is weighted equally in quantification because CA3 short-shafted cells are intrinsically more complex than CA3 long shafted cells (Fitch et al., 1989). One of our past reports found chronic stress to decrease the branch points in the basal dendritic tree of long-shafted neurons, but not short-shafted neurons (McLaughlin et al., 2005). It is tempting to speculate that perhaps the contribution of cell types in the analysis could have produced the differences in these reports. Alternatively, perhaps chronic CORT administration produces a different neuronal profile than that observed by chronic stress, and future studies will need to parse out these outcomes.
Serum from a subset of rats was analyzed and showed that 17β-estradiol administration produced detectable, circulating 17β-estradiol levels within the range found in cycling, proestrous female rats (~40 – 50 pg/ml, Smith M., 1975). These serum levels were also greater than circulating 17β-estradiol found in rats implanted with blank or cholesterol capsules. Females in the tap water treatment and implanted with 17β-estradiol Silastic capsules had the highest serum levels, which were significantly greater than the remaining groups (Fig. 4). Interestingly, both male and female gonadectomized rats with blank Silastic implants showed detectable 17β-estradiol levels, suggesting that the assay may have had some cross-reactivity. Consequently, the actual serum levels of 17β-estradiol in these groups were most likely lower than our assay detected. It is important to note that all groups receiving 25% 17β-estradiol implants showed significantly elevated serum 17β-estradiol compared to the cholesterol- and blank-filled capsules. These findings further validate effectiveness of 17β-estradiol administration through Silastic implants.
The mechanisms by which 25% 17β-estradiol in cholesterol and cholesterol alone prevented CORT-induced hippocampal dendritic changes are unclear; however, there are some intriguing possibilities. In our previous study (McLaughlin et al., 2010), we observed a dose-dependent increase in serum 17β-estradiol based upon the type of Silastic implant, such that cholesterol treatment elevated serum 17β-estradiol levels compared to ovariectomy, and these serum 17β-estradiol levels were further increased by capsules containing 25% 17β-estradiol in a cholesterol base. At that time, we hypothesized that one mechanism of action might be through the conversion of cholesterol into estradiol given the dose-dependent serum estradiol elevations and other studies showing that high cholesterol diets could significantly elevate 17β-estradiol serum levels in ovariectomized mice and improve cognition (see recent work, Li et al., 2012). However, unlike our past study, the current research showed that cholesterol implants may not have produced 17β-estradiol levels that were statistically elevated compared to those associated with the blank implants and yet, the cholesterol-treated rats were protected against CORT-induced CA3 dendritic retraction. This finding revealed a limitation of our study in that cholesterol alone produced similar neuroprotection against CORT-induced CA3 dendritic retraction as compared to the 17β-estradiol group. Consequently, the current design made it difficult to distinguish whether 17β-estradiol was neuroprotective alone. Specifically the 17β-estradiol implants also contained a percentage of cholesterol (75%), with cholesterol perhaps mediating the neuroprotective effects against CORT-induced CA3 dendritic retraction. Indeed, cholesterol has been shown to be important for synaptic activity (Mauch et al., 2001, Pfrieger, 2003; Suzuki et al., 2007, Frank et al., 2008), dendritic differentiation (Goritz et al., 2005), and cellular function (Tsui-Pierchala et al., 2002). Moreover, dysregulating cholesterol metabolism in the brain has been implicated in several neurodegenerative disorders that involve limbic structures, such as Huntington’s disease (Karasinska and Hayden, 2011) and Alzheimer’s disease (Vance et al., 2005). The importance of cholesterol in brain function is further emphasized by reports that the brain contains approximately 25% of the total quantity of cholesterol in the body (Dietschy and Turley, 2001). Therefore, the possibility exists that cholesterol (and not 17β-estradiol) may have mediated neuroprotection for CA3 dendritic architecture following chronic CORT treatment.
Another possible mechanism mediating protection against CORT-induced CA3 dendritic retraction may involve the conversion of cholesterol into another neurosteroid, as cholesterol is a steroid precursor and can give rise to brain estradiol, as well as other neurosteroids. It is important to recognize that steroid levels within the brain do not necessarily represent steroid plasma levels (Croft et al., 2008). Consequently, cholesterol-filled implants could have provided neuroactive steroids within the brain, even when the serum assay revealed statistically similar estradiol levels as found in the blank- implanted group. Hence, cholesterol from Silastic implants could have elevated neurosteroids and even brain estradiol levels. For instance,17β-estradiol can be synthesized de novo within the brain, including within the hippocampus (Baulieu 1998; Kretz et al., 2004; Hojo et al., 2004). Indeed, enzymes required for neurosteroidogenesis are abundant in many regions of the hippocampus (Kimoto et al., 2001; Hojo et al., 2004, 2008), leading to the intriguing possibility that cholesterol was locally converted into 17β-estradiol, for example. Estrogens can exert neuroprotective effects and enhance the survival of neurons (Behl 2003; Maggi et al., 2004; Turgeon et al., 2006), protect against traumatic brain injury, and benefit several neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, and schizophrenia (For review see: Behl, 2002; Dhandapani and Brann, 2002). In vitro studies show that 17β-estradiol can protect against amyloid-β damage (Marin et al., 2003) and glutamate neurotoxicity (Mize et al., 2003). Taken together, it is likely that cholesterol and 17β-estradiol may both block CORT-induced hippocampal dendritic remodeling.
The current data are of particular importance because hippocampal dendritic architecture may have implications on hippocampal function, such as learning and memory. We have previously shown that chronic stress and the resulting simplification of hippocampal CA3 dendritic arbors correspond with poor spatial learning and memory in males (for reviews, Conrad 2006, 2010). We have also found that chronic stress does not produce these same cognitive changes in females, whether they are gonadally intact or ovariectomized (for review, McLaughlin et al., 2009), perhaps due to a more complex interplay between the hypothalamic-pituitary-adrenal axis and the hypothalamic-pituitary-gonadal axis (Conrad and Bimonte-Nelson, 2010). These findings beg the question as to whether CA3 dendritic retraction is necessary for spatial learning and memory.
Based on the results from our laboratory, we suggest that CA3 dendritic retraction is an indicator of vulnerability to spatial learning and memory deficits, but the presence of CA3 dendritic retraction per se does not dictate impaired spatial memory. For example, chronic restraint stress that produces CA3 dendritic retraction in male rats typically leads to spatial memory deficits; however, these deficits can be prevented by merely one injection of metyrapone to reduce CORT prior to memory testing (Wright et al., 2006). In this scenario, the status of the stressed brain, including the expression of CA3 dendritic retraction, would still be present in the subsequent hours in which testing occurred, but the neurochemical profile would be altered by the metyrapone injection. Moreover, a theory about information processing in the hippocampus derived from place cells posits that the CA3 trisynaptic pathway contributes to information about memory retrieval or expectation, whereas the entorhinal cortical pathway to the CA1 neurons contributes information about the senses or the actual experience (Moser and Paulsen, 2001). One take home point is that while two main pathways contribute to CA1 information processing, one does not involve the CA3 neurons. Perhaps CA3 neurons contribute to refinement as opposed to being a functional necessity. Another point to contemplate is that estrogens increase CA1 spine density (Woolley and McEwen, 1993; McLaughlin et al., 2008, 2010; Conrad et al., 2012) and the combination of chronic stress and estradiol increases CA1 dendritic complexity (Conrad et al., 2012). Perhaps these plastic changes in the CA1 dendrites might be compensating for or even facilitating spatial abilities following chronic stress, whether or not CA3 dendritic complexity is altered. Regardless of the interpretation, the findings of the current study reveal that when both males and females are gonadectomized and provided with similar steroids (17β-estradiol, cholesterol, blank), the CORT effects on hippocampal CA3 dendritic architecture and neuroprotective mechanisms of 17β-estradiol/cholesterol no longer reveal sex differences. Similar findings are observed at pre-puberty when gonadal hormones have not fully matured following a prenatal and postnatal stress paradigm in which CA3 dendritic retraction is found in both males and females (Bock et al., 2011). Future studies are necessary to elucidate the specific cellular and molecular mechanisms behind the neuroprotection exerted by 17β-estradiol/cholesterol and how 17β-estradiol and cholesterol in combination with CORT impact neuronal function or activity.
Highlights.
Corticosterone induced hippocampal CA3 dendritic retraction in male and female rats.
Cholesterol protects against CORT-induced dendritic retraction in male and female rats.
25% 17β-Estradiol in choloestrol protects against CORT-induced dendritic retraction.
Acknowledgements
Funding was provided by NH74727, the Howard Hughes Medical Institute through the Undergraduate Science Education Program and from ASU School of Life Sciences (Ortiz) and the NIH IMSD Program via R25GM099650 (Ortiz). The authors gratefully acknowledge Laurence M. Demers, Jeffery Hanna, Agniezska Mika, and James Slinkey.
Abbreviations
- CA3
Cornu Ammonis region 3
- Chol
Cholesterol
- CORT
Corticosterone
- E
17β-estradiol
- GC
Glucocorticoid
- LS
Long Shaft
- SS
Short Shaft
- Tap
tap water
- X
blank Silastic implant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baulieu EE. Neurosteroids: A novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Beck SG, List TJ, Choi KC. Long- and short-term administration of corticosterone alters CA1 hippocampal neuronal properties. Neuroendocrinology. 1994;60:261–272. doi: 10.1159/000126758. [DOI] [PubMed] [Google Scholar]
- Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- Behl C. Estrogen Can Protect Neurons: Modes of Action. Biochemistry and Molecular Biology. 2003;83:195–197. doi: 10.1016/s0960-0760(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Bock J, Sriti Murmu M, Biala Y, Weinstock M, Braun K. Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience. 2011;193:34–43. doi: 10.1016/j.neuroscience.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35(5):694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Chronic High Corticosterone Reduces Neurogenesis in the Dentate Gyrus of Adult Male and Female Rats. Neuroscience. 2010;168:680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Bush VL, Middlemiss DN, Marsden CA, Fone KCF. Implantation of a slow release corticosterone pellet induces long-term alterations in serotonergic neurochemistry in the rat brain. J Neuroendocrinol. 2003;15:607–613. doi: 10.1046/j.1365-2826.2003.01034.x. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What Is the Functional Significance of Chronic Stress-Induced CA3 Dendritic Retraction Within the Hippocampus? Behavioral and Cognitive Neuroscience Reviews. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Progress in Neuro-Psychopharm & Bio Psychiatry. 2010;34(5):742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Progress in Brain Research. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. Journal of Neuroscience. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic Stress and a Cyclic Regimen of Estradiol Administration Separately Facilitate Spatial Memory: Relationship With Hippocampal CA1 Spine Density and Dendritic Complexity. Behavioral Neuroscience. 2012;126:142–156. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, MacMillan DD, Tskehanov S, Wright RL, Baran SE, Fuchs RA. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of Learning and Memory. 2004;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Croft AP, O’Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Research. 2008;1238:12–22. doi: 10.1016/j.brainres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Bio of Repro. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Current Opinion in Lipidology. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dumas TC, Gillette T, Ferguson D, Hamilton K, Sapolsky RM. Anti-Glucocorticoid Gene Therapy Reverses the Impairing Effects of Elevated Corticosterone on Spatial Memory, Hippocampal Neuronal Excitability, and Synaptic Plasticity. Journal of Neuroscience. 2010;30:1712–1720. doi: 10.1523/JNEUROSCI.4402-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evuarherhe O, Leggett J, Waite E, Kershaw Y, Lightman S. Reversal of the hypothalamo-pituitary-adrenal response to oestrogens around puberty. Journal of Endocrinology. 2009;202:279–285. doi: 10.1677/JOE-09-0175. [DOI] [PubMed] [Google Scholar]
- Frank C, Rufini S, Tancredi V, Forcina R, Grossi D, D’Arcangelo G. Cholesterol depletion inhibits synaptic transmission and synaptic plasticity in rat hippocampus. Experimental Neurology. 2008;212:407–414. doi: 10.1016/j.expneurol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Research. 1989;479(1):105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Heideman PD, Pittman JT, Schubert KA, Dubois CMR, Bowles J, Lowe SM, Price MR. Variation in levels of luteinizing hormone and reproductive photoresponsiveness in a population of white-footed mice (Peromyscus leucopus) Am J Physiol Regul Integr Comp Physiol. 2010;298:R1543–R1548. doi: 10.1152/ajpregu.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017 alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain - Role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, Hayden MR. Cholesterol metabolism in Huntington disease. Nature reviews. Neurology. 2011;10:561–572. doi: 10.1038/nrneurol.2011.132. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Campbell LW, Hao SY, Landfield PW. Corticosteroid Modulation of Hippocampal Potentials – Increased Effect With Aging. Science. 1989;245:1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura HO, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. In: Pacak K, et al., editors. Stress: Current Neuroendocrine and Genetic Approaches. vol. 1018. New York: New York Acad Sciences; 2004. pp. 323–327. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou LP, Brauckmann S, Zhao ST, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. Journal of Neuroscience. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF. Regulation of progestin receptors in medial amygdala: Estradiol, phytoestrogens and sex. Physiology and Behavior. 2009;97(2):146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xiao N, Yang X, Gao J, Ding J, Wang T, Hu G, Xiao M. A high cholesterol diet ameliorates hippocampus-related cognitive and pathological deficits in ovariectomized mice. Behavioural Brain Research. 2012;230:251–258. doi: 10.1016/j.bbr.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Luine VN, Spencer RL, McEwen BS. Effects of Chronic Corticosterone Ingestion on Spatial Memory Performance and Hippocampal Serotonergic Function. Brain Research. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Lussier AL, Romay-Tallón R, Caruncho HG, Kalynchuk LE. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience. 2013;231:38–48. doi: 10.1016/j.neuroscience.2012.11.037. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-Induced Atrophy of Apical Dendrites of Hippocampal CA3c Neurons – Comparison of Stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-Induced Atrophy of Apical Dendrites of Hippocampal CA3c Neurons – Involvement of Glucocorticoid Secretion and Excitatory Amino-Acid Receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain research. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. European Journal of Pharmacology. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: Mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Marin R, Guerra B, Hernandez-Jimenez JG, Kang XL, Fraser JD, Lopez FJ, Alonso R. Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience. 2003;121:917–926. doi: 10.1016/s0306-4522(03)00464-0. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective Retention of Corticosterone by Limbic Structure in Rat Brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Uptake of Corticosterone by Rat Brain and Its Concentration by Certain Limbic. Brain research. 1969;16:227–241. doi: 10.1016/0006-8993(69)90096-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Molecular neurobiology. 2009;40(2):166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17b-estradiol effectiveness in altering CA1 spines. Hormones and Behavior. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17 beta-Estradiol or Cholesterol Prevents Stress-Induced Hippocampal CA3 Dendritic Retraction in Ovariectomized Female Rats: Possible Correspondence Between CA1 Spine Properties and Spatial Acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- Moser EI, Paulsen O. New excitement in cognitive space: between place cells and spatial memory. Current Opinion in Neurobiology. 2001;11:745–751. doi: 10.1016/s0959-4388(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective Corticosteroid Antagonists Modulate Specific Aspects of Spatial Orientation Learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Overk CR, Lu PY, Wang YT, Choi J, Shaw JW, Thatcher GR, Mufson EJ. Effects of aromatase inhibition versus gonadectomy on hippocampal complex amyloid pathology in triple transgenic mice. Neurobio of Disease. 2012;45(1):479–487. doi: 10.1016/j.nbd.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Silva MTA, Weiss JM. Telemetered recording of hormone effects on hippocampal neurons. Science. 1971;171:394–395. doi: 10.1126/science.172.3981.394. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta-Biomembr. 2003;1610:271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Schubert MI, Kalisch R, Sotiropoulos I, Catania C, Sousa N, Almeida OFX, Auer DP. Effects of altered corticosteroid milieu on rat hippocampal neurochemistry and structure - An in vivo magnetic resonance spectroscopy and imaging study. J Psychiatr Res. 2008;42:902–912. doi: 10.1016/j.jpsychires.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Schachinger H, de Kloet ER, Oitzl MS. Stress impairs spatial but not early stimulus-response learning. Behav Brain Res. 2010;213:50–55. doi: 10.1016/j.bbr.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Smith M. The Control of Progesterone Secretion During the Estrous Cycle and Early Pseudopregnancy in the Rat: Prolactin, Gonadotropin and Steroid Levels Associated with Rescue or the Corpus Luteum of Pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Sousa N, Almeida OFX, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998a;2(4):237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain research. 1998b;794:199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sunanda, Meti BL, Raju TR. Entorhinal cortex lesioning protects hippocampal CA3 neurons from stress-induced damage. Brain research. 1997;770:302–306. doi: 10.1016/s0006-8993(97)00888-3. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kiyosue K, Hazama S, Ogura A, Kashihara M, Hara T, Koshimizu H, Kojima M. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J Neurosci. 2007;27:6417–6427. doi: 10.1523/JNEUROSCI.0690-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: Relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- Tsui-Perchala BA, Encinas M, Milbrandt J, Johnson EM. Lipid rafts in neuronal signaling and function. Trends in Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Estradiol impairs response inhibition in young and middle-aged, but not old rats. Neurotoxicology and Teratology. 2011;33(3):405–414. doi: 10.1016/j.ntt.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin Prevents Stress-Induced And Corticosterone-Induced Atrophy of CA3 Pyramidal Neurons. Hippocampus. 1992b;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress Induces Atrophy of Apical Dendrites of Hippocampal Ca3 Pyramidal Neurons. Brain Res. 1992a;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Waters P, McCormick CM. Caveats of chronic exogenous corticosterone treatments in adolescent rats and effects on anxiety-like and depressive behavior and hypothalamic-pituitary-adrenal (HPA) axis function. Biology of Mood & Anxiety Disorders. 2011;1:4. doi: 10.1186/2045-5380-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Rice R. Ethanol-induced conditioned partner preference in female mice. Behav Brain Res. 2013;243:273–277. doi: 10.1016/j.bbr.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to Excess Glucocorticoids Alters Dendritic Morphology of Adult Hippocampal Pyramidal Neurons. Brain research. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Workman JL, Brummelte S, Galea LAM. Postpartum corticosterone administration reduces dendritic complexity and increases the density of mushroom spines of hippocampal CA3 arbours in dams. J Neuroendocrinol. 2013;25:119–130. doi: 10.1111/j.1365-2826.2012.02380.x. [DOI] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]