Abstract
We recently described a family of experiments for R2nv Driven Spin Diffusion (RDSD) spectroscopy suitable for homonuclear correlation experiments under fast MAS conditions (J. Am. Chem. Soc., 133, 2011, 3943). In these RDSD experiments, since the broadened second-order rotational resonance conditions are dominated by the radio frequency field strength and the phase shifts, as well as the size of reintroduced dipolar couplings, the different R2nv sequences display unique polarization transfer behaviors and different recoupling frequency bandwidths. Herein, we present a series of modified R2nv sequences, dubbed COmbined R2nv-Driven (CORD), that yield broadband homonuclear dipolar recoupling and give rise to uniform distribution of cross peak intensities across the entire correlation spectrum. We report NMR experiments and numerical simulations demonstrating that these CORD spin diffusion sequences are suitable for broadband recoupling at a wide range of magnetic fields and MAS frequencies, including fast-MAS conditions (νr = 40 kHz and above). Since these CORD sequences are largely insensitive to dipolar truncation, they are well suited for the determination of long-range distance constraints, which are indispensable for the structural characterization of a broad range of systems. Using U-13C-alanine and U-13C,15N-histidine, we show that under fast-MAS conditions, the CORD sequences display polarization transfer efficiencies within broadband frequency regions that are generally higher than those offered by other existing spin diffusion pulse schemes. A 89-residue U-13C,15N-dynein light chain (LC8) protein has also been used to demonstrate that the CORD sequences exhibit uniformly high cross peak intensities across the entire chemical shift range.
Keywords: R2nv-Driven Spin Diffusion (RDSD), COmbined R2nv-Driven Spin Diffusion (CORD SD), symmetry sequences, solid-state NMR, fast MAS, broadband homonuclear correlation spectroscopy
INTRODUCTION
In recent decades, solid-state NMR (SS-NMR) spectroscopy has emerged as a powerful method for probing molecular structure and dynamics in inorganic materials, polymers, and biological solids.[1-10] Magic angle spinning (MAS) technique [11, 12] is widely used in SS-NMR for obtaining narrow spectral lines, and with the breakthroughs in MAS technologies starting in the late 1990’s, MAS frequencies of the order of νr = 40-110 kHz are now accessible to solid-state NMR spectroscopists.[13-15] Under these MAS frequencies, both the resolution and sensitivity per unit mass can be enhanced greatly due to: (i) the efficient suppression of various anisotropic NMR interactions, (ii) the increased coupling of the magnetization with the coil due to smaller rotor diameter, (iii) the related higher radio frequency (rf)-fields that allow for a better control of the magnetization, and (iv) the access to the proton detection in solids under high resolution.[16-21] With these advantages, detailed structural and dynamics analysis of very large proteins and protein assemblies containing either uniform or dilute isotopic labels has become feasible. Numerous SS-NMR sequences have been developed for recoupling anisotropic interactions, and with the aid of these methods structural and dynamics information can be obtained.[22-37] However, the majority of the existent recoupling techniques cannot work well at MAS frequencies above 30 kHz.
The conventional spin-diffusion-based recoupling techniques, including proton-driven spin diffusion (PDSD),[38, 39] RF-assisted diffusion (RAD),[40] and dipolar-assisted rotational resonance (DARR),[33, 41] have lead to extensive 13C (or 15N) applications for resonance assignments, determination of structural constraints, and quantitative SS-NMR measurements under the MAS frequencies of 30 kHz and lower.[42-58] These second-order rotary-resonance-recoupling (R3) techniques do not severely suffer from dipolar truncation and are particularly advantageous for structural determination in uniformly isotopically enriched systems. However, their performances deteriorate rapidly with increased MAS frequencies and in practice they do not work above νr ≥ 25-30 kHz, where the polarization transfer rates are more dependent on the R3 conditions, which cannot be effectively attained by conventional spin diffusion methods. Recently, several pulse sequences have been developed with modified 1H rf field (ν1H ) irradiation to obtain 13C-13C spin diffusion spectra at MAS frequencies of 30 kHz and above, including the mixed rotational and rotary resonance (MIRROR),[59] phase-alternated recoupling irradiation (PARIS) and its variants,[60, 61] and R2nv-symmetry driven spin diffusion (RDSD) [62] techniques. Within these pulse schemes, phase- or amplitude-modulated rf field irradiation is applied on protons in a periodic fashion, and the second order R3 conditions, Δνiso = ν1H ± nνr, are largely broadened resulting in more efficient polarization transfers at MAS frequencies faster than 30 kHz.[59, 62]
Within spin diffusion spectroscopy under MAS frequencies of 30 kHz and above, the polarization transfer efficiency between 13C spin pairs is more sensitive to their frequency differences, Δνiso. As shown in our recent RDSD report,[62] each R2nv symmetry sequence exhibits different polarization transfer behaviors with distinct dependencies on Δνiso because the recoupled bands are dominated not only by the reintroduced dipolar couplings, but also by the amplitude, v1H, and the phase shifts of the applied rf field. The R211 sequence (PARIS) is most efficient for accomplishing spin diffusion among the carbons with small Δνiso values, i.e. aliphatic-to-aliphatic, while the R222 sequence shows highest polarization transfer efficiencies for coupled spins with relatively large Δνiso value, i.e., aliphatic-to-carbonyl. The R221 sequence displays high transfer efficiency and uniform excitation for both aliphatic-to-aliphatic and aliphatic-to-carbonyl carbon regions, but the transfer efficiency for the 13C spin pairs with Δδiso = 60-90 ppm is much lower, in accordance with the numerically simulated 13C-13C transfer profiles.
To achieve broadband 13C-13C correlation spectroscopy at MAS frequencies of 30 kHz and above, several spin-diffusion schemes with super phase-cycling have been reported recently, including PARISxy [63] and SHANGHAI [64]. In this work, we present an alternative approach to broadband spin diffusion under fast-MAS conditions: a series of modified R2nv sequences dubbed COmbined R2nv-Driven (CORD). We demonstrate that CORD sequences yield broadband homonuclear dipolar recoupling and give rise to uniform distribution of cross peak intensities across the entire correlation spectrum spanning a 13C chemical shift range of 200 ppm. The variations in rf field strengths and phase shifts in these super phase-cycled CORD schemes and sequences derived from CORD assure significant broadening of the second-order R3 conditions, which helps driving 13C-13C spin diffusion in a truly broad frequency range, even for the spin systems with sparse proton content. We report here SS-NMR experiments and numerical simulations demonstrating that these CORD spin diffusion sequences are suitable for broadband recoupling over a wide range of magnetic fields and MAS frequencies including MAS frequencies of 40 kHz and above. The comparison to the recent spin-diffusion schemes (PARISxy (m = 2) and SHANGHAI) and to the fpRFDR [37] and DREAM [32] sequences is also included, both through numerical simulations and experimental measurements. We present experimental results on model systems U-13C,15N-alanine and U-13C,15N-histidine, as well as on a protein, U-13C,15N-dynein light chain (LC8) protein. These findings indicate that at νr = 40 kHz, the basic CORD sequence and the super-cycled CORD schemes display polarization transfer efficiencies that are generally higher than those attained by other existing spin diffusion pulse schemes, which give rise to uniformly high cross peak intensities across the entire 13C chemical shift range. Furthermore, these CORD sequences can also be used for the determination of long-range distance constraints because they are generally insensitive to dipolar truncation, in contrast with DREAM and fpRFDR techniques, and which is particularly important in uniformly labeled biological samples. Thus CORD-based sequences are well suited for truly broadband spin diffusion spectroscopy at MAS frequencies of 30 kHz and faster.
EXPERIMENTS AND METHODS
Materials
U-13C,15N-labeled L-alanine (Ala) and L-histidine (His) were purchased from Cambridge Isotope Laboratory. Ala powder sample was packed into the 1.8 mm MAS rotor for subsequent SS-NMR experiments without any further purification or re-crystallization. His sample was doped with 0.1 mol% CuCl2 and re-crystallized before packing into MAS rotor. U-13C,15N-enriched dynein light chain protein (LC8) was expressed in E.coli and purified as described previously.[65] This LC8 sample was prepared by controlled precipitation, via slow addition of a solution of 30% PEG-3350 to the solution of 11 mg of LC8 (30 mg/ml). Prior to the precipitation step, both PEG-3350 and LC8 were dissolved in 10 mM MES buffer (10 mM MgCl2, pH 6.0) and doped with 50 mM EDTA-Cu(II).
Solid-State NMR Spectroscopy
Experiments were conducted on a 14.1 T Varian Infinity-Plus and a 19.97 T Bruker AVIII SS-NMR spectrometers, with 1H and 13C Larmor frequencies of 599.8/850.4 and 150.8/213.8 MHz, respectively. A 1.8 mm triple-resonance HCN MAS probe developed in the Samoson laboratory was used for the experiments performed at 14.1 T on Ala and His samples, and all spectra were recorded at the MAS frequency of νr = 40 kHz, controlled to within ±5 Hz using a Varian MAS controller. The typical 90° pulse lengths were then of 2.8 (1H) and 4.1 μs (13C). A Bruker 1.3 mm triple-resonance HCN MAS probe was used for the experiments at 19.97 T. For these experiments, Cu(II)-EDTA doped LC8 protein sample was packed into the rotor and spun at the MAS frequency of νr = 40 kHz, controlled to within ±10 Hz using a Bruker MAS controller. The typical 90° pulse lengths were then of 1.4 (1H) and 3.0 μs (13C).
To reduce sample heating during fast MAS, nitrogen gas was used for cooling, resulting in a final sample temperature of 0 °C for the LC8 sample, and 20 °C for Ala and His samples. Low-power TPPM 1H decoupling with a rf field strength of 11 kHz was used during t1 and t2 periods. The 1H→13C cross polarization was performed with a 1H rf field of 55 kHz and a linear amplitude ramp (80-100%) on 13C with the center of the ramp Hartmann-Hahn matched to the first spinning sideband. During the mixing period, τmix, different composite pulse irradiations were applied on protons, and a series of corresponding 2D NMR spectra were recorded. The rf field strengths on the 1H channel during the mixing time were set to their nominal value of ν1H = 40 and 20 kHz for R21v and R22v symmetry sequences, respectively. Appropriate rf field strengths, corresponding to the n = 1 R3 condition, were used for PARISxy and SHANGHAI sequences, i.e., ν1H = νr. All 2D NMR data were processed with NMR-Pipe software [66] in a Mac environment using a standard protocol, including zero-filling to twice the number of points in the indirect dimension, Fourier transform, and phase correction in both dimensions.
Numerical Simulations
All numerical simulations were performed using the SIMPSON [67] and SPIN-EVOLUTION [68] software packages. 168 REPULSION angles (α, β) and 64 γ angles were used to generate a powder average. The atomic coordinates for the model spin systems employed in the simulations were taken from the SS-NMR structure of the leucine residue in the N-f-MLF-OH tri-peptide (PDB ID 1Q7O).[51] These atomic coordinates are generally regarded as a valid representation for spin systems of other amino acids, including Ala and His. The one-bond dipolar coupling constants for 1H-13C and 13C-13C were set as 22,690 and 2,251 Hz, respectively. Throughout the simulations, all possible pairwise dipolar couplings were taken into account. J couplings were ignored as their effects are negligible given their small sizes. Other parameters used in simulations were the same as in the corresponding experiments.
RESULTS AND DISCUSSION
Bandwidth and Polarization Transfer Efficiency in CORD Sequences: Numerical Simulations
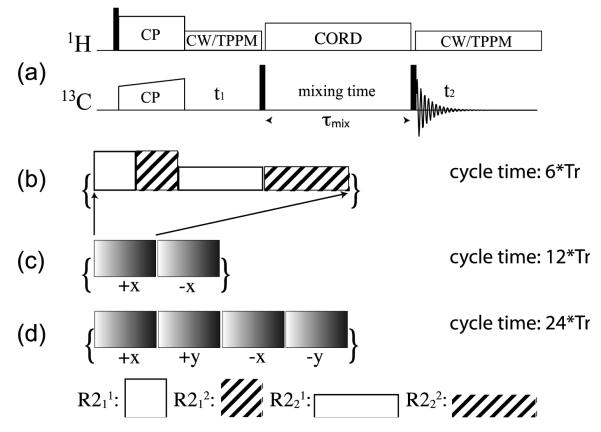
The pulse sequence for homonuclear CORD spin diffusion experiments is shown in Figure 1a. As illustrated in Figure 1b-d, during the mixing period, a series of combined R2nv symmetry pulses are applied on the 1H spins; these include the basic CORD (Figure 1b), CORDxix (Figure 1c) and CORDxy4 (Figure 1d). The basic CORD sequence shown in Figure 1b is composed of four types of R2nv symmetry pulses, R211, R212, R221, and R222. The order of these R2nv symmetry pulses does not influence the outcome of a particular experiment and can be set arbitrarily. As in our recent report, the nominal rf field strengths are set at ν1H = vr and ν1H = vr/2 for R21v and R22v sequences, respectively. Various super-cycled CORD versions can be derived as well, including CORD0CORD180 denoted as CORDxix (Figure 1c), CORD0CORD90CORD180CORD270 denoted as CORDxy4 (Figure 1d), CORD0CORD90 denoted as CORDxy (not shown), and CORD0CORD90CORD180 CORD270CORD0CORD270CORD180CORD90 denoted as CORDxy8 (not shown).
Figure 1.
(a) The general pulse sequence for 2D 13C-13C CORD homonuclear correlation experiments. An rf irradiation consisting of a series of composite pulses is applied on proton spins during the mixing time, τmix, with the irradiation sequences corresponding to: (b) basic CORD; (c) CORDxix; (d) CORDxy4. These irradiation schemes are composed of rotor-synchronized R2nv-type symmetry sequences: R211 (ν1H = νr), R212 (ν1H = νr), R221 (ν1H = νr/2), R242 (ν1H = νr/2).
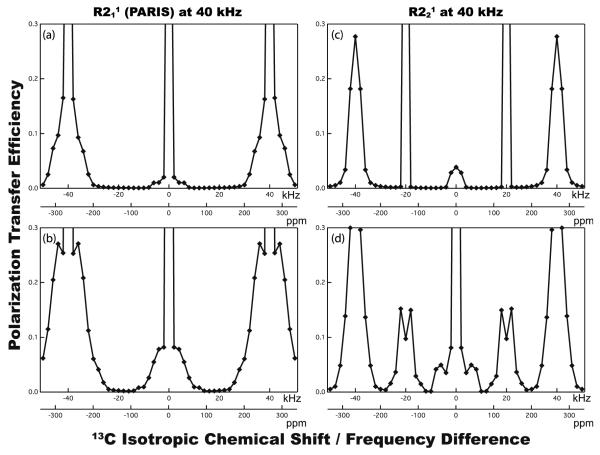
Protons play important roles in all varieties of 2nd-order 13C-13C spin diffusion experiments, where the 13C-13C polarization transfers can be driven by the recoupled 1H-13C hetero- and 1H-1H homonuclear dipolar couplings. Under MAS frequencies below 30 kHz, even the residual dipolar couplings can easily drive 13C-13C polarization transfers over a very broad frequency range. However, at MAS frequencies of 30 kHz and above, the first-order rotational resonance (R2) conditions, Δνiso = ±nνr, cannot be fulfilled, and the general PDSD technique fails. Under such high MAS frequencies, the homonuclear polarization transfers mostly depend on the second-order rotary resonance recoupling (R3) conditions, Δνiso = ν1H ± nνr, and broadband recoupling would be reached when the broadened R3 conditions are met, ν1H ± nνr - KscνDD ≤ Δνiso ≤ ν1H ± nνr + KscνDD, which can be accomplished by various recoupling sequences applied on protons. Ksc is the effective recoupling scaling factor, and νDD the 1H-13C dipolar coupling constant. In Figure 2, the simulated dependence of the 13C-13C polarization transfer efficiency is plotted versus the resonance frequency difference, Δνiso, for R211 (basic PARIS) and R221 symmetry sequences at the MAS frequency of νr = 40 kHz and magnetic field of 14.1 T. For the spin systems containing a small amount of protons (i.e. C2H in (a, c)), only 13C spin pairs belonging to very narrow frequency bands can be recoupled. However, it can be seen that the frequency bands are greatly broadened when spin systems contain more protons (i.e. C2H2 in (b, d)). When the rf pulse irradiation is of R211 symmetry (PARIS), only aliphatic-to-aliphatic correlations are observed, while aliphatic-to-aromatic and aliphatic-to-carbonyl correlations are very weak or absent. With R221 sequence, broaderband recoupling is attained, but the transfer efficiency for the 13C spin pairs with Δδiso ≈ 60-90 ppm (corresponding to aliphatic-to-aromatic correlations) is much lower than that for other regions, which would result in the missing cross peaks in the corresponding 13C-13C spin diffusion spectra.
Figure 2.
Simulated dependence of the 13C-13C polarization transfer efficiency versus the isotropic chemical shift difference for R211 (basic PARIS) and R211 symmetry sequences for C2Hn spin systems containing n = 1 proton (a, c) and n = 2 protons (b, d). The transfer efficiency is defined as 2I2Z/(I1Z + I2Z), where I1Z is the initial polarization on nucleus 1, and I2Z is the polarization on spin 2 after the polarization transfer. The MAS frequency is νr = 40 kHz, and the mixing time is τmix = 150 ms. The ν1H rf-field amplitude is equal to its nominal value of 20 (c,d) and 40 (a,b) kHz. The 13C-13C polarization transfer efficiency was simulated over the frequency difference of Δνiso = ±50 kHz at the magnetic field of 14.1 T.
In contrast, the 13C-13C recoupling bandwidths attained in the CORD schemes are expected to be much broader, and be the composite of the individual R2nv symmetry sequences. Moreover, the phase shifts introduced to the π pulses in the super-cycled CORD schemes are also expected to contribute to broadening the R3 conditions that determine the 13C-13C polarization transfers, in the same way as the improvements introduced by the PARISxy sequence over PARIS.
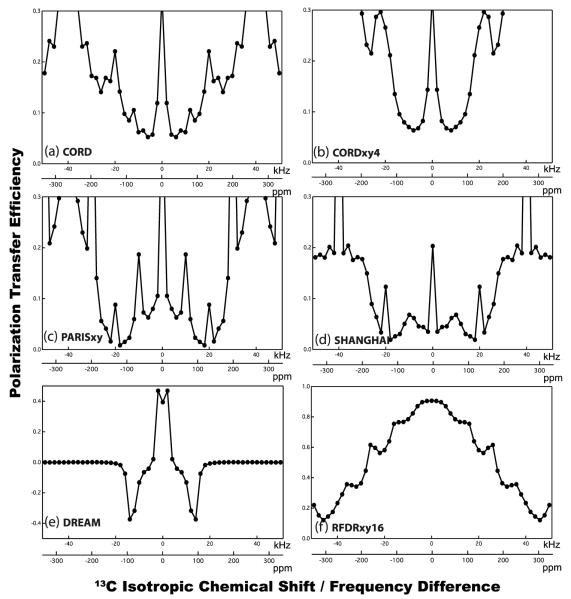
Figure 3 demonstrates the simulated dependence of the 13C-13C polarization transfer efficiency on the frequency difference for CORD, CORDxy4, PARISxy, SHANGHAI, DREAM and fpRFDR sequences, using the same C2H2 spin system model as in Figure 2b and 2d. As shown in Figure 3c, the phase-cycled PARISxy sequence exhibits much broader recoupling band-width compared with the basic PARIS sequence, in spite of the low efficiency of the aliphatic-to-aromatic and aliphatic-to-carbonyl polarization transfers. The modified PARISxy sequence with additional phase cycles, dubbed SHANGHAI, gives rise to a more broadband recoupling profile compared with PARISxy when standard rf field condition is used (ν1H = νr). Nevertheless, none of these sequences exhibits truly broadband behavior. In contrast, recoupling by both the basic CORD and the super phase-cycled CORDxy4 is fully broadbanded, covering all the possible correlations among 13C spins within the 0-200 ppm chemical shift range (Figure 3a and 3b). Notably, the numerical simulations reveal that in the super phase-cycled CORDxy4 the 13C-13C polarization transfer is much more uniform across the entire frequency (chemical shift) difference range than in the individual R2nv, PARISxy or SHANGHAI sequences, and this is also the case for other versions of phase-cycled CORD sequences (see Figure S1 of the Supporting Information). As discussed above, the basic CORD scheme is composed of four R2nv symmetry sequences that can be introduced in any order, such as R211R221R212R222. Regardless of the order in which the individual R2n elements are arranged, the composite CORD sequences give rise to broadband polarization transfer (Figure S1).
Figure 3.
Simulated dependence of the 13C-13C polarization transfer efficiency versus the isotropic chemical shift difference for various composite rf pulse irradiations: (a) CORD, (b) CORDxy4, (c) PARISxy, (d) SHANGHAI, (e) DREAM, and (f) fpRFDR. A C2H2 spin model (the same as in Figure 2b and 2d) was used for all the simulations. The MAS frequency is νr = 40 kHz, and the mixing time is τmix = 150 ms for (a-d), and 3 ms for (e) and (f). 13C-13C polarization transfer efficiency was simulated over the frequency difference of Δνiso = ±50 kHz at the magnetic field of 14.1 T.
Besides the spin-diffusion-based sequences, DREAM[32] and fpRFDR[65] also work well under fast MAS conditions, and high transfer efficiency can be easily attained provided sufficiently short mixing time is used. However, both sequences have limitations in the regimes where CORD is advantageous. For example, in the DREAM sequence, the polarization transfer efficiency is very sensitive to the isotropic chemical shift difference between the coupled spin pairs, and the recoupling frequency band is very limited (< 100 ppm), as seen in Figure 3e. Therefore, it is not possible to establish broadband polarization transfer across the entire spectrum in a single DREAM experiment. Both DREAM and fpRFDR sequences are very sensitive to dipolar truncation restricting their utility to intra-residue resonance assignments or acquisition of long-range constraints only in sparsely (but not uniformly) labeled systems. Furthermore, both DREAM and fpRFDR sequences suffer the sensitivity loss from various relaxation processes characterized by the respective time constants, including T1RF, T1ρ, and T1ZQ. For the spin-diffusion-based experiments, the strength of the detected signal is mostly dependent on the 13C spin-lattice relaxation times, and generally 13C T1 are of the order of seconds, therefore permitting determination of long-distance structural constraints with long mixing times of up to seconds.
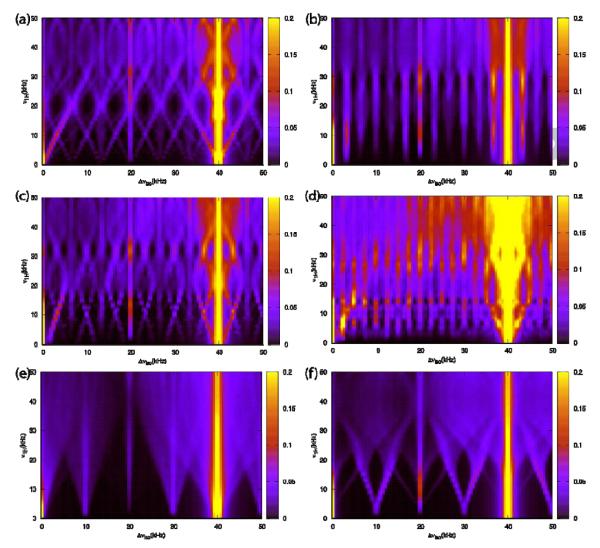
Another important consideration is the dependence of the rf field strength on the performance of the CORD sequences. The rf field strengths for the R2nv elements in CORD are typically defined according to their symmetry properties. However, the rf field strength of each R2nv block can also be set to an arbitrary value different from its nominal requirement (ν1H ≠r or 0.5*νr). Figure 4 shows the simulated dependence of the 13C-13C polarization transfer efficiency on the rf field strength and the frequency difference, Δviso, for various recoupling schemes: CORD, CORDxix, CORDxy4, and CORDxy8, PARISxy and SHANGHAI. The MAS frequency was 40 kHz and the magnetic field was 14.1 T. In these simulations, the C2H2 four-spin model was utilized, and the variable rf field strengths of v1H and v1H/2 were used for R21v and R22v sequences, respectively. It is clear that broadband polarization transfers can be easily attained by a series of CORD sequences, even with mismatched rf fields, in contrast with the PARISxy sequence. Notably, SHANGHAI sequence is more broadbanded than PARISxy, but the transfer efficiency is lower than that by the CORD sequences. The super phase-cycling of the CORD sequences improves the 13C-13C polarization transfer efficiency greatly, following the order: CORDxy8 > CORDxy4 > CORDxix > CORD. Interestingly, for CORDxy8, the positions of the recoupling bands are only very weakly (if at all) dependent on the rf field strength, and the polarization transfer efficiency should thus be largely insensitive to the rf-inhomogeneity. Another relevant observation emerging from the simulations is that due to the numerous resonance conditions, broadband recoupling can even be achieved in spin systems with low amount of protons, such as the C2H spin system (Figure S2), especially for CORDxy4 and CORDxy8 schemes. This property may be advantageous when using partly deuterated samples, and experiments are necessary to verify this prediction made on the basis of computational results. It should be noted that the standard rf field settings imposed by the R2nv symmetries were used for all the following SS-NMR experiments and simulations in the present report, while further explorations of other recoupling conditions will be the subject of future studies.
Figure 4.
Simulated dependence of the 13C-13C polarization transfer efficiency as a function of the rf field strength, v1H, and the resonant frequency difference, Δviso, for various composite rf pulse irradiations: (a) CORD, (b) CORDxix, (c) CORDxy4, (d) CORDxy8, (e) PARISxy and (f) SHANGHAI. A C2H2 spin model was used for 13C-13C spin diffusion simulations at MAS frequency of νr = 40 kHz and magnetic field of 14.1 T, with a mixing time of τmix = 150 ms.
Another important consideration is the performance of these various methods at the MAS frequencies higher than 40 kHz. As discussed above, CORD sequences perform very well at the spinning frequencies of 40 kHz, but it is more challenging to attain broadband recoupling at frequencies of 60 kHz and higher (the regime where 1H detection becomes useful). As demonstrated in Figure S3 of the Supporting Information, the super phase-cycled CORDxy4 and CORDxy8 sequences exhibit truly broadband polarization transfer at the MAS frequency of 60 kHz, where both PARISxy and SHANGHAI sequences cannot work well. This finding indicates that the amplitude and phase modulation in phase-cycled CORD sequences permit to fulfill the broadened second-order rotary resonance conditions, opening venues for 13C-13C spin-diffusion-based correlation spectroscopy in the “ultrafast” MAS regime.
Experimental Results
Guided by the above numerical simulations, we have hence first performed a series of 2D 13C-13C spin diffusion experiments on U-13C,15N-alanine at 14.1 T. The sample was spun at the MAS frequency of 40 kHz, and the various composite pulse irradiation sequences were applied during the mixing period. The results are summarized in Figure 5. An example of 2D 13C-13C CORD correlation spectrum acquired with the mixing time of τmix = 150 ms is given in Figure 5a. It can be seen that, even with the basic CORD sequence, all the expected correlations are present in the spectrum. Figure 5b shows the comparison of the three 1D traces extracted along the F1 dimension from the corresponding 2D correlation spectra obtained with various recoupling sequences, plotted on the same scale. PARISxy sequence exhibits the best transfer efficiency for the Cα ↔ Cβ correlations, but very weak polarization transfer for the Cα/CO and Cβ/CO regions characterized by large chemical shift differences. Notably, the polarization transfers by both CORD series and the SHANGHAI sequences are broadband, even though the Cα ↔ Cβ transfer rates are slower than that attained by PARISxy, which is consistent with the simulations shown in Figure 3. Moreover, the 13C-13C polarization transfer efficiencies by CORD sequences are similar to or higher than that by SHANGHAI in both aliphatic-to-aliphatic and aliphatic-to-carbonyl regions. All the CORD sequences display broadband recoupling with similar polarization transfer behaviors, and super phasecycled CORD sequences are only slightly better, which might be due to the abundance of protons (NH/NC > 1.5).
Figure 5.
(a) 2D 13C-13C CORD correlation spectra recorded on U-13C,15N-alanine spun at 14.1 T, with the MAS frequency of νr = 40 kHz, the mixing period of τmix = 150 ms and the rf fields of ν1H = 20 and 40 kHz. The first contour is set at 10×σ (σ is the noise rmsd) with the multiplier of 1.2. In (b), 1D traces extracted along the F1 dimension of the 2D 13C-13C correlation spectra are shown, together with 1D traces extracted from homonuclear correlation spectra recorded by other composite pulse sequences: CORD (red), CORDxix (blue), CORDxy4 (black), PARISxy (green) and SHANGHAI (brown)
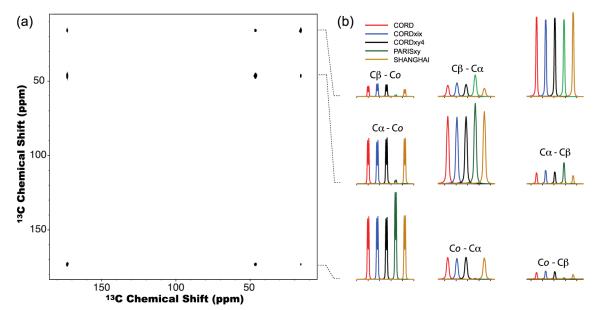
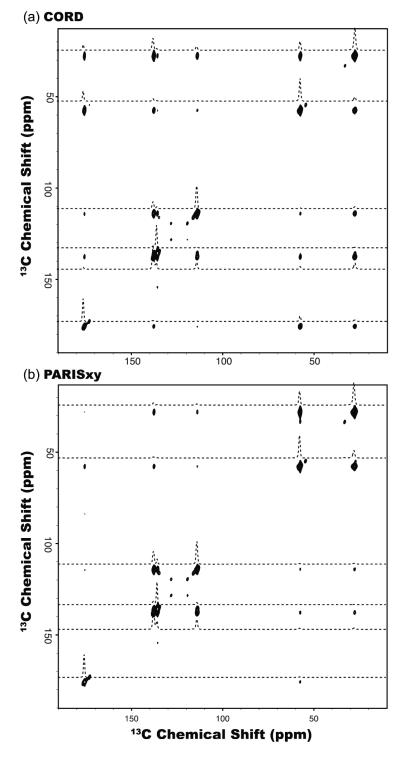
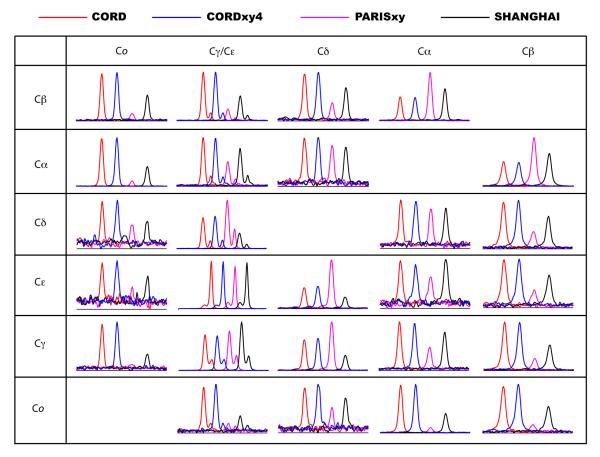
We have also examined the performance of the CORD series of sequences on His sample spun at 40 kHz. Figure 6 shows the comparison of 2D 13C-13C correlation spectra recorded at 14.1 T by CORD (a) and PARISxy (b) sequences, with a mixing time of τmix = 150 ms. All 13C-13C cross peaks for carbons separated by one to five bonds can be observed within 2D CORD spectrum. With the PARISxy rf field irradiation, the polarization transfer among 13C spin pairs is more sensitive to the frequency difference, Δviso, which results in missing cross peaks. Detailed comparisons of all cross peaks recorded by the various composite pulse sequences, including CORD, CORDxy4, PARISxy and SHANGHAI, are presented in Figure 7. The results clearly show that CORD and CORDxy4 sequences give the highest transfer efficiencies for most of the 13C frequency regions, indicating that they are suitable for broadband homonuclear recoupling at MAS frequency of 40 kHz. PARISxy sequence shows the highest transfer efficiencies for 13C spin pairs with small Δviso, but for spin pairs characterized by larger Δviso, the performance is much worse than for the other three sequences. Compared to the original PARISxy scheme, SHANGHAI sequence can drive 13C-13C polarization transfer across a much broader frequency range. However, its transfer efficiency is lower than for both CORD and CORD derivative sequences throughout the entire carbon region except for Cα-to-Cβ correlations (see Figure S4 for the traces extracted along the F1 indirect dimension of the 2D 13C-13C correlation spectra). The results on the two model compounds indicate that phase- and amplitude-modulated CORD pulse schemes are advantageous for broadband 13C-13C spin diffusion experiments at MAS frequencies of 40 kHz and potentially higher.
Figure 6.
2D 13C-13C correlation NMR spectra of U-13C,15N-enriched histidine spun at the MAS frequency of νr = 40 kHz recorded at 14.1 T with CORD (a) and PARISxy (b) sequences, with the mixing time was τmix = 150 ms. The 1D traces extracted along the F1 dimension are shown to illustrate the polarization transfer efficiency. The first contour in all spectra is set at 10×σ with the multiplier of 1.2.
Figure 7.
1D traces along F1 showing 13C-13C cross peaks extracted from 2D spin diffusion correlation NMR spectra of U-13C,15N-enriched histidine recorded at 14.1 T with various composite pulse sequences: CORD (red), CORDxy4 (blue), PARISxy (purple), and SHANGHAI (black). The spinning frequency of νr = 40 kHz and the mixing time of τmix = 150 ms were used for all spin diffusion experiments. The diagonal auto-correlation peaks are not shown.
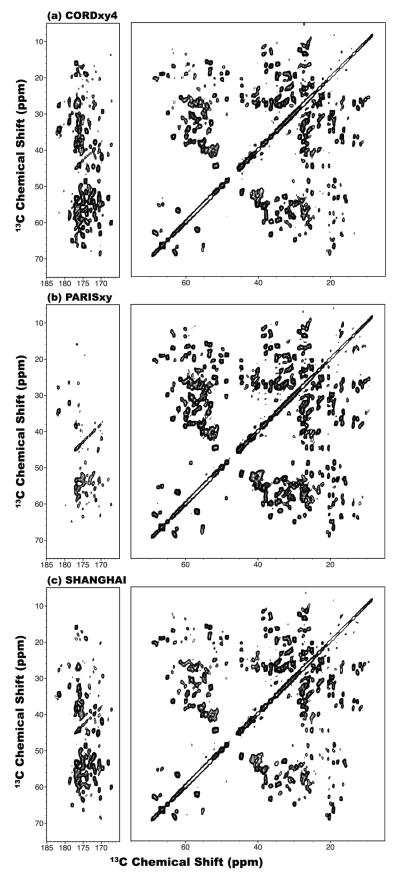
We have also examined the performances of super phase-cycled CORD sequences for obtaining broadband 13C-13C correlation spectra in proteins, using U-13C,15N-LC8 protein. Figure 8 displays the 2D 13C-13C correlation spectra of LC8 acquired with CORDxy4, PARISxy and SHANGHAI sequences at 14.1 T. The sample was spun at the MAS frequency of 40 kHz; and the mixing time of 150 ms was used for all NMR spectra. As demonstrated above on model compounds, PARISxy sequence shows low polarization transfer efficiency for carbon pairs with large Δviso values, and therefore the aliphatic-to-carbonyl and aliphatic-to-aromatic correlations are missing in the spectrum, as illustrated in Figures 8b and S5. With the SHANGHAI sequence, broadband 13C-13C transfer can be achieved, but the transfer efficiency is lower than that attained by CORDxy4 for most 13C spin pairs. As can be appreciated, CORDxy4 sequence exhibits uniformly broadband transfers giving rise to 13C-13C correlations through the entire carbon frequency range, including the aliphatic-to-aromatic cross peaks (Figure S5) missing in PARISxy and the aliphatic-to-aliphatic cross peaks (albeit the transfer efficiency is slightly lower compared to PARISxy).
Figure 8.
2D 13C-13C correlation NMR spectra of U-13C,15N-LC8 protein spun at the MAS frequency of νr = 40 kHz recorded at 14.1 T with CORDxy4 (a), PARISxy (b) and SHANGHAI (c) sequences. The mixing time was τmix = 150 ms. The first contour in all spectra is set at 5×σ with the multiplier of 1.2.
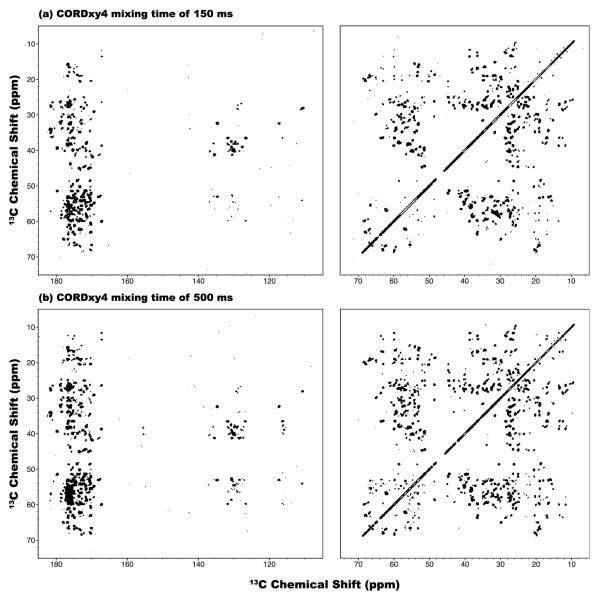
To compare the performance of CORD with that of other commonly used homonuclear recoupling sequences, we have collected a series of 2D 13C-13C CORDxy4, DREAM and fpRFDR spectra with various mixing times, at 19.97 T and the MAS frequency of 40 kHz. The results are summarized in Figures 9, 10 and 11. Consistent with our results at 14.1 T, with the CORD sequences, 13C-13C polarization transfers can be easily attained across the entire spectrum including the aromatic region with a mixing time of 150 ms (Figure 9a). Importantly, at a longer mixing time of 500 ms, numerous multiple-bond correlations can be observed, as shown in Figure 9b. This observation indicates that CORD sequences are well suited for recoding medium- and long-range constraints in the regime of long mixing times.
Figure 9.
2D CORDxy4 13C-13C correlation NMR spectra of U-13C,15N-LC8 protein spun at the MAS frequency of νr = 40 kHz and the magnetic field of 19.97 T, recorded with the mixing times of (a) 150 and (b) 500 ms. The first contour in all spectra is set at 5×σ with the multiplier of 1.2.
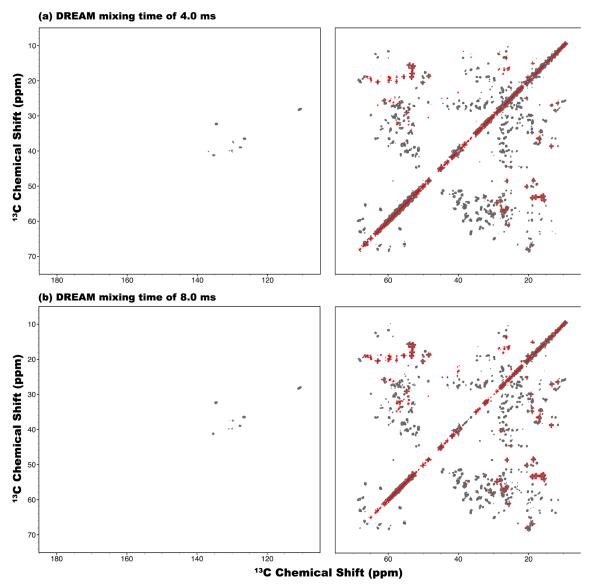
Figure 10.
2D DREAM 13C-13C correlation NMR spectra of U-13C,15N-LC8 protein spun at the MAS frequency of νr = 40 kHz and the magnetic field of 19.97 T, recorded with the mixing times of (a) 4 and (b) 8 ms. High power 1H decoupling with the rf field strength of 180 kHz was applied during DREAM mixing period. The first contour in all spectra is set at 5×σ with the multiplier of 1.2.
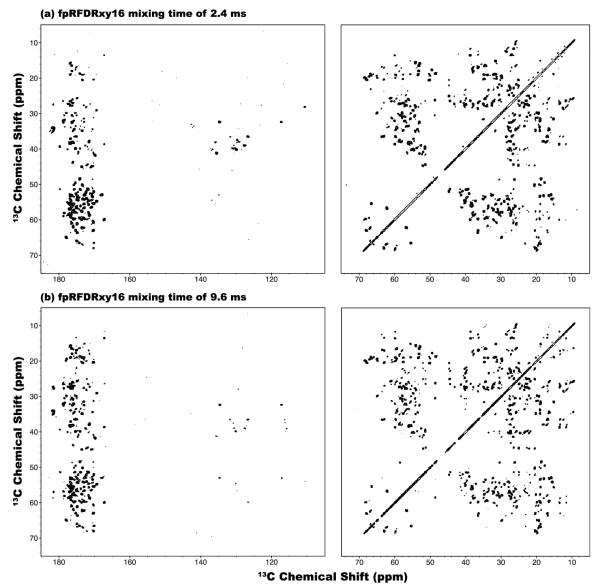
Figure 11.
2D fpRFDRxy16 13C-13C correlation NMR spectra of U-13C,15N-LC8 protein spun at the MAS frequency of νr = 40 kHz and the magnetic field of 19.97 T, recorded with the mixing times of (a) 2.4 and (b) 9.6 ms. No 1H decoupling was applied during the RFDR mixing period. The first contour in all spectra is set at 5×σ with the multiplier of 1.2.
As shown in Figure 10, even though 13C-13C correlations in aliphatic-to-aliphatic region can be readily obtained with the DREAM sequence, the correlations in aromatic and carbonyl regions are largely missing, which is consistent with the simulations shown in Figure 3. Furthermore, there are no new cross peaks in the DREAM spectrum acquired with long mixing time of 8 ms due to the effect of the dipolar truncation. In this 8-ms DREAM spectrum, some cross peaks actually disappear (e.g., the 35-to-18 ppm region) because of the fast signal decay due to the sensitivity to various relaxation times.
As illustrated in Figure 11, with fpRFDR sequence with xy-16 phase steps,[37, 69] broadband 13C-13C correlation spectrum can be obtained with a mixing time of 2.4 ms, although many fewer cross peaks are seen than in the CORD spectrum. Similar to the results observed in the DREAM experiment, when the fpRFDR mixing time is increased to 9.6 ms, very few new peaks appear in the fpRFDR spectrum and instead some cross peaks disappear. This may be due to the compounded effects of (i) dipolar truncation, (ii) signal decay due to relaxation, and (iii) insufficient super-cycling.[70]
Taken together, the numerical simulations and the experimental results on two model systems and a protein discussed in this report indicate that CORD series of sequences are advantageous for spin diffusion experiments at MAS frequencies of 40 kHz and above, compared with the previously developed methods. The uniform broadband and efficient polarization transfers across the entire frequency range, as well as lack of sensitivity to dipolar truncation, makes CORD series of sequences well suited for various applications in 13C-13C correlation spectroscopy in biological systems, including resonance assignments and measurement of distance restraints for protein structure calculation.
CONCLUSIONS
In summary, a series of combined R2nv-symmetry (CORD) sequences have been presented for obtaining broadbanded 13C-13C homonuclear correlation spectra under MAS frequencies of 40 kHz and above. Since the broadened second-order rotational resonance conditions are determined by the rf field strength, the phase shifts and the size of reintroduced dipolar couplings, the individual R2nv sequences display different polarization transfer behaviors and different recoupling band-widths. On the contrary, the combination of rf fields and phase shifts in the super phase-cycled composite CORD pulse schemes results in efficient broadening of the second-order rotary resonance conditions, and produces truly broadbanded 13C-13C spin diffusion. Compared to the original PARISxy and SHANGHAI sequences, the super phase-cycled CORD schemes display higher transfer efficiency throughout the entire 13C spectrum. That CORD sequences are largely insensitive to dipolar truncation makes them appropriate for recording long-range distance constraints in uniformly labeled systems. The CORD series of sequences are therefore well suited for broadbanded one- and multi-bond homonuclear correlation spectroscopy under a wide range of MAS frequencies up to 40 kHz and above, even at moderate or low magnetic fields, and the CORDxy4 and CORDxy8 sequences with super phase cycling are expected to work best for the uniform broadband correlation spectroscopy.
Supplementary Material
HIGHLIGHTS.
We present COmbined R2nv-Driven (CORD) sequences for spin diffusion at fast MAS (νr ≥ 40 kHz).
We show that CORD sequences exhibit broadband homonuclear dipolar recoupling.
CORD sequences display uniform cross peak intensities across the entire spectrum.
CORD sequences are advantageous for homonuclear spectroscopy in biological solids.
ACKNOWLEGEMENTS
This work was supported by the National Institutes of Health (NIH Grants R01GM085396 and 8P30GM103519-03 from NIGMS). We thank Bingwen Hu (East China Normal University) for the helpful discussions on numerical simulations. J. Trébosc, and J.P. Amoureux are grateful for funding provided by Region Nord/Pas de Calais, Europe (FEDER), CNRS, French Minister of Science, USTL, ENSCL, CortecNet, Bruker BIOSPIN, and contract ANR-2010-jcjc-0811-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. A structural model for Alzheimer’s beta-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. USA. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ma C, Marassi FM, Jones DH, Straus SK, Bour S, Strebel K, Schubert U, Oblatt-Montal M, Montal M, Opella SJ. Expression, purification, and activities of full-length and truncated versions of the integral membrane protein Vpu from HIV-1. Protein Sci. 2002;11:546–557. doi: 10.1110/ps.37302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nishimura K, Kim S, Zhang L, Cross TA. The closed state of a H+ channel helical bundle combining precise orientational and distance restraints from solid state NMR. Biochemistry. 2002;41:13170–13177. doi: 10.1021/bi0262799. [DOI] [PubMed] [Google Scholar]
- [4].Tycko R. Applications of solid state NMR to the structural characterization of amyloid fibrils: methods and results. Prog. Nucl. Mag. Reson. Spectr. 2003;42:53–68. [Google Scholar]
- [5].Franks WT, Wylie BJ, Stellfox SA, Rienstra CM. Backbone conformational constraints in a microcrystalline U-15N-labeled protein by 3D dipolar-shift solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006;128:3154–3155. doi: 10.1021/ja058292x. [DOI] [PubMed] [Google Scholar]
- [6].Lorieau JL, Day LA, McDermott AE. Conformational dynamics of an intact virus: order parameters for the coat protein of Pf1 bacteriophage. Proc. Natl. Acad. Sci. USA. 2008;105:10366–10371. doi: 10.1073/pnas.0800405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu J, Durr UH, Im SC, Gan Z, Waskell L, Ramamoorthy A. Bicelle-enabled structural studies on a membrane-associated cytochrome B5 by solid-state MAS NMR spectroscopy. Angew. Chem. Int. Ed. 2008;47:7864–7867. doi: 10.1002/anie.200801338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qiang W, Bodner ML, Weliky DP. Solid-state NMR Spectroscopy of human immunodeficiency virus fusion peptides associated with host-cell-like membranes: 2D correlation spectra and distance measurements support a fully extended conformation and models for specific antiparallel strand registries. J. Am. Chem. Soc. 2008;130:5459–5471. doi: 10.1021/ja077302m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Byeon IJL, Hou GJ, Han Y, Suiter CL, Ahn J, Jung J, Byeon CH, Gronenborn AM, Polenova T. Motions on the Millisecond Time Scale and Multiple Conformations of HIV-1 Capsid Protein: Implications for Structural Polymorphism of CA Assemblies. J. Am. Chem. Soc. 2012;134:6455–6466. doi: 10.1021/ja300937v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McDermott AE. Structural and dynamic studies of proteins by solid-state NMR spectroscopy: rapid movement forward. Curr. Opin. Struct. Biol. 2004;14:554–561. doi: 10.1016/j.sbi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [11].Andrew ER, Bradbury A, Eades RG. Removal of Dipolar Broadening of Nuclear Magnetic Resonance Spectra of Solids by Specimen Rotation. Nature. 1959;183:1802–1803. [Google Scholar]
- [12].Lowe IJ. Free Induction Decays of Rotating Solids. Phys. Rev. Lett. 1959;2:285–287. [Google Scholar]
- [13].Samoson A, Tuherm T, Gan Z. High-field high-speed MAS resolution enhancement in 1H NMR spectroscopy of solids. Solid-State Nucl. Magn. Reson. 2001;20:130–136. doi: 10.1006/snmr.2001.0037. [DOI] [PubMed] [Google Scholar]
- [14].Ernst M, Meier MA, Tuherm T, Samoson A, Meier BH. Low-power high-resolution solid-state NMR of peptides and proteins. J. Am. Chem. Soc. 2004;126:4764–4765. doi: 10.1021/ja0494510. [DOI] [PubMed] [Google Scholar]
- [15].Samoson A, Tuherm T, Past J, Reinhold A, Anupold T, Heinmaa N. New horizons for magic-angle spinning NMR. New Techniques in Solid-State NMR. 2005;246:15–31. doi: 10.1007/b98647. [DOI] [PubMed] [Google Scholar]
- [16].Reif B, Jaroniec CP, Rienstra CM, Hohwy M, Griffin RG. 1H-1H MAS correlation spectroscopy and distance measurements in a deuterated peptide. J. Magn. Reson. 2001;151:320–327. doi: 10.1006/jmre.2001.2354. [DOI] [PubMed] [Google Scholar]
- [17].Reif B, Griffin RG. H-1 detected H-1, N-15 correlation spectroscopy in rotating solids. J. Magn. Reson. 2003;160:78–83. doi: 10.1016/s1090-7807(02)00035-6. [DOI] [PubMed] [Google Scholar]
- [18].Zhou D, Rienstra C. Rapid Analysis of Organic Compounds by Proton-Detected Heteronuclear Correlation NMR Spectroscopy with 40[notdef]kHz Magic-Angle Spinning. Angew. Chem. Int. Ed. 2008;47:7328–7331. doi: 10.1002/anie.200802108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ishii Y, Yesinowski JP, Tycko R. Sensitivity enhancement in solid-state C-13 NMR of synthetic polymers and biopolymers by H-1 NMR detection with high-speed magic angle spinning. J. Am. Chem. Soc. 2001;123:2921–2922. doi: 10.1021/ja015505j. [DOI] [PubMed] [Google Scholar]
- [20].Ishii Y, Tycko R. Sensitivity enhancement in solid state N-15 NMR by indirect detection with high-speed magic angle spinning. J. Magn. Reson. 2000;142:199–204. doi: 10.1006/jmre.1999.1976. [DOI] [PubMed] [Google Scholar]
- [21].Zhou DH, Shah G, Mullen C, Sandoz D, Rienstra CM. Proton-detected solid-state NMR spectroscopy of natural-abundance peptide and protein pharmaceuticals. Angew. Chem. Int. Ed. 2009;48:1253–1256. doi: 10.1002/anie.200801029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bennett AE, Ok JH, Griffin RG, Vega S. Chemical-Shift Correlation Spectroscopy in Rotating Solids - Radio Frequency-Driven Dipolar Recoupling and Longitudinal Exchange. J. Chem. Phys. 1992;96:8624–8627. [Google Scholar]
- [23].Sun BQ, Rienstra CM, Costa PR, Williamson JR, Griffin RG. 3D N-15-C-13-C-13 chemical shift correlation spectroscopy in rotating solids. J. Am. Chem. Soc. 1997;119:8540–8546. [Google Scholar]
- [24].Baldus M, Petkova AT, Herzfeld J, Griffin RG. Cross polarization in the tilted frame: assignment and spectral simplification in heteronuclear spin systems. Molecular Physics. 1998;95:1197–1207. [Google Scholar]
- [25].Rienstra CM, Hohwy M, Hong M, Griffin RG. 2D and 3D N-15-C-13-C-13 NMR chemical shift correlation spectroscopy of solids: Assignment of MAS spectra of peptides. J. Am. Chem. Soc. 2000;122:10979–10990. [Google Scholar]
- [26].Baldus M. Correlation experiments for assignment and structure elucidation of immobilized polypeptides under magic angle spinning. Prog. Nucl. Mag. Reson. Spectr. 2002;41:1–47. [Google Scholar]
- [27].Jaroniec CP, Filip C, Griffin RG. 3D TEDOR NMR experiments for the simultaneous measurement of multiple carbon-nitrogen distances in uniformly C-13, N-15-labeled solids. J. Am. Chem. Soc. 2002;124:10728–10742. doi: 10.1021/ja026385y. [DOI] [PubMed] [Google Scholar]
- [28].Igumenova TI, McDermott AE. Homonuclear C-13 J-decoupling in uniformly C-13-enriched solid proteins. J. Magn. Reson. 2005:11–20. doi: 10.1016/j.jmr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [29].Chen L, Kaiser JM, Polenova T, Yang J, Rienstra C, Mueller L. Backbone assignments in solid-state proteins using J-based 3D Heteronuclear correlation spectroscopy. J. Am. Chem. Soc. 2007;129:10650–10651. doi: 10.1021/ja073498e. [DOI] [PubMed] [Google Scholar]
- [30].Tian Y, Chen L, Niks D, Kaiser JM, Lai J, Rienstra C, Dunn MF, Mueller L. J-Based 3D sidechain correlation in solid-state proteins. Phys. Chem. Chem. Phys. 2009;11:7078–7086. doi: 10.1039/b911570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hou G, Paramasivam S, Byeon IJ, Gronenborn AM, Polenova T. Determination of relative tensor orientations by gamma-encoded chemical shift anisotropy/heteronuclear dipolar coupling 3D NMR spectroscopy in biological solids. Phys. Chem. Chem. Phys. 2010;12:14873–14883. doi: 10.1039/c0cp00795a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Verel R, Ernst M, Meier BH. Adiabatic dipolar recoupling in solid-state NMR: The DREAM scheme. J. Magn. Reson. 2001;150:81–99. doi: 10.1006/jmre.2001.2310. [DOI] [PubMed] [Google Scholar]
- [33].Takegoshi K, Nakamura S, Terao T. C-13-H-1 dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 2001;344:631–637. [Google Scholar]
- [34].McDermott A, Polenova T, Bockmann A, Zilm KW, Paulson EK, Martin RW, Montelione GT. Partial NMR assignments for uniformly (13C, 15N)-enriched BPTI in the solid state. J. Biomol. NMR. 2000;16:209–219. doi: 10.1023/a:1008391625633. [DOI] [PubMed] [Google Scholar]
- [35].Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H. Backbone and side-chain C-13 and N-15 signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 tesla. ChemBioChem. 2001;2:272–281. doi: 10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [36].Hou GJ, Byeon IJL, Ahn J, Gronenborn AM, Polenova T. H-1-C-13/H-1-N-15 Heteronuclear Dipolar Recoupling by R-Symmetry Sequences Under Fast Magic Angle Spinning for Dynamics Analysis of Biological and Organic Solids. J. Am. Chem. Soc. 2011;133:18646–18655. doi: 10.1021/ja203771a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ishii Y. C-13-C-13 dipolar recoupling under very fast magic angle spinning in solid-state nuclear magnetic resonance: Applications to distance measurements, spectral assignments, and high-throughput secondary-structure determination. J. Chem. Phys. 2001;114:8473–8483. [Google Scholar]
- [38].Bloembergen N. On the Interaction of Nuclear Spins in a Crystalline Lattice. Physica. 1949;15:386–426. [Google Scholar]
- [39].Meier BH. Polarization transfer and spin diffusion in solid-state NMR. Academic Press; New York: 1994. [Google Scholar]
- [40].Morcombe CR, Gaponenko V, Byrd RA, Zilm KW. Diluting abundant spins by isotope edited radio frequency field assisted diffusion. J. Am. Chem. Soc. 2004;126:7196–7197. doi: 10.1021/ja047919t. [DOI] [PubMed] [Google Scholar]
- [41].Takegoshi K, Nakamura S, Terao T. C-13-H-1 dipolar-driven C-13-C-13 recoupling without C-13 rf irradiation in nuclear magnetic resonance of rotating solids. J. Chem. Phys. 2003;118:2325–2341. [Google Scholar]
- [42].Hong M. Structure, topology, and dynamics of membrane peptides and proteins from solid-state NMR Spectroscopy. J. Phys. Chem. B. 2007;111:10340–10351. doi: 10.1021/jp073652j. [DOI] [PubMed] [Google Scholar]
- [43].Loquet A, Bardiaux B, Gardiennet C, Blanchet C, Baldus M, Nilges M, Malliavin T, Böckmann A. 3D structure determination of the Crh protein from highly ambiguous solid-state NMR restraints. J. Am. Chem. Soc. 2008;130:3579–3589. doi: 10.1021/ja078014t. [DOI] [PubMed] [Google Scholar]
- [44].McDermott A, Polenova T. Solid state NMR: new tools for insight into enzyme function. Curr. Opin. Struct. Biol. 2007;17:617–622. doi: 10.1016/j.sbi.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [45].Yang J, Paramasivam S, Marulanda D, Cataldi M, Tasayco ML, Polenova T. Magic angle spinning NMR spectroscopy of thioredoxin reassemblies. Magn. Reson. Chem. 2007;45(Suppl 1):S73–83. doi: 10.1002/mrc.2092. [DOI] [PubMed] [Google Scholar]
- [46].Han Y, Ahn J, Concel J, Byeon IJ, Gronenborn AM, Yang J, Polenova T. Solid-state NMR studies of HIV-1 capsid protein assemblies. J. Am. Chem. Soc. 2010;132:1976–1987. doi: 10.1021/ja908687k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Marulanda D, Tasayco ML, McDermott A, Cataldi M, Arriaran V, Polenova T. Magic angle spinning solid-state NMR spectroscopy for structural studies of protein interfaces. Resonance assignments of differentially enriched Escherichia coli thioredoxin reassembled by fragment complementation. J. Am. Chem. Soc. 2004;126:16608–16620. doi: 10.1021/ja0464589. [DOI] [PubMed] [Google Scholar]
- [48].Jehle S, Hiller M, Rehbein K, Diehl A, Oschkinat H, Rossum B. Spectral editing: selection of methyl groups in multidimensional solid-state magic-angle spinning NMR. J. Biomol. NMR. 2006;36:169–177. doi: 10.1007/s10858-006-9078-x. [DOI] [PubMed] [Google Scholar]
- [49].Higman V, Flinders J, Hiller M, Jehle S, Markovic S, Fiedler S, Rossum B, Oschkinat H. Assigning large proteins in the solid state: a MAS NMR resonance assignment strategy using selectively and extensively 13C-labelled proteins. J. Biomol. NMR. 2009;44:245–260. doi: 10.1007/s10858-009-9338-7. [DOI] [PubMed] [Google Scholar]
- [50].Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM. Magic-angle spinning solid-state NMR spectroscopy of the beta 1 immunoglobulin binding domain of protein G (GB1): N-15 and C-13 chemical shift assignments and conformational analysis. J. Am. Chem. Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]
- [51].Rienstra CM, Tucker-Kellogg L, Jaroniec CP, Hohwy M, Reif B, McMahon MT, Tidor B, Lozano-Perez T, Griffin RG. De novo determination of peptide structure with solid-state magic-angle spinning NMR spectroscopy. Proc. Natl. Acad. Sci. USA. 2002;99:10260–10265. doi: 10.1073/pnas.152346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Siemer A, Ritter C, Ernst M, Riek R, Meier B. High-Resolution Solid-State NMR Spectroscopy of the Prion Protein HET-s in Its Amyloid Conformation. Angew. Chem. Int. Ed. 2005;44:2441–2444. doi: 10.1002/anie.200462952. [DOI] [PubMed] [Google Scholar]
- [53].Nadaud P, Helmus J, Kall SL, Jaroniec C. Paramagnetic Ions Enable Tuning of Nuclear Relaxation Rates and Provide Long-Range Structural Restraints in Solid-State NMR of Proteins. J. Am. Chem. Soc. 2009;131:8108–8120. doi: 10.1021/ja900224z. [DOI] [PubMed] [Google Scholar]
- [54].Weingarth M, Bodenhausen G, Tekely P. Broadband Carbon-13 Correlation Spectra of Microcrystalline Proteins in Very High Magnetic Fields. J. Am. Chem. Soc. 2009;131:13937–13938. doi: 10.1021/ja9036143. [DOI] [PubMed] [Google Scholar]
- [55].Xu J, Struppe J, Ramamoorthy A. Two-dimensional homonuclear chemical shift correlation established by the cross-relaxation driven spin diffusion in solids. J. Chem. Phys. 2008;128:052308. doi: 10.1063/1.2826323. [DOI] [PubMed] [Google Scholar]
- [56].Hou GJ, Deng F, Ye CH, Ding SW. Towards uniform enhancement in solid-state cross polarization magnetic angle spinning NMR: A scheme incorporating cross polarization with rotational resonance. J. Chem. Phys. 2006;124:234512. doi: 10.1063/1.2206787. [DOI] [PubMed] [Google Scholar]
- [57].Hou G, Ding S, Zhang L, Deng F. Breaking the T1 constraint for quantitative measurement in magic angle spinning solid-state NMR spectroscopy. J. Am. Chem. Soc. 2010;132:5538–5539. doi: 10.1021/ja909550f. [DOI] [PubMed] [Google Scholar]
- [58].Takeda K, Noda Y, Takegoshi K, Lafon O, Trebosc J, Amoureux JP. Quantitative cross-polarization at magic-angle spinning frequency of about 20 kHz. J. Magn. Reson. 2012;214:340–345. doi: 10.1016/j.jmr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- [59].Scholz I, Huber M, Manolikas T, Meier B, Ernst M. MIRROR recoupling and its application to spin diffusion under fast magic-angle spinning. Chem. Phys. Lett. 2008;460:278–283. [Google Scholar]
- [60].Weingarth M, Demco DE, Bodenhausen G, Tekely P. Improved magnetization transfer in solid-state NMR with fast magic angle spinning. Chem. Phys. Lett. 2009;469:342–348. [Google Scholar]
- [61].Weingarth M, Bodenhausen G, Tekely P. Broadband magnetization transfer using moderate radio-frequency fields for NMR with very high static fields and spinning speeds. Chem. Phys. Lett. 2010;488:10–16. [Google Scholar]
- [62].Hou G, Yan S, Sun SJ, Han Y, Byeon IJ, Ahn J, Concel J, Samoson A, Gronenborn AM, Polenova T. Spin diffusion drive by R-symmetry sequencs: applications to homonuclear correlation spectroscopy in MAS NMR of biological and organic solids. J. Am. Chem. Soc. 2011;133:3943–3953. doi: 10.1021/ja108650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Weingarth M, Masuda Y, Takegoshi K, Bodenhausen G, Tekely P. Sensitive C-13-C-13 correlation spectra of amyloid fibrils at very high spinning frequencies and magnetic fields. J. Biomol. NMR. 2011;50:129–136. doi: 10.1007/s10858-011-9501-9. [DOI] [PubMed] [Google Scholar]
- [64].Hu BW, Lafon O, Trebosc J, Chen Q, Amoureux JP. Broadband homonuclear correlations assisted by H-1 irradiation for bio-molecules in very high magnetic field at fast and ultra-fast MAS frequencies. J. Magn. Reson. 2011;212:320–329. doi: 10.1016/j.jmr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- [65].Sun S, Butterworth AH, Paramasivam S, Yan S, Lightcap CM, Williams JC, Polenova T. Resonance assignments and secondary structure analysis of dynein light chain 8 by magic-angle spinning NMR spectroscopy. Canadian Journal of Chemistry. 2011;89:909–918. doi: 10.1139/v11-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- [67].Bak M, Rasmussen JT, Nielsen NC. SIMPSON: A general simulation program for solid-state NMR spectroscopy. J. Magn. Reson. 2000;147:296–330. doi: 10.1006/jmre.2000.2179. [DOI] [PubMed] [Google Scholar]
- [68].Veshtort M, Griffin RG. SPINEVOLUTION: A powerful tool for the simulation of solid and liquid state NMR experiments. J. Magn. Reson. 2006;178:248–282. doi: 10.1016/j.jmr.2005.07.018. [DOI] [PubMed] [Google Scholar]
- [69].Gullion T, Baker DB, Conradi MS. New, Compensated Carr-Purcell Sequences. J. Magn. Reson. 1990;89:479–484. [Google Scholar]
- [70].Shen M, Hu BW, Lafon O, Trebosc J, Chen Q, Amoureux JP. Broadband finite-pulse radio-frequency-driven recoupling (fp-RFDR) with (XY8)4(1) super-cycling for homonuclear correlations in very high magnetic fields at fast and ultra-fast MAS frequencies. J. Magn. Reson. 2012;223:107–119. doi: 10.1016/j.jmr.2012.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.