Abstract
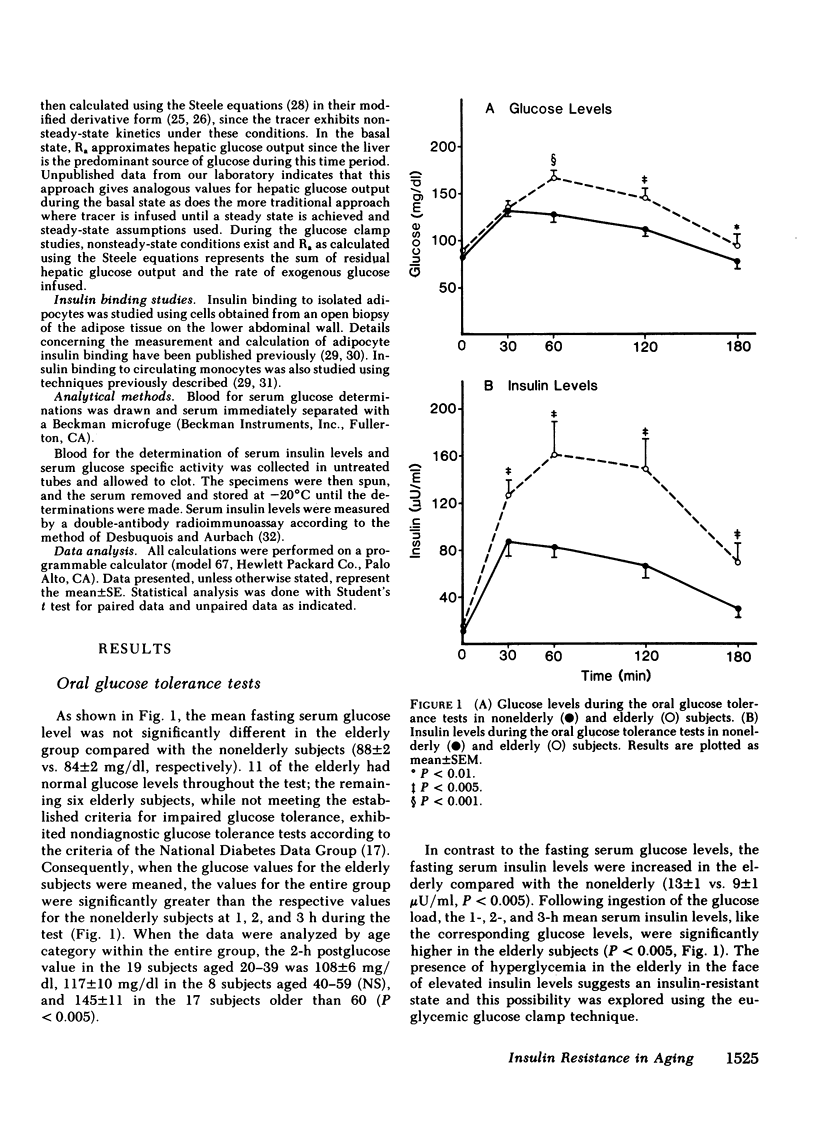
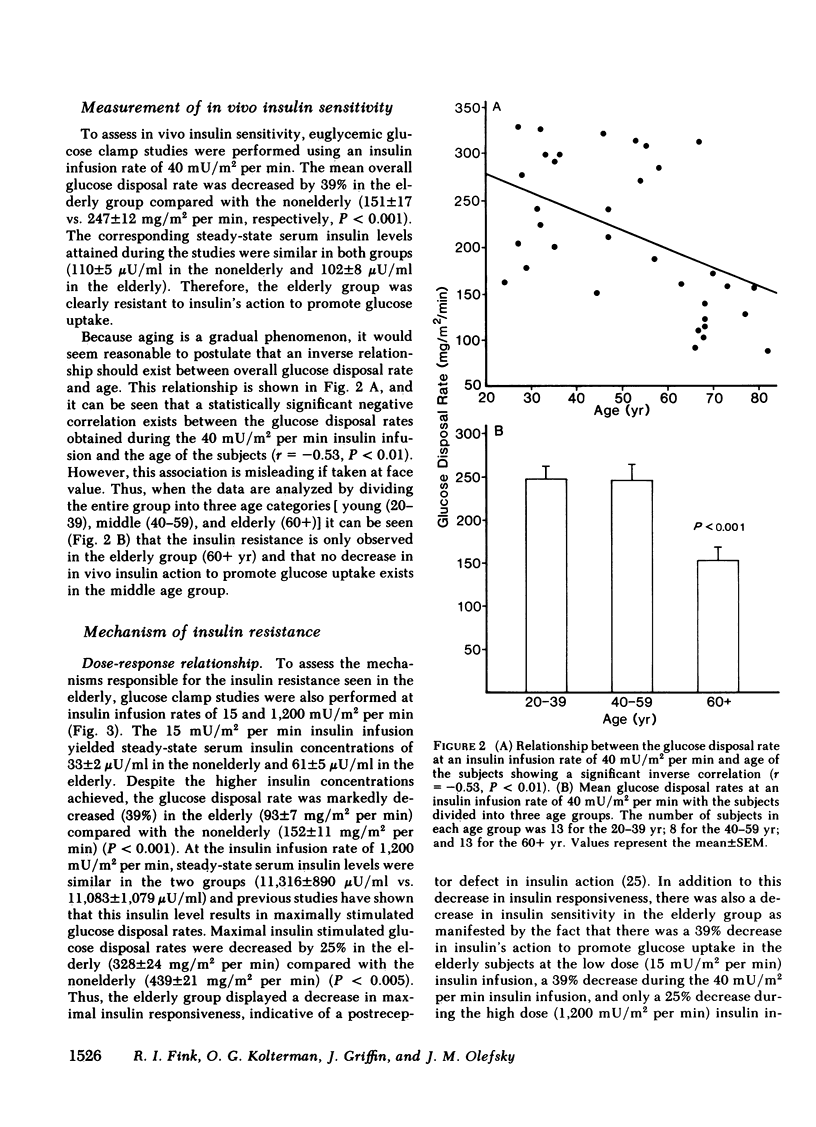
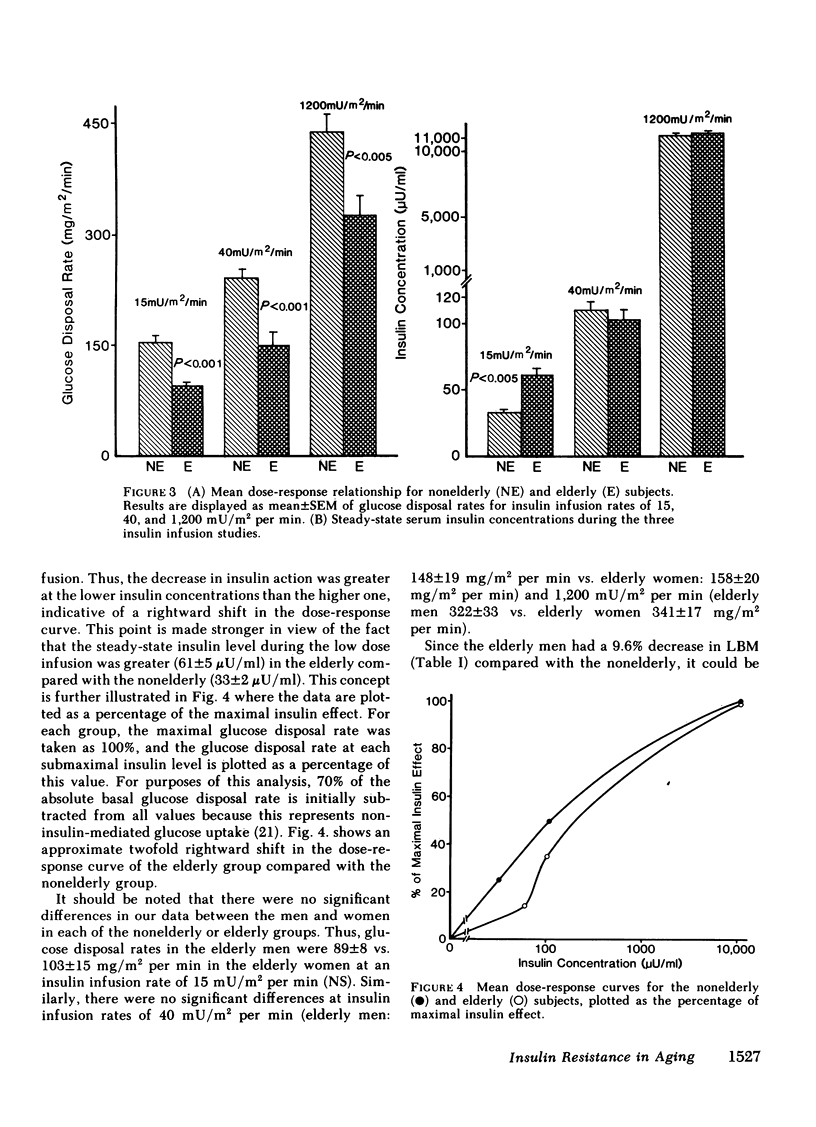
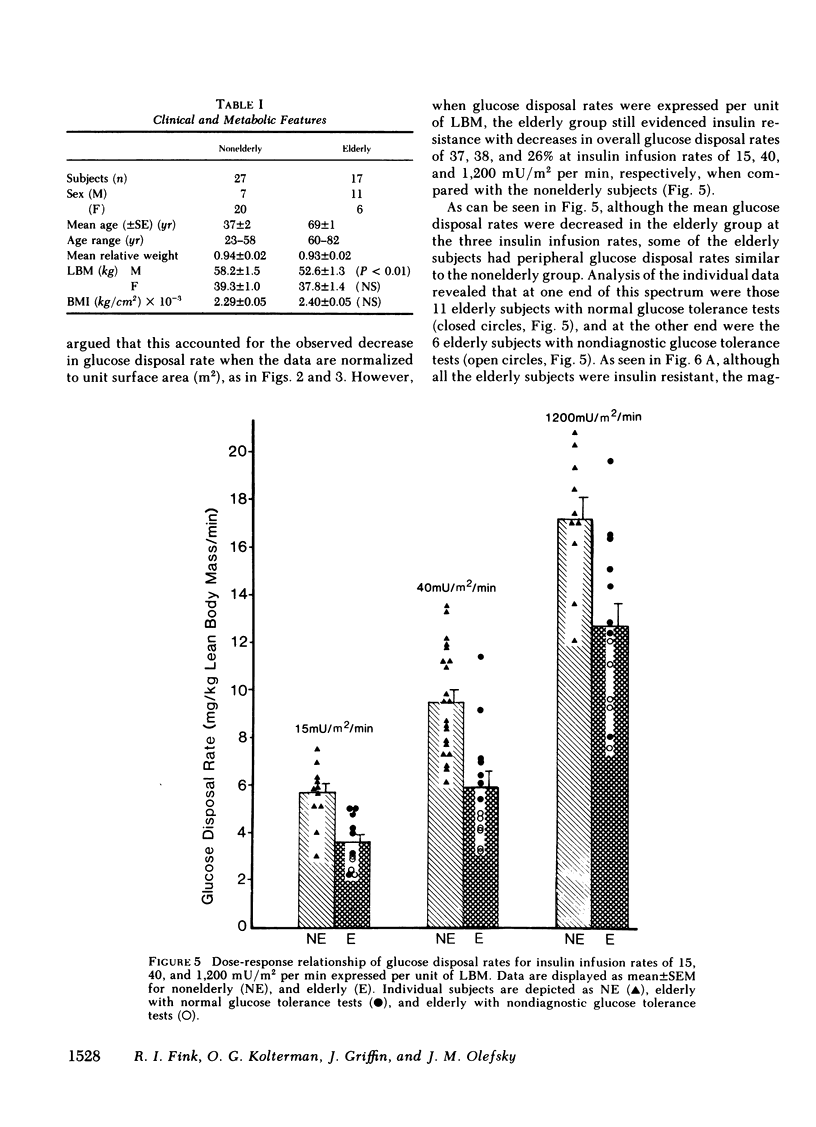
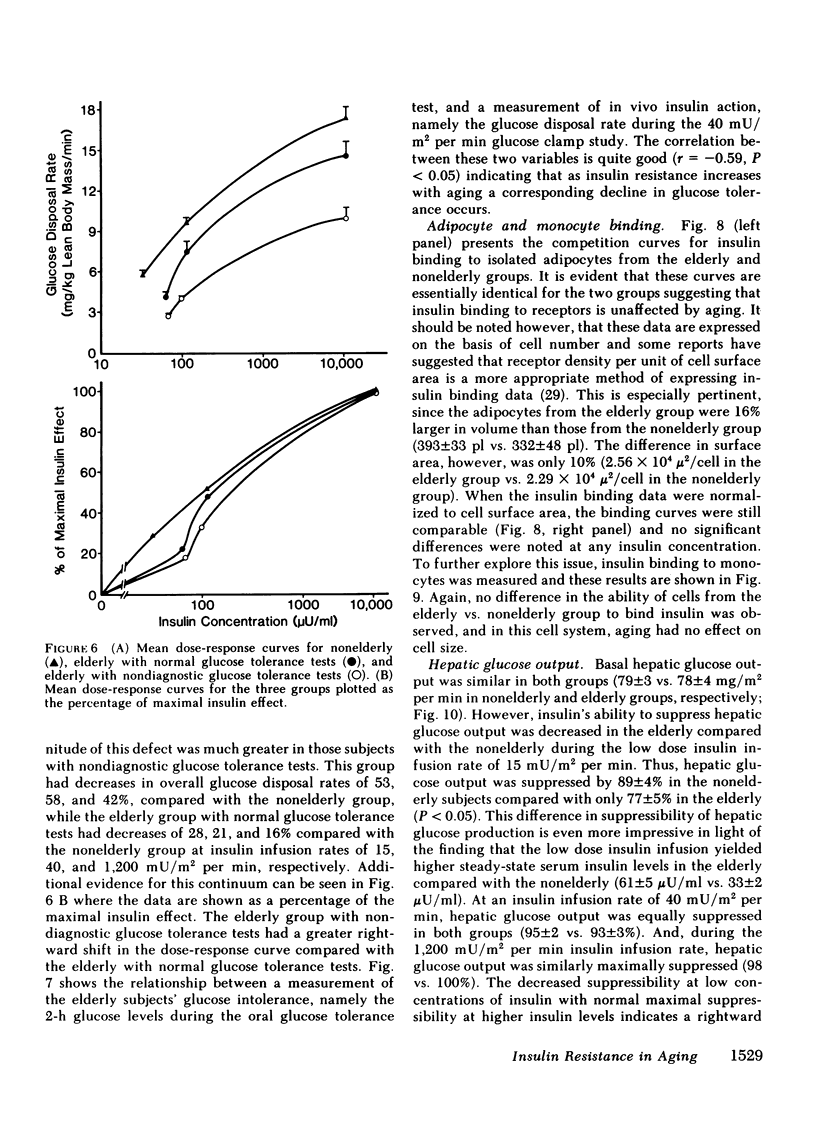
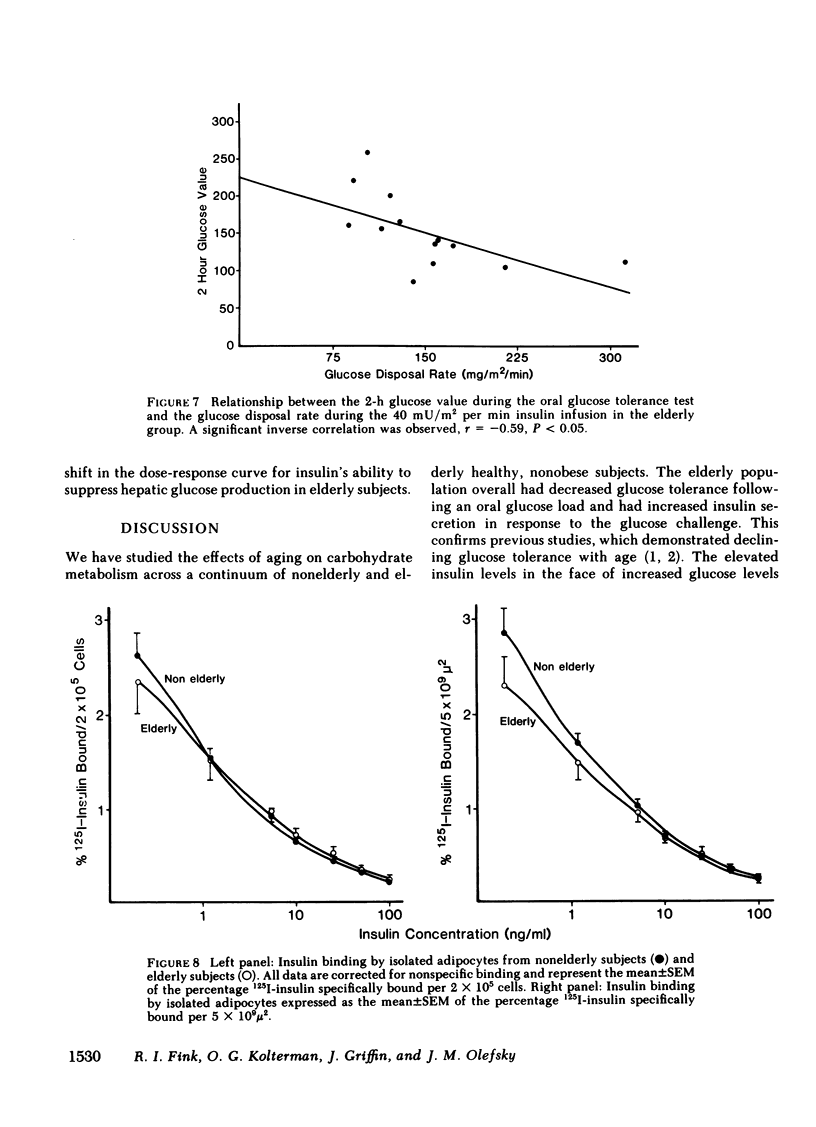
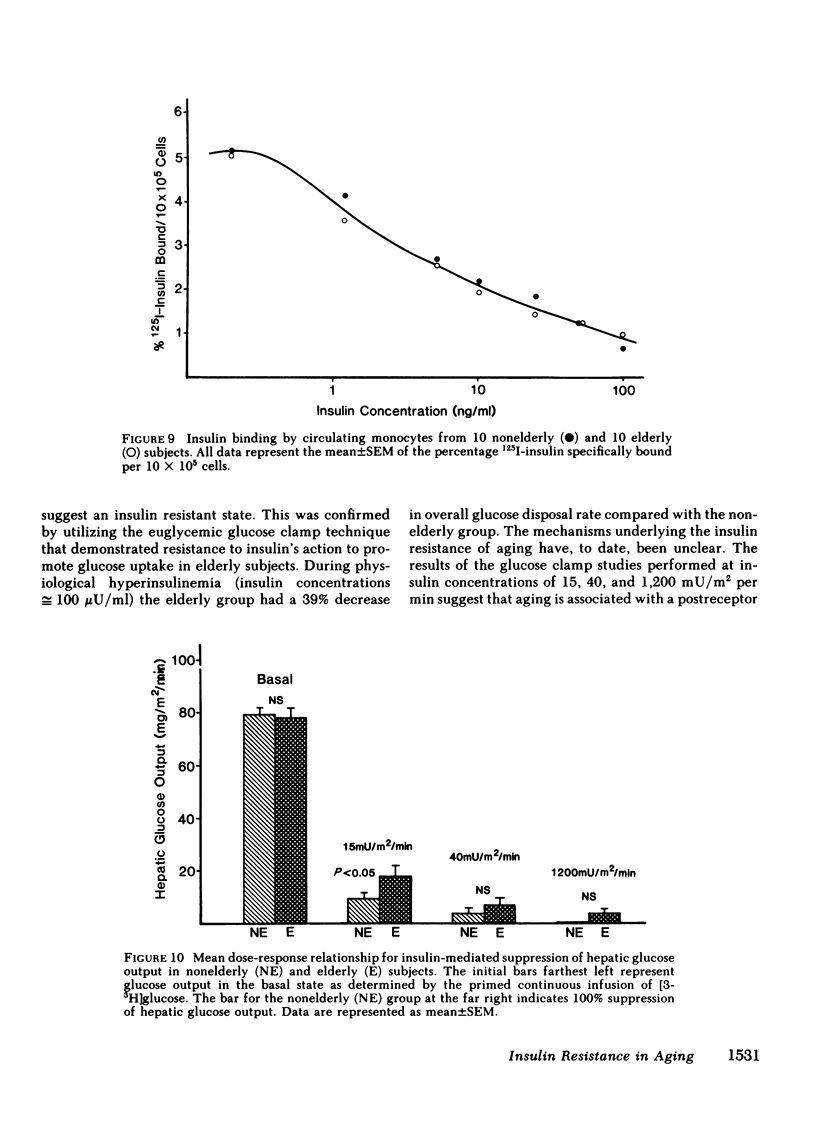
We have studied 17 elderly and 27 non-elderly, nonobese subjects (mean age 69±1 and 37±2 yr, respectively) to assess the mechanisms responsible for the abnormal carbohydrate tolerance associated with aging. Serum glucose and insulin levels were significantly elevated in the elderly subjects compared with the nonelderly subjects during a 75-g oral glucose tolerance test, suggesting an insulin resistant state. Peripheral insulin sensitivity was assessed in both groups using the euglycemic glucose clamp technique during an insulin infusion rate of 40 mU/m2 per min. Similar steady-state serum insulin levels led to a peripheral glucose disposal rate of 151±17 mg/m2 per min in the elderly compared with a value of 247±12 mg/m2 per min in the nonelderly, thus documenting the presence of insulin resistance in the elderly subjects. Insulin binding to isolated adipocytes and monocytes was similar in the elderly and nonelderly groups (2.34±0.33 vs. 2.62±0.24% and 5.04±1.10 vs. 5.12±1.07%), respectively. Thus, insulin resistance in the presence of normal insulin binding suggests the presence of a postreceptor defect in insulin action. This was confirmed by performing additional euglycemic clamp studies using infusion rates of 15 and 1,200 mU/m2 per min to assess the contours of the dose-response relationship. These studies revealed a 39 and 25% decrease in the glucose disposal rate in the elderly subjects, respectively. The results confirm the presence of a postreceptor defect as well as a rightward shift in the dose-response curve. Insulin's ability to suppress hepatic glucose output was less in the elderly subjects during the 15 mU/m2 per min insulin infusion (77±5 vs. 89±4% suppression), but hepatic glucose output was fully and equally suppressed in both groups during the 40 and 1,200 mU/m2 per min infusion. Finally, a significant inverse relationship was observed between the degree of glucose intolerance in the individual elderly subjects, as reflected by the 2-h serum glucose level during the oral glucose tolerance test, and the degree of peripheral insulin resistance as assessed by the glucose disposal rate during the 40 mU/m2 per min insulin infusion (r = 0.59, P < 0.01).
We conclude that carbohydrate intolerance develops as part of the aging process. This carbohydrate intolerance appears to be the consequence of peripheral insulin resistance caused by a postreceptor defect in target tissue insulin action, which causes both a decrease in the maximal rate of peripheral glucose disposal and a rightward shift in the insulin action dose-response curve. In elderly subjects, the severity of the abnormality in carbohydrate tolerance is directly correlated to the degree of peripheral insulin resistance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbagallo-Sangiorgi G., Laudicina E., Bompiani G. D., Durante F. The pancreatic beta-cell response to intravenous administration of glucose in elderly subjects. J Am Geriatr Soc. 1970 Jul;18(7):529–538. doi: 10.1111/j.1532-5415.1970.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Chumlea W. C., Roche A. F., Siervogel R. M., Knittle J. L., Webb P. Adipocytes and adiposity in adults. Am J Clin Nutr. 1981 Sep;34(9):1798–1803. doi: 10.1093/ajcn/34.9.1798. [DOI] [PubMed] [Google Scholar]
- Cunningham J. J. An individualization of dietary requirements for energy in adults. J Am Diet Assoc. 1982 Apr;80(4):335–338. [PubMed] [Google Scholar]
- Davidson M. B. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism. 1979 Jun;28(6):688–705. doi: 10.1016/0026-0495(79)90024-6. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Glucose intolerance and aging. Diabetes Care. 1981 Jul-Aug;4(4):493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe B. I., Vinik A. I., Jackson W. P. Insulin reserve in elderly subjects. Lancet. 1969 Jun 28;1(7609):1292–1293. doi: 10.1016/s0140-6736(69)92226-0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kimmerling G., Javorski W. C., Reaven G. M. Aging and insulin resistance in a group of nonobese male volunteers. J Am Geriatr Soc. 1977 Aug;25(8):349–353. doi: 10.1111/j.1532-5415.1977.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. A., Streeten D. H., Doisy R. J. Effects of oral chromium supplementation on the glucose tolerance of elderly human subjects. Metabolism. 1968 Feb;17(2):114–125. doi: 10.1016/0026-0495(68)90137-6. [DOI] [PubMed] [Google Scholar]
- Malina R. M. Quantification of fat, muscle and bone in man. Clin Orthop Relat Res. 1969 Jul-Aug;65:9–38. [PubMed] [Google Scholar]
- Matter S., Stamford B. A., Weltman A. Age, diet, maximal aerobic capacity and serum lipids. J Gerontol. 1980 Jul;35(4):532–536. doi: 10.1093/geronj/35.4.532. [DOI] [PubMed] [Google Scholar]
- Metz R., Surmaczynska B., Berger S., Sobel G. Glucose tolerance, plasma insulin, and free fatty acids in elderly subjects. Ann Intern Med. 1966 May;64(5):1042–1048. doi: 10.7326/0003-4819-64-5-1042. [DOI] [PubMed] [Google Scholar]
- Nolan S., Stephan T., Chae S., Vidalon C., Gegick C., Khurana R. C., Danowski T. S. Age-related insulin patterns in normal glucose tolerance. J Am Geriatr Soc. 1973 Mar;21(3):106–111. doi: 10.1111/j.1532-5415.1973.tb00854.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J. B., Mahan C. M., Freedlender A. E., Williams R. F. Effect of age on corbohydrate metabolism. J Clin Endocrinol Metab. 1971 Oct;33(4):619–623. doi: 10.1210/jcem-33-4-619. [DOI] [PubMed] [Google Scholar]
- Offenbacher E. G., Pi-Sunyer F. X. Beneficial effect of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects. Diabetes. 1980 Nov;29(11):919–925. doi: 10.2337/diab.29.11.919. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Jen P., Reaven G. M., Alto P. Insulin binding to isolated human adipocytes. Diabetes. 1974 Jul;23(7):565–571. doi: 10.2337/diab.23.7.565. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding to monocytes and total mononuclear leukocytes from normal and diabetic patients. J Clin Endocrinol Metab. 1976 Jul;43(1):226–231. doi: 10.1210/jcem-43-1-226. [DOI] [PubMed] [Google Scholar]
- RAPOPORT M. I., HURD H. F. THIAZIDE-INDUCED GLUCOSE INTOLERANCE TREATED WITH POTASSIUM. Arch Intern Med. 1964 Mar;113:405–408. doi: 10.1001/archinte.1964.00280090091014. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Cummins J. C., Wolfe R. R., Durkot M., Matthews D. E., Zhao X. H., Bier D. M., Young V. R. Quantitative aspects of glucose production and metabolism in healthy elderly subjects. Diabetes. 1982 Mar;31(3):203–211. doi: 10.2337/diab.31.3.203. [DOI] [PubMed] [Google Scholar]
- SCHROEDER H. A., BALASSA J. J., TIPTON I. H. Abnormal trace metals in man--chromium. J Chronic Dis. 1962 Oct;15:941–964. doi: 10.1016/0021-9681(62)90114-5. [DOI] [PubMed] [Google Scholar]
- SILVERSTONE F. A., BRANDFONBRENER M., SHOCK N. W., YIENGST M. J. Age differences in the intravenous glucose tolerance tests and the response to insulin. J Clin Invest. 1957 Mar;36(3):504–514. doi: 10.1172/JCI103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sandberg H., Yoshimine N., Maeda S., Symons D., Zavodnick J. Effects of an oral glucose load on serum immunoreactive insulin, free fatty acid, growth hormone and blood sugar levels in young and elderly subjects. J Am Geriatr Soc. 1973 Oct;21(10):433–439. doi: 10.1111/j.1532-5415.1973.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Schroeder H. A., Nason A. P., Tipton I. H. Chromium deficiency as a factor in atherosclerosis. J Chronic Dis. 1970 Aug;23(2):123–142. doi: 10.1016/0021-9681(70)90071-8. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R., DeFronzo R., Wahren J., Felic P. Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci U S A. 1977 Jan;74(1):348–352. doi: 10.1073/pnas.74.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Hall M. R. Carbohydrate tolerance in the very aged. Diabetologia. 1973 Oct;9(5):387–390. doi: 10.1007/BF01239433. [DOI] [PubMed] [Google Scholar]
- Soman V. R., Koivisto V. A., Deibert D., Felig P., DeFronzo R. A. Increased insulin sensitivity and insulin binding to monocytes after physical training. N Engl J Med. 1979 Nov 29;301(22):1200–1204. doi: 10.1056/NEJM197911293012203. [DOI] [PubMed] [Google Scholar]
- TIPTON I. H., COOK M. J. Trace elements in human tissue. II. Adult subjects from the United States. Health Phys. 1963 Feb;9:103–145. doi: 10.1097/00004032-196302000-00002. [DOI] [PubMed] [Google Scholar]


