Abstract
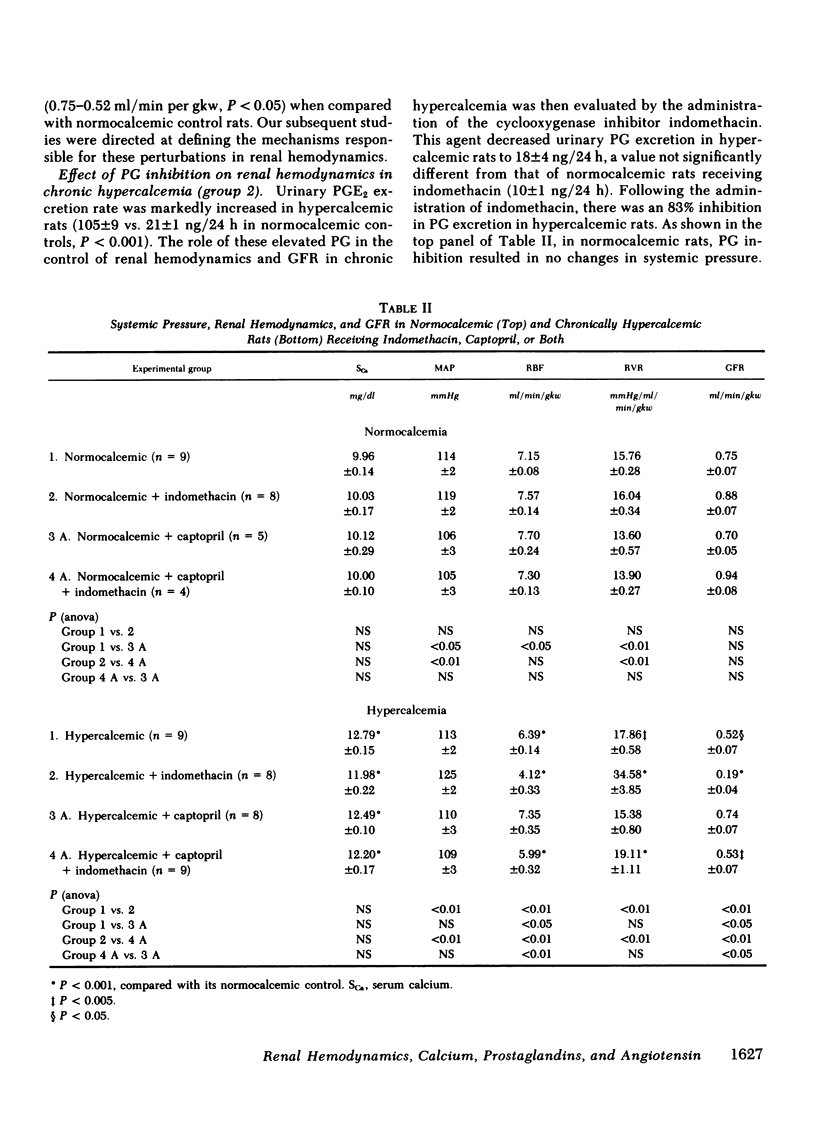
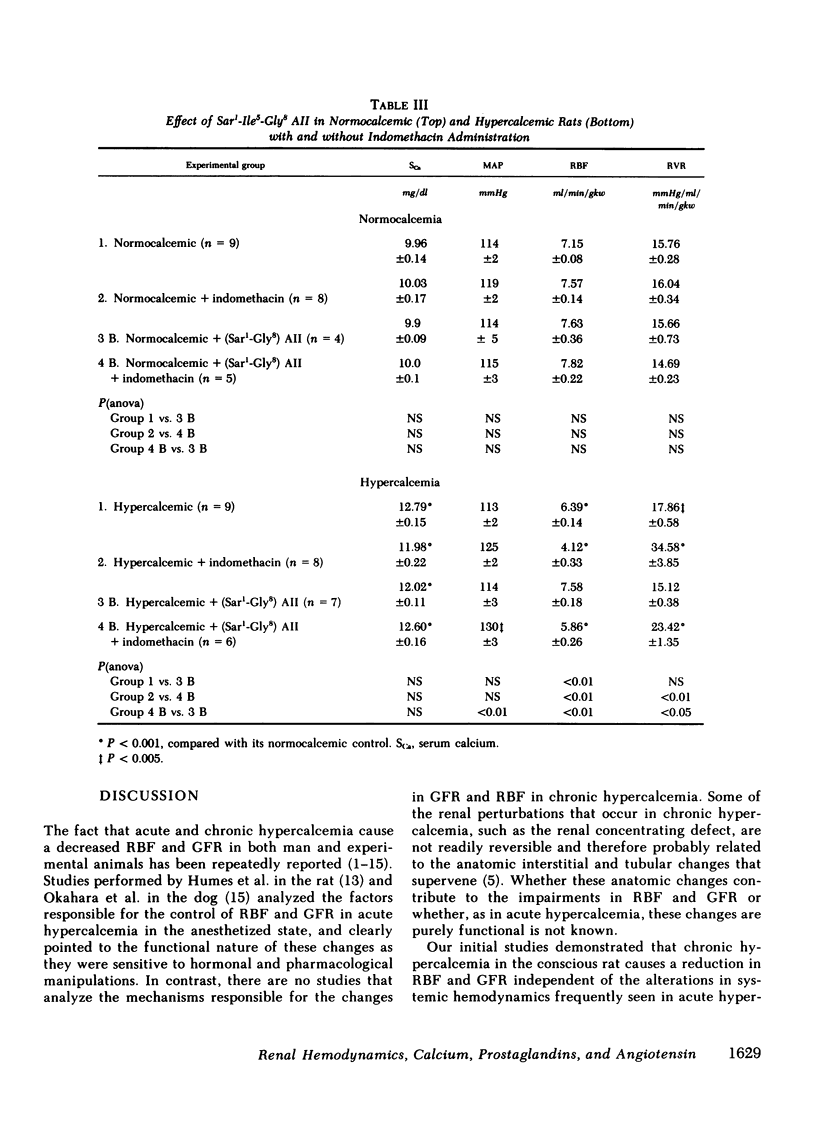
The role of prostaglandins (PG), renin-angiotensin system (RAS) and calcium (Ca) in the control of renal hemodynamics and glomerular filtration rate (GFR) in chronic hypercalcemia (serum Ca 12.8 mg%) was studied. Renal blood flow (RBF, 6.39 ml/min per gram kidney weight [gkw]) and GFR (0.52 ml/min per gkw) were significantly decreased in hypercalcemic rats when compared with normocalcemic rats (7.15, P < 0.001 and 0.74, P < 0.05, respectively). These changes in RBF and GFR occurred independent of any significant alterations in systemic hemodynamics, blood and plasma volume. Inhibition of the renal PG with indomethacin resulted in marked decrements in both RBF (6.39-4.12 ml/min per gkw, P < 0.01) and GFR (0.52-0.19 ml/min per gkw, P < 0.01) in hypercalcemic rats, whereas there was no significant alterations in normocalcemic rats. Inhibition of the RAS with captopril resulted in marked increments in both RBF (6.39-7.35 ml/min per gkw, P < 0.05) and GFR (0.52-0.74 ml/min per gkw, P < 0.05) in hypercalcemic rats. In fact, there was no significant difference from the RBF and GFR of similarly treated normocalcemic rats. Similar results were also obtained with the competitive angiotensin II (AII) antagonist (sarcosyl1-isoleucyl5-glycyl8) AII. Since both the renal PG and the RAS are involved in the control of RBF and GFR in hypercalcemia, the role of each is best revealed in the absence of the other. Hence, comparison of the RBF and GFR in the PG-inhibited hypercalcemic rats in the presence of AII (4.12 and 0.19 ml/min per gkw, respectively) and absence of AII (5.99 and 0.53 ml/min per gkw, P < 0.01 for both) reveals the vasoconstrictive role for AII in hypercalcemia. On the other hand, comparison of the RBF and GFR in the AII-inhibited hypercalcemic rats in the presence of PG (7.35 and 0.74 ml/min per gkw, respectively) and absence of PG (5.99 and 0.53 ml/min per gkw, P < 0.01 and P < 0.05, respectively) reveals the vasodilatory role for PG in hypercalcemia. Finally, comparison of the RBF and GFR in both PG- and AII-inhibited hypercalcemic rats (5.99 and 0.53 ml/min per gkw, respectively) with similarly treated normocalcemic rats (7.30 and 0.94 ml/min per gkw, P < 0.001 and P < 0.005, respectively) reveals the vasoconstrictive role for Ca in chronic hypercalcemia. Our study therefore demonstrates that in chronic hypercalcemia the RBF and GFR are controlled by an active interplay of the vasoconstrictive effect of AII, the vasodilatory effect of renal PG, and the direct vasoconstrictive effect of Ca, independent of either AII or PG. The sum total of these forces produces a modest but significant decrease in RBF and GFR.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. W., Vane J. R. Intrarenal prostaglandin release attenuates the renal vasoconstrictor activity of angiotensin. J Pharmacol Exp Ther. 1973 Mar;184(3):678–687. [PubMed] [Google Scholar]
- Arisz L., Donker A. J., Brentjens J. R., van der Hem G. K. The effect of indomethacin on proteinuria and kidney function in the nephrotic syndrome. Acta Med Scand. 1976;199(1-2):121–125. doi: 10.1111/j.0954-6820.1976.tb06701.x. [DOI] [PubMed] [Google Scholar]
- BANK N., AYNEDJIAN H. S. ON THE MECHANISM OF HYPOSTHENURIA IN HYPERCALCEMIA. J Clin Invest. 1965 Apr;44:681–693. doi: 10.1172/JCI105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis C., Brenner B. M. Modulation by prostaglandin synthesis inhibitors of the action of exogenous angiotensin II on glomerular ultrafiltration in the rat. Circ Res. 1978 Dec;43(6):889–898. doi: 10.1161/01.res.43.6.889. [DOI] [PubMed] [Google Scholar]
- Bennett C. M. Urine concentration and dilution in hypokalemic and hypercalcemic dogs. J Clin Invest. 1970 Jul;49(7):1447–1457. doi: 10.1172/JCI106362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette M. G., Vary J., Carrière S. Hyposthenuria in hypercalcemia. A possible role of intrarenal blood-flow (IRBF) redistribution. Pflugers Arch. 1974;350(1):9–23. doi: 10.1007/BF00586735. [DOI] [PubMed] [Google Scholar]
- CARONE F. A., EPSTEIN F. H., BECK D., LEVITIN The effects upon the kidney of transienthypercalcemia induced by parathyroid extract. Am J Pathol. 1960 Jan;36:77–103. [PMC free article] [PubMed] [Google Scholar]
- COHEN S. I., FITZGERALD M. G., FOURMAN P., GRIFFITHS W. J., DE WARDENER H. E. Polyuria in hyperparathyroidism. Q J Med. 1957 Oct;26(104):423–431. [PubMed] [Google Scholar]
- Chomdej B., Bell P. D., Navar L. G. Renal hemodynamic and autoregulatory responses to acute hypercalcemia. Am J Physiol. 1977 Jun;232(6):F490–F496. doi: 10.1152/ajprenal.1977.232.6.F490. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Studer R. K., Derubertis F. R. Renal inner medullary prostaglandin synthesis. A calcium-calmodulin-dependent process suppressed by urea. J Clin Invest. 1981 Sep;68(3):722–732. doi: 10.1172/JCI110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Radioimmunoassay of prostaglandins Falpha, E1 and E2 in human plasma. Eur J Clin Invest. 1975 Jul 29;5(4):311–318. doi: 10.1111/j.1365-2362.1975.tb00459.x. [DOI] [PubMed] [Google Scholar]
- EDVALL C. A. Renal function in hyperparathyroidism; a clinical study of 30 cases with special reference to selective renal clearance and renal vein catheterization. Acta Chir Scand Suppl. 1958;114(Suppl 229):1–56. [PubMed] [Google Scholar]
- EPSTEIN F. H., BECK D., CARONE F. A., LEVITIN H., MANITIUS A. Changes in renal concentrating ability produced by parathyroid extract. J Clin Invest. 1959 Jul;38(7):1214–1221. doi: 10.1172/JCI103896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards B. R., Sutton R. A., Dirks J. H. Effect of calcium infusion on renal tubular reabsorption in the dog. Am J Physiol. 1974 Jul;227(1):13–18. doi: 10.1152/ajplegacy.1974.227.1.13. [DOI] [PubMed] [Google Scholar]
- FROHLICH E. D., SCOTT J. B., HADDY F. J. Effect of cations on resistance and responsiveness of renal and forelimb vascular beds. Am J Physiol. 1962 Sep;203:583–587. doi: 10.1152/ajplegacy.1962.203.3.583. [DOI] [PubMed] [Google Scholar]
- Flamenbaum W., Kotchen T. A., Nagle R., McNeil J. S. Effect of potassium on the renin-angiotensin system and HgCl 2 -induced acute renal failure. Am J Physiol. 1973 Feb;224(2):305–311. doi: 10.1152/ajplegacy.1973.224.2.305. [DOI] [PubMed] [Google Scholar]
- Foidart J., Sraer J., Delarue F., Mahieu P., Ardaillou R. Evidence for mesangial glomerular receptors for angiotensin II linked to mesangial cell contractility. FEBS Lett. 1980 Dec 1;121(2):333–339. doi: 10.1016/0014-5793(80)80375-9. [DOI] [PubMed] [Google Scholar]
- GILL J. R., Jr, BARTTER F. C. On the impairment of renal concentrating ability in prolonged hypercalcemia and hypercalciuria in man. J Clin Invest. 1961 Apr;40:716–722. doi: 10.1172/JCI104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich W. L., Anderson R. J., Berns A. S., McDonald K. M., Paulsen P. J., Berl T., Schrier R. W. The role of renal nerves and prostaglandins in control of renal hemodynamics and plasma renin activity during hypotensive hemorrhage in the dog. J Clin Invest. 1978 Mar;61(3):744–750. doi: 10.1172/JCI108988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kurtz T. W., Waldinger T. P. Cardiac output and renal blood flow in glycerol-induced acute renal failure in the rat. Circ Res. 1977 Feb;40(2):178–182. doi: 10.1161/01.res.40.2.178. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Ichikawa I., Troy J. L., Brenner B. M. Evidence for a parathyroid hormone-dependent influence of calcium on the glomerular ultrafiltration coefficient. J Clin Invest. 1978 Jan;61(1):32–40. doi: 10.1172/JCI108922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I., Miele J. F., Brenner B. M. Reversal of renal cortical actions of angiotensin II by verapamil and manganese. Kidney Int. 1979 Aug;16(2):137–147. doi: 10.1038/ki.1979.115. [DOI] [PubMed] [Google Scholar]
- Levi M., Peterson L., Berl T. Mechanism of concentrating defect in hypercalcemia. Role of polydipsia and prostaglandins. Kidney Int. 1983 Mar;23(3):489–497. doi: 10.1038/ki.1983.46. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Anderson R. J., Guggenheim S. J., Robertson G. L., Berl T. Role of vasopressin in impaired water excretion in conscious rats with experimental cirrhosis. Kidney Int. 1981 Aug;20(2):173–180. doi: 10.1038/ki.1981.119. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Berl T., Aisenbrey G. A., Better O. S., Anderson R. J. The effect of anesthesia on hemodynamics and renal function in the rat. Pflugers Arch. 1980 Mar;384(2):135–141. doi: 10.1007/BF00584429. [DOI] [PubMed] [Google Scholar]
- Lins L. E. Renal function in hypercalcemia. A clinical and experimental study. Acta Med Scand Suppl. 1979;632:1–46. [PubMed] [Google Scholar]
- Okahara T., Abe Y., Imanishi M., Yukimura T., Yamamoto K. Effect of calcium on prostaglandin E2 release in dogs. Am J Physiol. 1981 Jul;241(1):F77–F84. doi: 10.1152/ajprenal.1981.241.1.F77. [DOI] [PubMed] [Google Scholar]
- Oliver J. A., Sciacca R. R., Pinto J., Cannon P. J. Participation of the prostaglandins in the control of renal blood flow during acute reduction of cardiac output in the dog. J Clin Invest. 1981 Jan;67(1):229–237. doi: 10.1172/JCI110018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULOS P. P. The renal tubular reabsorption and urinary excretion of calcium by the dog. J Lab Clin Med. 1957 Feb;49(2):253–257. [PubMed] [Google Scholar]
- Paiva A. C., Paiva T. B., Miyamoto M. E., Nakaie C. R. The role of calcium in the response of rabbit aorta to angiotensin. Mayo Clin Proc. 1977 Jul;52(7):427–429. [PubMed] [Google Scholar]
- Stockigt J. R., Collins R. D., Biglieri E. G. Determination of plasma renin concentration by angiotensin I immunoassay. Diagnotic import of precise measurement of subnormal renin in hyperaldosteronism. Circ Res. 1971 May;28(5 Suppl):175–191. doi: 10.1161/01.res.28.5.ii-175. [DOI] [PubMed] [Google Scholar]
- Thiel G., Wilson D. R., Arce M. L., Oken D. E. Glycerol induced hemoglobinuric acute renal failure in the rat. II. The experimental model, predisposing factors, and pathophysiologic features. Nephron. 1967;4(5):276–297. doi: 10.1159/000179588. [DOI] [PubMed] [Google Scholar]
- Vanherweghem J. L., Ducobu J., d'Hollander A., Toussaint C. Effects of hypercalcemia on water and sodium excretion by the isolated dog kidney. Pflugers Arch. 1976 May 6;363(1):75–80. doi: 10.1007/BF00587405. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Davis B. B. Effects of calcium on prostaglandin E2 synthesis by rat inner medullary slices. Am J Physiol. 1978 Sep;235(3):F213–F218. doi: 10.1152/ajprenal.1978.235.3.F213. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Herman C. A., Davis B. B. Effects of calcium and A23187 on renal inner medullary prostaglandin E2 synthesis. Am J Physiol. 1980 Apr;238(4):E371–E376. doi: 10.1152/ajpendo.1980.238.4.E371. [DOI] [PubMed] [Google Scholar]
- Zipser R. D., Hoefs J. C., Speckart P. F., Zia P. K., Horton R. Prostaglandins: modulators of renal function and pressor resistance in chronic liver disease. J Clin Endocrinol Metab. 1979 Jun;48(6):895–900. doi: 10.1210/jcem-48-6-895. [DOI] [PubMed] [Google Scholar]


