Abstract
Background and Purpose
Constraint-Induced Movement therapy is a set of treatments for rehabilitating motor function after CNS damage. We assessed the roles of its two main components.
Methods
A 2×2 factorial components analysis with random assignment was conducted. The two factors were type of training and presence/absence of a set of techniques to facilitate transfer of therapeutic gains from the laboratory to the life situation (Transfer Package; TP). Participants (N=40) were outpatients ≥1-year post-stroke with hemiparesis. The different treatments, which in each case targeted the more-affected arm, lasted 3.5 hr/day for 10 weekdays. Spontaneous use of the more-affected arm in daily life and maximum motor capacity of that arm in the laboratory were assessed with the Motor Activity Log (MAL) and the Wolf Motor Function Test (WMFT), respectively.
Results
Use of the TP, regardless of the type of training received, resulted in MAL gains that were 2.4 times as large as the gains in its absence (P<0.01). These clinical results parallel previously reported effects of the TP on neuroplastic change. Both the TP and training by shaping enhanced gains on the WMFT (Ps<0.05). The MAL gains were retained without loss one year post-treatment. An additional substudy (N=10) showed that a single component of the TP, weekly telephone contact with participants for one month after treatment, doubled MAL scores at 6-month follow-up.
Conclusions
The TP is a method for enhancing both spontaneous use of a more-affected arm after chronic stroke and its maximum motor capacity. Shaping enhances the latter.
Keywords: Constraint-Induced Movement therapy, transfer package, shaping, task practice, hemiplegia, stroke rehabilitation
Introduction
Constraint-Induced Movement therapy (CI therapy) has been found in multiple randomized controlled trials (RCTs) to be efficacious for rehabilitating upper-extremity function in chronic and subacute stroke in adults (reviewed in 1) and cerebral palsy in children from 1 year through adolescence (reviewed in 2). Case series support the efficacy of CI therapy for rehabilitating upper-extremity function in traumatic brain injury (TBI)3 and multiple sclerosis (MS)4 and lower-extremity function in chronic stroke,5 TBI,6 and MS.7 The magnitude of the treatment effect that has been reported, however, has been markedly variable.
The upper-extremity CI therapy protocol, as practiced in this laboratory, consists of three basic components:8–10 1) intensive training of the more-affected arm for multiple days following shaping principles (see Interventions); 2) the transfer package (TP), a set of behavioral techniques to facilitate transfer of therapeutic gains from the treatment setting to daily life (see Interventions and Supplemental Methods); and 3) prolonged motor restriction of the less-affected arm.
In a representative RCT of the full CI therapy protocol from this laboratory with 41 chronic stroke patients, the value of the effect size index d for post-treatment gains in real-world spontaneous use of the more-affected arm was 3.6.8 For comparison, 0.8 is considered a large value in the meta-analysis literature.11 All but two of the over 300 CI therapy studies published by other laboratories report a positive treatment effect, but it is usually smaller than that obtained here. For example, a widely-cited meta-analysis reports a mean d value of 0.8 for 21 CI therapy studies (total N=508), approximately 1/3 the d value reported here.12 However, most of these studies used attenuated or partial versions of our method. The usual missing component is the TP. In contrast, the results from this laboratory have been largely duplicated in studies from four laboratories that adhered to our method and whose therapists were trained here.13–16
Previous studies have found prolonged restraint of the less-affected arm is not necessary to obtain a full treatment effect.5, 17, 18 This paper reports on a study testing the contribution of the two other components: training with shaping and the TP. In a previous paper derived from this study employing voxel based morphometry (VBM), we reported that treatment with the full CI therapy protocol including the TP resulted in a profuse increase in grey matter in motor areas of the brain. Use of the same protocol but with no TP did not produce any detectable neuroplastic changes.19 The clinical findings from the subjects in that study are reported here; subjects were recruited between 2005–2007.
Study 1: Methods
Participants, Randomization, and Informed Consent
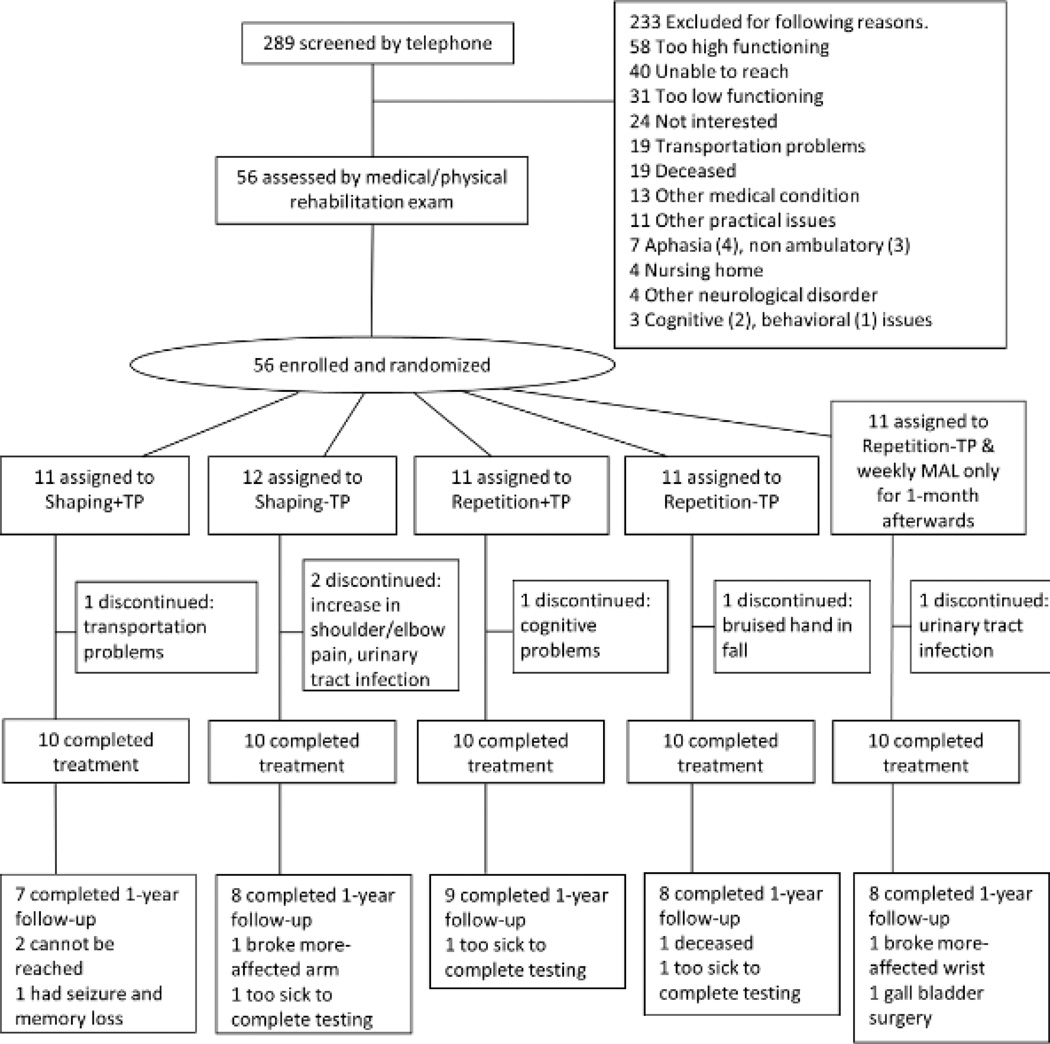
Forty-five community residents ≥1-year post-stroke with upper-extremity hemiparesis were enrolled; 40 completed treatment (see Figure 1). All had mild-to-moderate motor impairment of the more-affected arm, which is categorized as a Grade 2 deficit according to a classification schema used in CI therapy studies (see Supplemental Table 1).20 Specifically, participants were required to have extension of ≥10° at the metacarpophalangeal and one of the interphalangeal joints of each finger, ≥10° extension or abduction of the thumb, and ≥ 20° degrees extension of the wrist from a fully flexed starting position.8, 21 Exclusion criteria were: 1) presence of medical conditions severe enough to interfere with participation in treatment; 2) profound bilateral hearing loss with use of hearing aids (90 dB or worse); 3) legally blind status; 4) ferrous metal in the body or any condition that would preclude an MRI; 5) uncontrolled seizures; 6) pharmacological treatment for motor disability ≤3-months before treatment, e.g., botulinum toxin or oral/intrathecal baclofen; 7) previous CI therapy.
Figure 1.
Trial profile. Shaping=training with shaping, Repetition=repetitive task practice, +TP=presence of Transfer Package, -TP=absence of Transfer Package.
All participants provided signed informed consent prior to randomization. The study was performed at The University of Alabama at Birmingham, whose Institutional Review Board for human research approved this research. Participants were informed that they would be enrolling in a project to test the importance of different components of CI therapy. Participants were randomized in equal numbers using a computer-generated random numbers table to receive one of four possible combinations of the two factors to be tested: presence vs. absence of the transfer package (+TP vs. –TP) and training with shaping vs. repetitive task practice (shaping vs. repetition; see Figure 1).
Interventions
A components analysis was conducted with a 2×2 factorial design. The possible combinations of the two treatment factors were represented by four separate groups: shaping+TP, repetition+TP, shaping-No TP, repetition-No TP.
For all groups, training took place for 10 consecutive weekdays; 3 hr/day training + 0.5 hr/day TP for the 2 +TP groups, and 3.5 hr/day training for the 2 -TP groups. The amount of in-laboratory treatment and participant-therapist interaction was thus equivalent between groups. In the +TP groups, participants wore a heavily padded safety mitt on their less-affected arm to prevent use of that hand for a target of 90% of waking hours for the entire 14-day treatment period (10 training days plus 4 weekend days). In the –TP groups, participants wore the safety mitt for only in-laboratory treatment.
Shaping is a training method in which a motor or behavioral objective is approached in small steps by successive approximations (i.e., a task is gradually made more difficult with respect to a participant’s motor capabilities). Its principles were explicitly formulated by Skinner22, 23 and they have been applied to the rehabilitation of movement.21, 24 A more detailed description of the shaping process is presented in the Supplemental Methods section.
Repetitive Task Practice
The same or similar tasks were used with the same schedule of administration as in shaping and the participants were encouraged to keep trying, but no feedback was given and tasks of increasing difficulty were not introduced.
Transfer Package
The TP consists of a set of techniques in common use in the behavioral analysis field for the treatment of a variety of conditions, but they have not been used systematically in rehabilitation. The techniques used here are: behavioral contracts, daily home diary, daily administration of the Motor Activity Log to track amount and quality of use of the more-affected arm in 30 important ADL, problem solving to overcome perceived barriers to more-affected arm use in ADL performance, written assignment of practice at home of both tasks carried out in the laboratory and use of the more-affected arm in specified ADL, post-treatment home skill practice assignments, weekly telephone calls for the first month after laboratory treatment in which the MAL is given and problem solving carried out. The procedures are described in detail in Supplemental Methods.
Measures
The Motor Activity Log (MAL) is a scripted, structured interview21 that is reliable and valid.25 Among evidence for validity is a strong correlation (r, range=0.71–0.91, Ps≤0.01) with an objective measure of amount of movement in the life situation, accelerometry.26 Participants are asked to rate the quality of movement and amount of use of their more-affected arm in daily life on 30 upper-extremity activities over a specified period (e.g., last week, yesterday). Only the quality of movement rating, named the Arm Use scale, is reported frequently because the two ratings are highly correlated as is the case here, (r=0.95, P=0.0001), and hence redundant.8, 19, 20, 25 The minimum detectable change (MDC) on the MAL Arm Use scale is 0.5 points (10% of full scale range).25, 26 The test score is the mean of the item scores. The Wolf Motor Function Test (WMFT) is a valid and reliable measure of in-laboratory motor capacity, (i.e., maximum ability) when a participant is asked to complete a task with the more-affected arm.27, 28 Time to complete each of 15 upper-extremity actions or tasks is recorded. The test score is the mean of the item Performance Time scores after transforming them into a rate (repetitions/minute).29 The MRI results from the subjects in this study have been reported previously; they were recruited from 2005–2007.19
Data Analysis
Mixed model, repeated measures analyses of variance (ANOVAs) were used to test the independent and interdependent effects, if any, of presence of the TP and type of training on pre- to post-treatment outcomes. Parallel models, which substituted test scores at 1-year follow-up for for the post-treatment values, were used to evaluate the long-term effects of these components of CI therapy. Inspection of the group means and corresponding confidence intervals permitted description of the differences in outcomes between particular groups and testing occasions. The analysis was conducted on a per-protocol basis because the purpose of this components analysis was to identify the contribution of receiving particular components of CI therapy on treatment outcome. Two-tailed tests with an α of 0.05 were used. To control for study-wide inflation of Type I error, simple contrasts, e.g., comparing individual groups to one another, were only conducted if the relevant omnibus test was significant.30 The f statistic11 was used to index the effect size of the differences in treatment gains between the groups; values ≥0.4 are considered large. The d′ statistic was used to index the effect size of the changes within each group or combination of groups; values ≥0.57 are considered large.
Trial Profile and Initial Participant Characteristics
Out of 289 candidates screened by telephone, 56 were enrolled. Out of this number, 45 were randomized to one of the four groups in Study 1 and 11 were randomized to the single group in Study 2 (see Study 2: Methods and Results). In Study 1, 89% completed treatment and 80% completed MALs at 1-year follow-up. There was no difference in drop-out between groups at either post-treatment (P=0.793) or one-year follow-up (P=0.741). Figure 1 shows the trial profile and numbers randomized to and completing treatment in each group, along with reasons for drop-out.
Study 1: Results
Participants were, on average, 63 years old (range=29–88) and 3.9 years after stroke (range=1.0–11.0). Thirty-eight were right dominant before stroke; sixteen had paresis of the right side. There were no significant differences at pre-treatment between the Study 1 groups on any of the characteristics listed in Supplemental Table 2 (P, range=0.16-.36), including expectation of benefit from treatment (P=0.20). Nor were there pre-treatment differences on the MAL (P=0.92; see Supplemental Table 3) or WMFT (P=0.74; see Supplemental Table 4).
Changes from Pre- to Post-treatment
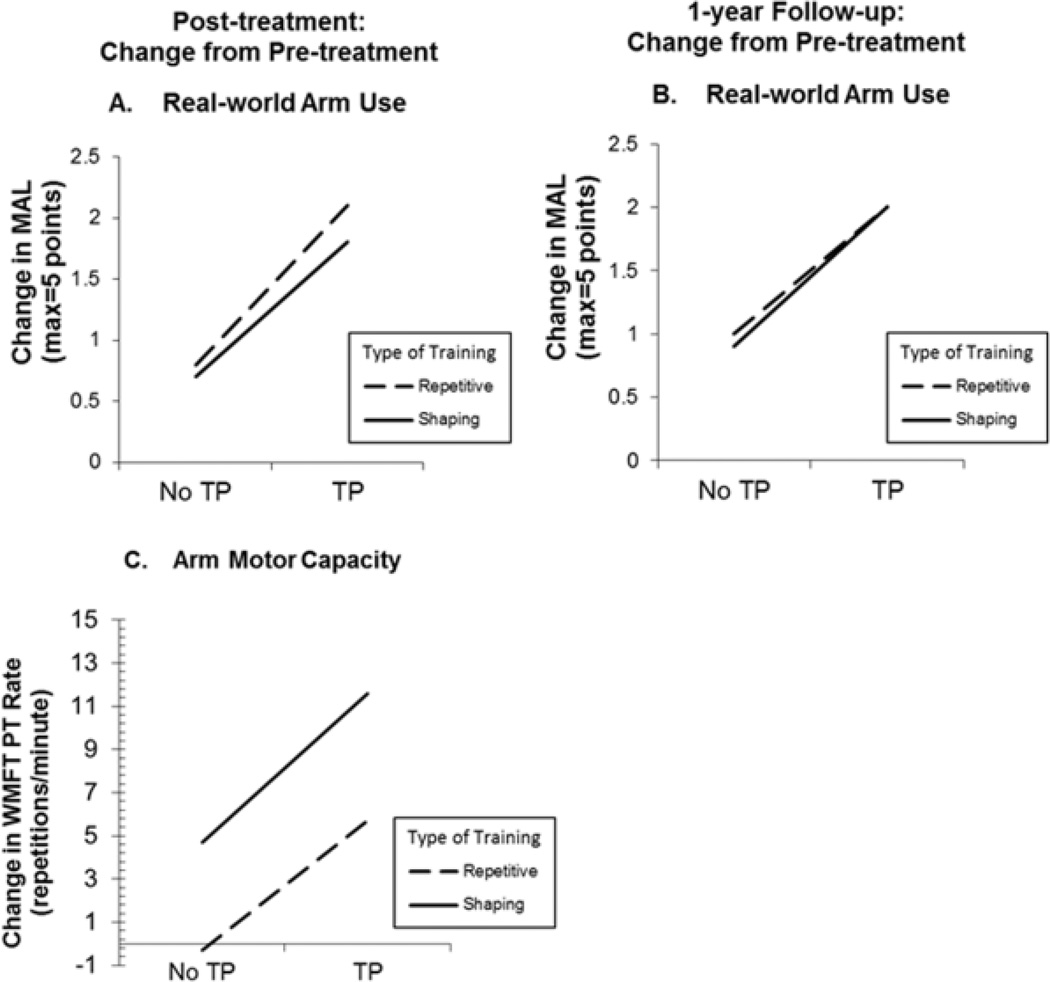
Supplemental Table 3 and Figure 2, Panel A show changes at post-treatment on the MAL. Use of the TP, regardless of type of training received, resulted in gains in spontaneous use of the more-affected arm in the life situation that were significantly larger than those observed in its absence (mean difference in MAL gains=1.2 points, P<0.01). Type of training received did not affect MAL gains (mean difference=0.2, P=0.495). Inspection of the mean changes in each group reveals that although the –TP groups had MAL gains that were greater than the MDC on this test (Shaping-TP, mean=0.7; Repetition-TP, mean=0.8); the +TP groups had changes that were more than twice as large (Shaping+TP, mean=1.8; Repetition+TP, mean=2.1).
Figure 2.
Treatment outcome for real-world spontaneous use of more-affected arm (MAL) and the maximum motor capacity of that extremity (WMFT Performance Rate). MAL outcomes at post- treatment and 1-year follow-up are graphed in Panel A and Panel B, respectively. WMFT post-treatment outcomes are graphed in Panel C. For all 3 panels, change from pre-treatment is plotted. MAL=Motor Activity Log, WMFT=Wolf Motor Function Test.
Supplemental Table 4 and Figure 2, Panel C show post-treatment changes on the WMFT, which, as noted, measures maximum motor capacity in the laboratory. Use of the TP and training with shaping each made independent contributions to post-treatment WMFT Performance Rate gains (+TP vs. –TP, mean difference=6.4 repetitions/minute, P<0.05; Shaping vs. Repetition, mean difference=5.4, P<0.05). Inspection of the mean changes within each group suggests that effects of these two factors were additive. Absence of both the TP and shaping resulted in no gains: Repetition-TP group, mean=-0.3. Presence of the TP or of shaping resulted in similar gains: Repetition+TP group, mean=5.7, Shaping-TP group, mean=4.7. Presence of both factors resulted in gains that were nearly double those when one factor alone was present: Shaping+TP group, mean=11.6.
The magnitude of the enhancement in treatment outcome produced by the TP in spontaneous use of the more-affected arm in the real world (MAL) was twice as large as that for maximum motor capacity of that arm (WMFT; f=0.8 vs 0.4, Supplemental Tables 3 & 4), which is consistent with previous data from this laboratory.5, 8, 9, 21 Notwithstanding the difference between these two aspects of motor function in magnitude of treatment gains, there was a moderate correlation between them both before treatment (r=0.44, P<0.01) and with respect to treatment change (r=0.55, P<0.001) across all participants.
Changes from Pre-treatment to 1-year After Treatment
As may be seen from Supplemental Table 3 and Figure 2, Panel B, there was no decrement in MAL scores after treatment ended in any of the four groups. Thus, the pattern of findings with respect to real-world outcome was the same at 1-year follow-up as at post-treatment.
Study 2: Methods and Results
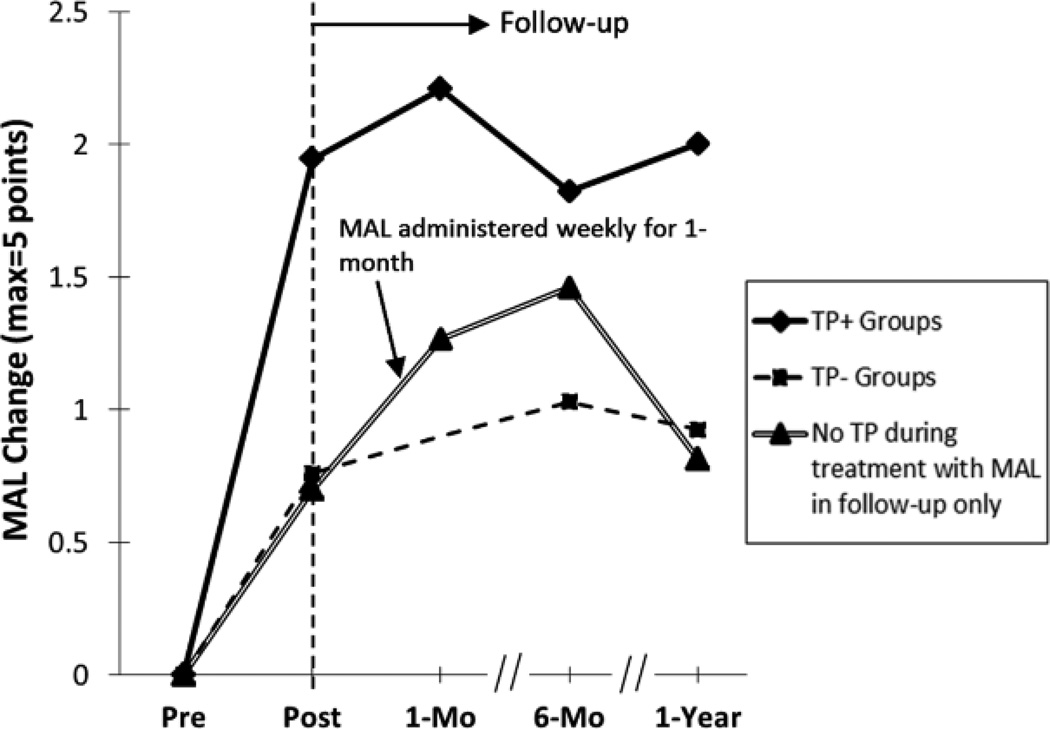
A separate study was carried out to assess the effect of a single component of the TP, weekly phone contact with participants for the first month after treatment, on post-treatment retention. The participants were randomly selected from the same pool of potential participants as used in Study 1 (see Figure 1). None of their characteristics were significantly different than those of the participants in Study 1 (see Supplemental Table 2 and Supplemental Table 5). During treatment they were given repetitive task practice and no TP just as the Repetition-TP group in Study 1. Figure 3 shows that pre- to post-treatment change on the MAL was virtually the same for these two groups. However, the addition of four weekly phone contacts for the first month after treatment substantially increased the spontaneous use of the more-affected arm in the life situation. Six months after treatment, the MAL gains in this group bridged approximately one half the gap in MAL gains at post-treatment between the repetitive task practice groups with and without the TP. At 1-year follow-up, the MAL score in this group had decreased to the level of the Repetition-TP group at that occasion, suggesting that other elements of the TP are needed to sustain MAL gains over the longer time interval at the higher level.
Figure 3.
Real-world CI therapy outcome for groups from study 1 with the TP (Shaping+TP, Repetition+TP), no TP (Shaping-TP, Repetition-TP), and no TP during treatment but with phone contact in follow-up only (Study 2 participants). Study 2 participants, who did not receive the TP during treatment, had virtually the same pre- to post-treatment MAL gains as the -TP groups in Study 1, which were less than half of the MAL gains made by +TP groups in Study 1.. After treatment, Study 2 participants had four weekly phone calls for the first month post-treatment in which the MAL and problem solving were carried out. Six months after treatment approximately half the difference in spontaneous use of the more-affected arm between the - TP and +TP groups had been bridged. Change from pre-treatment is plotted.
Overall Discussion
The current consensus in physical rehabilitation, including the perspective of patients, researchers, clinicians, and health care payers is that functional activity in the life situation is the most important outcome to pursue.31–33 In the present experiment, training in the laboratory/clinic by itself produced a substantial real-world effect. However, the TP was by far more important for inducing transfer of training from the treatment setting to the activities of daily living, increasing real-world treatment effect by a factor of almost two and one-half.
The two TP groups scored a mean of 1.2 on the MAL Arm Use scale at the beginning of treatment and ended treatment 2-weeks later scoring 3.1. A rating of 3, according to the verbal anchor presented to participants, represents an ability to carry out a daily life activity independently. A post-treatment test score of 3.1 indicates that after treatment participants were carrying out approximately half the 30 ADLs tracked by the MAL without the aid of the less-affected arm or an external source. Converting the mean scores to the percentile scale presented to participants, the spontaneous use of the more-affected arm improved from 12% before treatment to 53% after treatment, an increase of 4.4 times. This is consistent with previous research from this laboratory.8, 34
Improvement on the MAL at post-treatment was 2.4 times greater in the groups that received the TP than in the groups that did not, even though all groups received more-affected arm training of the same duration and intensity. Moreover, this advantage persisted for the entire year of follow-up; there was no decrement in MAL gains in any of the Study 1 groups. The power of the TP is further indicated by the fact that the introduction of a single one of its components in Study 2, weekly phone contacts for the first month after treatment, resulted in bridging the performance gap between groups with and without the TP at post-treatment by half 6 months afterwards. In future research it would be of value to carry out a components analysis to determine the role of each of the individual elements of the TP.
The question arises as to whether the TP increases treatment effect by increasing the amount of practice of more-affected arm use. Alternatively, it is possible that the TP promotes integration of therapeutic gains achieved in the laboratory into real-world activities so that more-affected arm use becomes habitual. These two possibilities are not mutually exclusive. Addressing this question in future research would be of mechanistic and theoretical interest; however from the point of view of practical therapeutics, the resolution of this important issue does not really matter. The TP appears to be a means of increasing real-world treatment outcome that does not involve increasing costly therapist time; this would be of considerable value whatever the mechanism by which the TP achieved its effect.
Supplementary Material
Acknowledgments
Source of Funding
This research was supported by Grant HD34273 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None of the authors have financial relationships relevant to this article.
References
- 1.Langhorne L, Coupar F, Pollack A. Motor recovery after stroke: a systematic review. Lancet Neurology. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 2.Hoare B, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004149.pub2. Art. No.: CD004149. [DOI] [PubMed] [Google Scholar]
- 3.Shaw SE, Morris DM, Uswatte G, McKay S, Meythaler JM, Taub E. Constraint-Induced Movement therapy for recovery of upper-limb function following traumatic brain injury. J Rehab Res Devel. 2005;42:769–778. doi: 10.1682/jrrd.2005.06.0094. [DOI] [PubMed] [Google Scholar]
- 4.Mark V, Taub E, Bashir K, Uswatte G, Delgado A, Bowman M, et al. Constraint-Induced Movement therapy can improve hemiparetic progressive multiple sclerosis. preliminary findings. Mult Scler. 2008;14:992–994. doi: 10.1177/1352458508090223. PMID18573826. [DOI] [PubMed] [Google Scholar]
- 5.Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement therapy: a new family of techniques with broad application to physical rehabilitation--a clinical review. J Rehab Res Devel. 1999;36:237–251. [PubMed] [Google Scholar]
- 6.Morris D, Shaw S, Mark V, Uswatte G, Barman J, Taub E. The influence of neuropsychological characteristics on the use of CI therapy with persons with traumatic brain injury. NeuroRehabilitation. 2006;21:131–137. [PubMed] [Google Scholar]
- 7.Mark VW, Taub E, Uswatte G, Bashir K, Cutter GR, Bryson C, et al. Constraint-Induced Movement Therapy for the lower extremities in multiple sclerosis: case series with 4-year follow-up. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2012.09.032. in press. [DOI] [PubMed] [Google Scholar]
- 8.Taub E, Uswatte G, King DK, Morris D, Crago J, Chatterjee A. A placebo-controlled trial of Constraint-Induced Movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. PMID16514097. [DOI] [PubMed] [Google Scholar]
- 9.Taub E, Uswatte G, Mark VW, Morris D. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–255. PMID17039223. [PubMed] [Google Scholar]
- 10.Morris D, Taub E, Mark V. Constraint-Induced Movement therapy (CI therapy): Characterizing the intervention protocol. Eura Medicophys. 2006;42:257–268. PMID17039224. [PubMed] [Google Scholar]
- 11.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 12.Sirtori V, Corbetta D, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in stroke patients (Review) Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004433.pub2. Art. No.: CD004433. [DOI] [PubMed] [Google Scholar]
- 13.Miltner WHR, Bauder H, Sommer M, Dettmers C, Taub E. Effects of Constraint-Induced Movement therapy on chronic stroke patients: a replication. Stroke. 1999;30:586–592. doi: 10.1161/01.str.30.3.586. PMID10066856. [DOI] [PubMed] [Google Scholar]
- 14.Kunkel A, Kopp B, Muller G, Villringer K, Villringer A, Taub E, et al. Constraint- Induced Movement therapy: a powerful new technique to induce motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–628. doi: 10.1016/s0003-9993(99)90163-6. PMID10378486. [DOI] [PubMed] [Google Scholar]
- 15.Sterr A, Elbert T, Berthold I, Kölbel S, Rockstroh B, Taub E. Longer versus shorter daily Constraint-Induced Movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–1377. doi: 10.1053/apmr.2002.35108. PMID12370871. [DOI] [PubMed] [Google Scholar]
- 16.Dettmers C, Teske U, Hamzei F, Uswatte G, Taub E, Weiller C. Distributed form of Constraint-Induced Movement therapy improves functional outcome and quality of life after stroke. Arch Phys Med Rehabil. 2005;86:204–209. doi: 10.1016/j.apmr.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Uswatte G, Taub E, Morris D, Barman J, Crago J. Contribution of the shaping and restraint components of Constraint-Induced Movement therapy to treatment outcome. NeuroRehabilitation. 2006;21:147–156. PMID16917161. [PubMed] [Google Scholar]
- 18.Ploughman M, Corbett D. Can forced-use therapy be clinically applied after stroke? An exploratory randomized controlled trial. Arch Phys Med Rehabil. 2004;85:1417–1423. doi: 10.1016/j.apmr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. PMC2574634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taub E, Uswatte G, Bowman MH, Mark VW, Delgado A, Bryson C, et al. Effect of CI therapy on arm use in chronic stroke patients with plegic hands. Arch Phys Med Rehabil. 2013;94:86–94. doi: 10.1016/j.apmr.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. PMID8466415. [PubMed] [Google Scholar]
- 22.Skinner BF. The Behavior of Organisms. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- 23.Skinner BF. The Technology of Teaching. New York: Appleton-Century-Crofts; 1968. [Google Scholar]
- 24.Taub E, Crago J, Burgio L, Groomes T, Cook EW, DeLuca S, et al. An operant approach to overcoming learned nonuse after CNS damage in monkeys and man: the role of shaping. J Exp Anal Behav. 1994;61:281–293. doi: 10.1901/jeab.1994.61-281. PMC1334416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uswatte G, Taub E, Morris D, Light K, Thompson P. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. PMID17030751. [DOI] [PubMed] [Google Scholar]
- 26.Uswatte G, Foo B, Olmstead H, Lopez K, Holand A, Simms ML. Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch Phys Med Rehabil. 2005;86:1498–1501. doi: 10.1016/j.apmr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Wolf S, Catlin P, Ellis M, Link Archer A, Morgan B, et al. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 28.Morris D, Uswatte G, Crago J, Cook EW, III, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity motor function following stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 29.Hodics TM, Nakatsuka K, Upreti B, Alex A, Smith PS, Pezzullo JC. Wolf Motor Function Test for characterizing moderate to severe hemiparesis in stroke patients. Arch Phys Med Rehabil. 2012:93. doi: 10.1016/j.apmr.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keppel G, Zedeck S. Data Analysis for Research Designs: Analysis of Variance and Multiple Regression/Correlation Approaches. New York: W. H. Freeman and Company; 1989. [Google Scholar]
- 31.Uswatte G, Taub E. Implications of the learned nonuse formulation for measuring rehabilitation outcomes: lessons from Constraint-Induced Movement therapy. Rehabil Psychol. 2005;50:34–42. [Google Scholar]
- 32.Keith RA. Conceptual basis of outcome measures. Am J Phys Med Rehabil. 1995;74:73–80. doi: 10.1097/00002060-199501000-00013. PMID7873118. [DOI] [PubMed] [Google Scholar]
- 33.Granger CV. The emerging science of functional assessment: our tool for outcomes analysis. Arch Phys Med Rehabil. 1998;79:235–240. doi: 10.1016/s0003-9993(98)90000-4. PMID9523772. [DOI] [PubMed] [Google Scholar]
- 34.Taub E, Lum PS, Hardin P, Mark VW, Uswatte G. AutoCITE: automated delivery of CI therapy with reduced effort by therapists. Stroke. 2005;36:1301–1304. doi: 10.1161/01.STR.0000166043.27545.e8. PMID15879335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.