Abstract
The function of P. falciparum chloroquine resistance transporter (PfCRT) can be quantified using a S. cerevisiae model system (Baro, N. K., Pooput C and Roepe P.D. Biochemistry. 50, 6701 – 6710). We further optimize this system to distinguish PfCRT isoforms found in P. falciparum strains and isolates from across the globe. We create and express 13 naturally occurring pfcrt alleles associated with a range of chloroquine resistant (CQR) phenotypes. Using galactose induction of PfCRT we quantify PfCRT and chloroquine (CQ) dependent yeast growth inhibition, and [3H]-CQ transport specifically due to a given PfCRT isoform. Surprisingly, we find poor correlation between these parameters vs CQ IC50 observed in strains of malaria harboring the same isoforms. This suggests that increased CQ transport due to PfCRT mutation is necessary, but not sufficient, for the range of CQ IC50 observed in globally distributed CQR P. falciparum isolates.
Keywords: malaria, drug resistance, chloroquine, PvCRT, PfCRT
Mutation of the pfcrt gene causes cytostatic chloroquine resistance (CQRCS) in P. falciparum malaria, typically characterized by a 7 – 10 fold increase in CQ IC50 [1-3]. The pfcrt mutations confer amino acid substitutions in the encoded PfCRT protein, which resides within the digestive vacuolar (DV) membrane of the intraerythrocytic parasite and which is believed to be a transporter [2, 3]. CQR phenotypes are further characterized by cross resistance patterns to other drugs that may be influenced by additional genes [4-6]. Our current model for how mutant PfCRT confers CQR envisions increased electrochemically downhill transport of CQ out of the DV [2,7-9], combined with DV osmolyte dysequilibria that perturbs CQ – heme binding via changes in DV volume and perhaps pH [2,10,11].
A complete molecular description of all CQR phenomena is likely more complex. There are at least 23 different PfCRT isoforms that have been found in distinct CQR parasite isolates [3,12], originating from at least five geographically distinct loci (Colombia, Peru, P.N.G., Phillipines, S.E.A.). These parasite isolates exhibit different phenotypes and their cognate mutant PfCRTs harbor different patterns of 4 to 10 amino acid substitutions. Reproducible, statistically valid CQ IC50 data are only available for approximately half of these isolates, since many have not yet been established as stably growing laboratory strains. Nonetheless, the IC50 reveal a wide range in CQ sensitivity across CQR parasites ([12], Table 1) that is either solely or in part due to PfCRT mutation. It is currently not known precisely how much various PfCRT isoforms contribute to the various CQ IC50 shifts seen around the globe. Based on early P. falciparum allelic exchange experiments [13] it is often assumed that the PfCRT amino acid substitutions in these isoforms are necessary and sufficient for the shift in CQ IC50 that is observed in the cognate CQR strain. Close inspection of the data in [13] shows that for the Dd2 (S.E. Asia) and 7G8 (S. America) CQR associated PfCRT isoforms, this is a reasonable assumption since 70% - 90% of the shift in CQ IC50 for laboratory CQR strains Dd2 and 7G8 is recapitulated by allelic exchange of wild type pfcrt with Dd2 or 7G8 pfcrt. However, no other naturally occurring, CQR associated, PfCRT isoforms have yet been expressed in allelic exchange models, so it is unclear if this is the case for all CQR isoforms. Also, we have recently reported that CQ IC50 (quantification of cytostatic or growth inhibitory potential) and CQ LD50 (quantification of cytocidal or parasite cell kill activity) are considerably different for CQR strains of P. falciparum [33]. A consequence is that the well characterized CQR strain Dd2 is 10 – fold CQRCS as measured by IC50 ratio vs CQS strain HB3, but is 125-fold cytocidal CQR (CQRCC) when LD50 for the same strains are ratio’ed. These and related data indicate that additional genetic events could perhaps complement PfCRT mutation to confer CQR [33,34].
Table 1. Strains and isolates of Plasmodium facliparum: PfCRT haplotypes and CQ IC50.
Residues mutated relative to wild type are highlighted green. Empty cells denote residues for which sequence data is not available and (−) denotes a deletion mutation. Where multiple IC50 values (in nM) were found in the literature, the high and low values were reported. Sources of low IC50 value, high IC50 value (if applicable), and PfCRT sequencing data are referenced in order (far right).


|
In sum, it is not yet entirely clear precisely how much altered CQ transport due to PfCRT amino acid substitutions contribute to all CQR phenomena (e.g. various IC50 vs LD50 phenomena), and even though PfCRT mutations likely confer the majority of the shift in CQ IC50 for some CQR strains of P. falciparum [32], it is not yet known if PfCRT mutations alone are responsible for the CQ IC50 shifts seen across all globally distributed isolates. Parasite allelic exchange experiments that might help to clarify these issues are very difficult, and there appear to be poorly understood compensatory genetic events that are required for expression of certain CQR PfCRTs in various parasite genetic backgrounds [16]. To further explore these issues we have improved upon a previously described approach [35] in order to rapidly distinguish even subtle differences between the function of CQR associated PfCRT isoforms.
Materials and Methods
Materials
Yeast DOB media and DOB with galactose and raffinose were obtained in powder form from MP Biomedicals, Solon, OH. Cell culture plastics were from BD Falcon. Glass beads for yeast cell lysis were from B. Braun Biotech, Allentown, PA. Anti-HexaHis-HRP and anti-V5-HRP antibodies were from Qiagen (Valencia, CA) and Invitrogen (Carlsbad CA), respectively. 3H – CQ was from American Radiolabelled Chemicals Inc (St. Louis MO). All other chemicals were reagent grade or better, were purchased from Sigma (St. Louis MO) and used without additional purification.
Yeast Strains and Methods
CH1305 (MATa ade2 ade3 ura3-52 leu2 lys2-801) was supplied by J.F. Cannon [36]. Solid and liquid media were prepared as described in Sherman et al. [37], and included synthetic complete (SC) media lacking one or more specified amino acids, as well as rich medium (YPAD or YPD). Induction of CRT protein expression, standard yeast growth methods, yeast transfections, and other routine methods were as described [35].
Plasmids
The pYES2 backbone containing PfHB3vh, PfDd2vh and Pf7G8vh was constructed previously [35] and used as template DNA in subsequent rounds of multi-site directed mutagenesis via the Agilent QUICKChange method to create the various isoforms of PfCRT (see Table 2). All constructs were confirmed by direct DNA sequencing of the full pfcrt gene.
Table 2.
Oligonucleotides used in the present study.
| Name | Sequence (5′ – 3′) |
|---|---|
| A220S | CATCTACCTGTCAGTTTGCGTGATAGAGACGATCTTCGCTAAGAGAACCTTGAA |
| K76T | CAGTTTGCGTGATGAACACGATCTTCGCTAAGAGAAC |
| N75E | CCTGTCAGTTTGCGTGATGGAGAAGATCTTCGCTAAGAGAA |
| A144T | CTTGCAGCGTCATCTTGACCTTCATCGGTCTTACC |
| A220S | CAACCTAGTCCTGATTAGCAGTCTGATCCCTGTCTGTTTC |
| AL-144,148-FI | CTTGCAGCGTCATCTTGTTCTTCATCGGTATTACCAGAACCACAGGT |
| CK-72,76-ST | CCATCATCTACCTGTCAGTTAGCGTGATGAACACGATCTTCGC |
| CMNK-72,74-6-RIEI | CATCTACATCCTGTCCATCATCTACCTGTCAGTTCGCGTGATAGAGA TAATCTTCGCTAAGAGAACC |
| E75D | CCTGTCAGTTTGCGTGATCGATACCATCTTCGC |
| H97L | CGTGACTAGTGAAACCCTCAACTTCATCTGCATGA |
| H97Q | CGTGACTAGTGAAACCCAGAACTTCATCTGCATGATC |
| I194T | CAGTAATCATCGTAGTCACAACCGCATTGGTGGAAATG |
| I356T | CATCCAGGGTCCCGCAACCGCTATTGCCT |
| I356V | GCATCCAGGGTCCCGCAGTCGCTATTGC |
| I371R | CTTAGCAGGTGATGTCGTAAGAGAACCACGTTTGTTG |
| K76N | CCTGTCAGTTTGCGTGATGAACAATATCTTCGCTAAGA |
| L148I | CTTGGCCTTCATCGGTATTACCAGAACCACAGG |
| L160Y | CAGGTAACATTCAGTCCTTCGTCTATCAACTATCAATTCCAATCAACATG |
| M74I | CATCTACCTGTCAGTTTGCGTGATAAACAAGATCTTCG |
| MN_7-45_IE | CCATCATCTACCTGTCAGTTTGCGTGATAGAGAAGATCTTCGCTAAG |
| MNK_7-456_IEI | TCCTGTCCATCATCTACCTGTCAGTTTGCGTGATAGAGATAATCTTCG CTAAGAGAACC |
| MNK74-76IET | CATCTACCTGTCAGTTTGCGTGATAGAGACGATCTTCGCTAAGAGA ACCTTGAA |
| N326D | CGCCTTGTTCTCATTCTTCGACATCTGTGATAACCTGAT |
| N326S | GCCTTGTTCTCATTCTTCAGCATCTGTGATAACCTGATC |
| NK_7-56_ET | ACCTGTCAGTTTGCGTGATGGAGACGATCTTCGCTAAGAGAACCT |
| P275L | CTGAAGGAGTTACACTTGCTATACAACGAAATCTGGACC |
| Q271E | CACACTACCATTCCTGAAGGAGTTACACTTGCCATACAACG |
| Q352K | TATTGTGAGTTGCATCAAGGGTCCCGCAATCGC |
| R371I | CTTAGCAGGTGATGTCGTAATAGAACCACGTTTGTTG |
| R371T | TTCTTAGCAGGTGATGTCGTAACGGAACCACGTTTGTTGG |
| S163R | TCAGTCCTTCGTCTTGCAACTAAGAATTCCAATCAACATGTTCTTC |
| S220A | CCTAGTCCTGATATCCGCTCTGATCCCTGTCTG |
| S326SN | TTCGCCTTGTTCTCATTCTTCAACATCTGTGATAACCTGATCAC |
| S72C | GTCCATCATCTACCTGTCAGTTTGCGTGATGAACAC |
| T152A | TCGGTCTTACCAGAACCGCAGGTAACATTCAGTCC |
| T333S | ATCTGTGATAACCTGATCAGCAGCTACATCATCGATAAG |
| T356I | ATCCAGGGGCCCGCAATCGCTATTGCC |
| T76I | CAGTTTGCGTGATCGAAATCATCTTCGCGAAGAGAA |
| T76K | GTCAGTTTGCGTGATCGAAAAGATCTTCGCGAAGAGAACCT |
| T76N | CAGTTTGCGTGATCGAAAACATCTTCGCGAAGAGAA |
| E75K | CCTGTCAGTTTGCGTGATCGAAACCATCTTCGCG |
| H123R | GGGTAACAGCAAGGAACGTCGTAGGAGCTTCAAC |
| T205A | GAAGCTGAGCTTCGAAGCACAGGAAGAGAACTC |
| C350R | CACTATTGTGAGTCGCATCCAGGGGCCCGC |
Western Blotting
Was as described previously [35].
Colony Formation Assays and Quantitative Growth Analysis
Was performed under CRT – inducing and non – inducing conditions as described [35] with some modifications. For ΔpH and Δψ dependent growth curve analysis yeast harboring different isoforms of CRT were assayed in synthetic complete media containing additional 100 mM KCl and buffered with 100 mM HEPES (pH 6.75) (see Results). Growth under each condition was measured in triplicate via back dilution of the strain grown under normal non inducing conditions (SD media lacking uracil). Under normal conditions (medium external pH (pHex) ~ 5.0) the yeast PM maintains a high ΔpH and low Δψ, but alkalinization of the external medium lowers ΔpH and increases Δψ concomitantly such that substantial Δψ compensates for loss of ΔpH (see [35]).
[3H] CQ Whole Cell Accumulation Assays
3H-CQ transport specific to PfCRT was assayed as previously described [35]. Within, steady state accumulation (at 30 min incubation, pH 7.5) of CQ is reported.
RESULTS
Previously, we found that elevating plasma membrane electrical potential (ΔΨ) in yeast that were expressing CQR – associated PfCRT protein increased CQ accumulation for the yeast [35]. In contrast, elevated ΔΨ did not increase CQ transport mediated by CQS – associated PfCRT or by the P. vivax CRT orthologue PvCRT [35]. We thus hypothesized that optimizing assay conditions such that yeast plasma membrane ΔΨ was at maximum would facilitate distinction between CQS (e.g. HB3) vs CQR (e.g. Dd2) PfCRT isoforms. Indeed, Fig. 1A shows results from plate spotting assays and Figs. 1B, 2A, 2B shows quantitative growth curve analyses wherein ΔΨ is clamped to higher values by growth in high K+/pH = 6.75 medium (which depresses ΔpH and elevates ΔΨ [2,35]). At higher ΔΨ, since CQ transport by CQR (Dd2) PfCRT is stimulated [35] lower concentrations of CQ are sufficient to assay PfCRT function and to distinguish CQS (HB3) from CQR (Dd2) isoforms (Fig. 1B, 2B).
Figure 1.
Improved growth assays. (A) HB3 (CQS) and Dd2 (CQR) PfCRT dependent growth on solid agar plates over a range of CQ concentrations (mM CQ, left hand side; “EV” = “empty vector” or no PfCRT expressed) and (B) in liquid growth medium for yeast expressing PfCRTHB3 (black) or PfCRTDd2 (gray). Note that expression of either HB3 or Dd2 isoforms slows growth specifically in the presence of CQ, and that the effect is greatest for Dd2, particularly at high pH (high ΔΨ; see also [35]). (C) P. falciparum strains and isolates (and their CQ IC50 values) whose cognate CRT isoforms were analyzed within. * = strain also known as TM93-C1088. ** denotes that IC50 shown has been normalized to IC50 reported by Mu and colleagues [14] by using CQS control data from the same study (c.f. Table 1).
Figure 2.
Growth of yeast harboring pYES2 (dashed), pYES2/HB3CRT(black), or pYES2/Dd2CRT (gray) in medium containing 16 mM CQ under non-inducing (glucose, panel A top left) vs inducing (galactose, panel B top right) conditions. (C,D) Quantification of PfCRT isoform-dependent growth delay in Saccharomyces cerevisiae by subtracting the time to reach maximum growth rate (as identified by the time corresponding to growth curve maximum slope) under non-inducing conditions (B: HB3T1, Dd2T1) from the corresponding time measured under inducing conditions (C: HB3T2, Dd2T2). Results from quantification for each yeast strain are found in Table 4.
We wondered if this increased sensitivity would facilitate clearer distinction between different CQR-associated PfCRT isoforms found in geographically distinct CQR isolates. We expressed 13 distinct PfCRT isoforms using the galactose inducible system described previously [35]. All isoforms express to similar levels (Fig. 3, Table 3). Screening these yeast for growth defects due to PfCRT expression in the presence of 16 mM CQ (Fig. 2C, 2D; Table 4) reveals variable responses due to PfCRT mediated accumulation of toxic CQ [35].
Figure 3.
Western blot analysis of S. cerevisiae yeast membranes expressing the indicated PfCRT isoform. Each lane contains 10 μg total protein. The α-V5 blot shows similar levels of protein expression are found for each PfCRT isoform, see Table 3 for quantification vs ≥ 3 blots for each isoform.
Table 3.
Quantification of expression of different PfCRT isoforms in yeast (see also Figure 3). Densitometry data are the average of at least three separate western blots (+/− S.E.M.) where each lane was loaded to the same total protein content as defined by amido black assay (see [35]).
| Isoform | Average Band Density |
SEM |
|---|---|---|
| HB3 | 1.00 | |
| S106/1 | 0.99 | 0.04 |
| Dd2 | 1.02 | 0.09 |
| 7G8 | 1.00 | 0.12 |
| GB4 | 1.00 | 0.04 |
| FCB | 0.98 | 0.05 |
| TM93 | 0.98 | 0.11 |
| TM6 | 1.03 | 0.07 |
| Ecu1110 | 0.95 | 0.13 |
| JAV | 0.95 | 0.08 |
| PNG4 | 0.99 | 0.12 |
| IP2300 | 1.01 | 0.01 |
| H209 | 1.01 | 0.08 |
Table 4. Growth delay data for yeast expressing all different CRT isoforms analyzed in present study.
Growth delay is expressed as the difference in hours (see Fig. 2C,D) for growth in the presence of 16 mM CQ under PfCRT inducing vs non-inducing conditions (see [35]).
| Isoform | Growth Delay (Hours) |
SEM |
|---|---|---|
| HB3 | 13.9 | 1.1 |
| S106/1 | 11.8 | 1.5 |
| Dd2 | 25.5 | 2.5 |
| 7G8 | 19.4 | 2.6 |
| GB4 | 19.3 | 2.4 |
| TM93 | 21.8 | 1.7 |
| TM6 | 18.9 | 0.2 |
| FCB | 22.4 | 1.8 |
| Ecu1110 | 30.7 | 3.6 |
| JAV | 29.2 | 3.2 |
| PNG4 | 23.4 | 1.8 |
| H209 | 31.9 | 0.3 |
| IP2300 | 13.1 | 0.1 |
Not all CQR P. falciparum isolates from around the globe have been established as stable laboratory strains. Thus conventional IC50 assays cannot be done for some isolates, rather, single passage quantification of 3H-hypoxanthine accumulation is used to assess sensitivity to CQ. These assays are difficult, highly operator dependent, and often do not allow for reproducible quantification of IC50. We therefore focused our efforts at further quantifying PfCRT isoforms to those 11 found in isolates that have been established as stable laboratory strains (Fig. 1C). Fortuitously, these strains show the full range of CQ IC50 that have so far been observed for all strains and isolates (Table 1) and one laboratory has quantified IC50 for nearly all of them using identical technique [14].
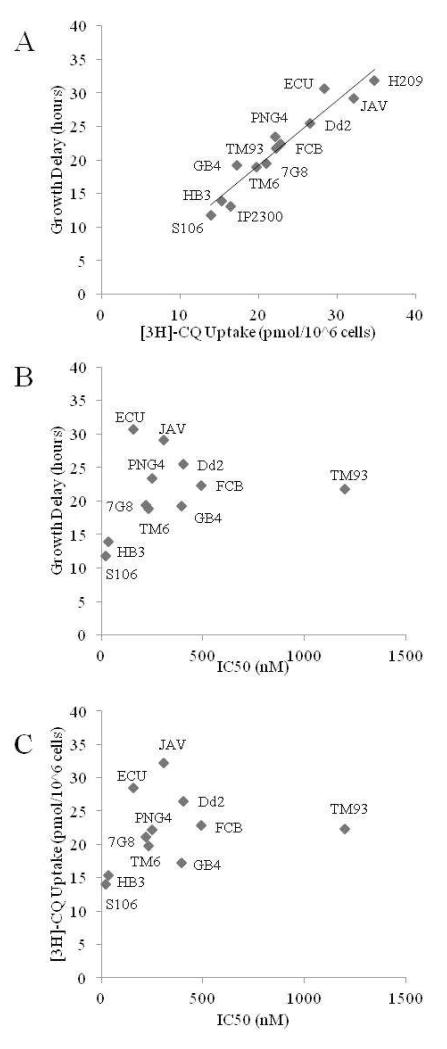
Correspondingly, yeast harboring these isoforms show variable sensitivity to the growth inhibitory effects of external CQ (Fig. 2C, 2D; Table 4). Not surprisingly, these yeast also show variable levels of 3H – CQ accumulation that is due to variable drug transport function of the different CQR PfCRT isoforms [35]. When we plot relative 3H – CQ accumulation vs the degree of CQ – induced growth inhibition for yeast expressing these PfCRTs, a linear correlation is obtained (Fig. 4A). This very strongly supports our simple model proposed earlier [35] wherein yeast strain growth inhibition due to external CQ is due to PfCRT mediated transport of toxic CQ into the yeast. Interestingly however, when either relative growth (Fig. 4B), or relative 3H-CQ uptake (Fig. 4C) for yeast harboring a given CQR PfCRT isoform is plotted vs the CQ IC50 observed for the P. falciparum strain harboring the same isoform, no correlation is obtained.
Figure 4.
(A) Linear correlation between isoform induced growth delay (see Fig. 3) vs isoform facilitated CQ uptake (R2 = 0.91). (B) Isoform induced growth delay plotted vs CQ IC50 for corresponding strains. No apparent correlation is found either with the TM93 “outlier” (R2 = 0.05) or with TM93 removed (R2 = 0.23). (C) PfCRT Isoform specific CQ transport plotted vs CQ IC50 for corresponding strains. No apparent correlation is found either with the TM93 “outlier” (R2 = 0.05) or with TM93 removed (R2 = 0.19).
DISCUSSION
Initial quantification of the contribution of amino acid substitutions in Dd2 and 7G8 isoforms of PfCRT to the elevated CQ IC50 observed in CQR strains Dd2 and 7G8 suggested that most, if not all, of the variable CQR phenotype in these strains was due to the different PfCRT mutations [32]. Additional studies over the past 10 years have suggested that elevated IC50 in these strains is likely due to heightened CQ transport by the mutant PfCRTs (reviewed in [2,3]). Since then, additional distinct PfCRT mutants have been discovered in other CQR isolates. These have evolved under different selective drug pressure and exhibit different CQ IC50 relative to strains Dd2 and 7G8. Some of these isolates have been established as laboratory strains wherein other aspects of the CQR phenotype have also been found to quantitatively differ relative to strains Dd2 and 7G8.
Two competing hypotheses for the molecular basis of these differences exist. One is that the different mutation patterns in the PfCRTs yield transporters with variable drug transport efficiencies, producing different levels of intra DV drug that then result in different IC50. In this case, we would expect the relative ability of mutant PfCRTs to either transport CQ or to induce CQ dependent yeast growth inhibition to correlate in some fashion with the CQ IC50 found for the cognate P. falciparum strain. This is not found to be the case (c.f. Fig. 4B, 4C). The second hypothesis is that different CQ transport by various mutant PfCRTs is only partly responsible for CQ IC50 variability, and that other genome mutations (and/or other mutant PfCRT functions, see [10,11]) complement PfCRT CQ transport to produce different CQ IC50. Direct data in support of either hypothesis has not been attainable until now, other than through P. falciparum allelic exchange experiments and quantitative trait loci (QTL) analysis of genetic cross progeny. The former experiments are technically quite difficult, due to low frequency site – specific recombination in malarial parasites, and the latter generally do not allow for analysis of the role of individual genes. The alternate yeast-based approach presented here provides much more rapid quantification of variable PfCRT isoform function. This approach should also be useful for screening PfCRT inhibitor preferences vs different PfCRT isoforms, and for further dissecting the function of PfCRT orthologues such as PvCRT. It is also useful for analyzing PfCRTs found in isolates that have not been (or cannot be) established as strains; we include two such isoforms (IP2300, H209 [16]) whose degree of function is somewhat controversial, since the yeast data cleanly provide a 3H-CQ transport phenotype.
We have quantified subtle differences in the function of mutant PfCRT isoforms from geographically distinct CQR P. falciparum isolates. For isoforms found in isolates that have been established as stable strains, and for which reliable CQ IC50 thus exist, we have quantified PfCRT function in two ways (Fig. 4). We find, not surprisingly, that PfCRT mediated CQ uptake very strongly correlates with CQ-dependent growth inhibition for yeast expressing different PfCRT isoforms (Fig. 4A). The molecular basis of yeast CQ growth inhibition is not fully elucidated, but it is probably due to the well-known lysosomatropic actions of CQ at these doses. Importantly, clinically relevant doses of CQ will yield ~ mM levels of CQ within the parasite DV (see [34] and references within), and the magnitude and polarity of ΔpH and ΔΨ as well as appropriate PfCRT membrane topology, are all preserved in yeast plasma membrane vs the DV membrane. Thus, CQ transport from outside yeast to inside in our model system directly mimics CQ transport from inside the DV to parasite cytosol [35].
We find that regardless how the data are plotted, good correlation is not observed between PfCRT isoform function and CQ IC50 in the cognate P. falciparum strain. This demonstrates that drug transport due to amino acid substitutions in PfCRT is necessary, but not sufficient, for influencing CQ IC50, and that additional gene mutations, or additional mutant PfCRT functions, in a variety of isolates likely influences CQ IC50 values. Such a conclusion is not as surprising as it might initially appear; mutation or altered expression of PfMDR1 protein has also been associated with mild (2 – 3 fold) changes in drug IC50’s [38 - 40] and our studies with recombinant PfMDR1 suggest that the protein binds CQ with high affinity [41]. In some strains, PfMDR1 alleles may contribute in pairwise fashion to PfCRT mutations in shifting CQ IC50. We suggest other genes or physiologic phenomena also contribute to CQ IC50, and that due to different drug selection histories in the regions from which these isolates originate, contribution from other genes will also vary in a geographically distinct fashion. Perhaps relatedly, we note that two laboratories have reported that altered CQ transport in drug resistant P. falciparum parasites does not necessarily correlate with the degree of parasite resistance measured at high dose CQ [33, 34, 42]. This may reflect altered targets for cytostatic vs cytocidal doseages of CQ as proposed [2,33,34], and/or be a consequence of an additional or overlapping mechanism for CQR that is not yet defined.
Finally, we note that strain TM93 appears particularly interesting. This strain is reported to have an unusually high CQ IC50 [14], yet TM93 PfCRT shows only average CQ transport function, hence it appears as somewhat of an outlier in Fig. 4B, 4C. The molecular features in addition to CQ transport by mutant PfCRT that contribute to the very high IC50 in strain TM93 deserve additional study. Strains for which PfCRT drug transport contributions to resistance appear low, which can be easily identified via the methods in this paper (e.g. TM93), should provide particularly fertile ground for additional studies of antimalarial drug resistance phenomena.
Acknowledgements
Supported by NIH grants AI056312 and AI090832 to PDR.
ABBREVIATIONS
- CQ
chloroquine
- CQR(S)
chloroquine resistant(sensitive)
- PNG
Papua New Guinea
- SEA
Southeast Asia
Footnotes
Author Contributions
N.K.B. and P.S.C. contributed equally to this work.
REFERENCES
- 1.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naudé B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roepe PD. Biochemistry. 2011;50:163–171. doi: 10.1021/bi101638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ecker A, Lehane AM, Clain J, Fidock DA. Trends Parasitol. 2012;28:504–501. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su XZ, Wellems TE. Mol. Microbiol. 2004;52:985–997. doi: 10.1111/j.1365-2958.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 5.Patel J, Thacker JD, Tan JC, Pleeter P, Checkley L, Gonzales JM, Deng B, Roepe PD, Cooper RA, Ferdig MT. Mol Microbiol. 201078:770–787. doi: 10.1111/j.1365-2958.2010.07366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez CP, Mayer S, Nurhasanah A, Stein WD, Lanzer M. Mol Microbiol. 2011;82:865–878. doi: 10.1111/j.1365-2958.2011.07855.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 8.Paguio MF, Cabrera M, Roepe PD. Biochemistry. 2009;48:9482–9491. doi: 10.1021/bi901035j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papakrivos J, Sá JM, Wellems TE. PLoS One. 2012;7:e39569. doi: 10.1371/journal.pone.0039569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett TN, Kosar AD, Ursos LMB, Dzekunov S, Sidhu ABS, Fidock DA, Roepe PD. Mol. & Biochem. Parasitol. 2004;133:99–114. doi: 10.1016/j.molbiopara.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Glikoreijevic B, McAllister R, Urbach J, Roepe PD. Biochemistry. 2006;45:12411–12423. doi: 10.1021/bi0610348. [DOI] [PubMed] [Google Scholar]
- 12.Summers RL, Nash MN, Martin RE. Cell Mol Life Sci. 2012;69:1967–1995. doi: 10.1007/s00018-011-0906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sa J, Twu O, Hayton K, Reyes S, Fay M, Ringwald P, Wellems T. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu J, Ferdig M, Feng X, Joy D, Duan J, Furuya T, Subramanian G, Ararind L;, Cooper R, Wooton J, Xiong M, Su X-z. Mol. Micro. 2003;49:977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- 15.Su X-z., Kirkman L, Fujioka H, Wellems T. Cell. 1997;91:592–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 16.Valerramos S, Valderramos J, Musset L, Purcell L, Mercereau-Puijalon O, Legrand E, Fidock D. PloS Path. 2010;6:e1000887. doi: 10.1371/journal.ppat.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D, Fidock D, Mungthin M, Lakshmanan V, Sidhu A, Bray P, Ward S. Mol. Cell. 2004;15:867–877. doi: 10.1016/j.molcel.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N, Kyle D, Pasay C, Fowler E, Baker J, Peters J, Cheng Q. Anti Microb. Agents Chemother. 2003;47:3500–3505. doi: 10.1128/AAC.47.11.3500-3505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayoumi R, Creasey A, Babiker H, Carlton J, Sultan A, Satti G, Sohal A, Walliker D, Jensen J, Arnot D. Trans. R. Soc. Trop. Med. Hyg. 1993;87:454–458. doi: 10.1016/0035-9203(93)90034-n. [DOI] [PubMed] [Google Scholar]
- 20.Cooper R, Ferdig M, Su X-z., Ursos L, Mu J, Nomura T, Fujioka H, Fidock D, Roepe P, Wellems T. Mo.l Pharma. 2002;61:35–42. doi: 10.1124/mol.61.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Chaijarkoenkul W, Ward S, Mungthin M, Johnson D, Owen A, Bray P, Na-Bangchang K. Malaria J. 2011;10:42–47. doi: 10.1186/1475-2875-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wooton J, Feng X, Ferdig M, Cooper R, Mu J, Baruch D, Magill A, Su X-z. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 23.Mungthin M, Bray P, Ward S. Am J Trop Med Hyg. 1999;60:469–474. doi: 10.4269/ajtmh.1999.60.469. [DOI] [PubMed] [Google Scholar]
- 24.Stead A, Bray P, Edwards G, Dekoning H, Elford B, Stocks P, Ward S. Mol. Pharm. 2001;59:1298–1306. doi: 10.1124/mol.59.5.1298. [DOI] [PubMed] [Google Scholar]
- 25.Cooper R, Hartwig C, Ferdig M. Acta. Trop. 2005;94:170–180. doi: 10.1016/j.actatropica.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Durrand V, Berry A, Sem R, Glaziou P, Beaudou J, Fandeur T. Mol. Biochem. Parasit. 2004;136:273–285. doi: 10.1016/j.molbiopara.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Echeverry D, Holmgren G, Murillo C, Higuita J, Bjorkman A, Gil J, Osorio L. Am. J. Trop. Med. Hyg. 2007;77:1034–1038. [PubMed] [Google Scholar]
- 28.Yang Z, Zhang Z, Sun X, Wan W, Cui L, Zhang X, Zhong D, Yan G, Cui L. Trop. Med. and Int. Health. 2007;12:1051–1060. doi: 10.1111/j.1365-3156.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagesha H, Casey G, Rieckman K, Fryauff D, Laksana B, Reeder J, Maguire J, Baird K. Am. J. Trop. Med. Hyg. 2003;68:398–402. [PubMed] [Google Scholar]
- 30.Mehlotra R, Fujioka H, Roepe P, Janneh O, Ursos L, Jacobs-Lorena V, McNamara D, Bockarie M, Kazura J, Kyle D, Fidock D, Zimmerman P. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12689–12694. doi: 10.1073/pnas.221440898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen N, Russell Bruce, Staley J, Kotecka B, Nasueld P, Cheng Qin. J Infect. Dis. 2001;183:1543–1545. doi: 10.1086/320206. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu AB, Verdier-Pinard D, Fidock DA. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paguio MF, Bogle KL, Roepe PD. Mol Biochem Parasitol. 2011;178:1–6. doi: 10.1016/j.molbiopara.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrera M, Paguio MF, Xie C, Roepe PD. Biochemistry. 2009;48:11152–11154. doi: 10.1021/bi901765v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baro NK, Pooput C, Roepe PD. Biochemistry. 2011;50:6701–6710. doi: 10.1021/bi200922g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nigavekar SS, Cannon JF. Yeast. 2002;19:115–122. doi: 10.1002/yea.807. [DOI] [PubMed] [Google Scholar]
- 37.Sherman F, Baim SB, Hampsey DM, Gooodhue CT, Friedman LR, Stiles JI. In: Translational Control. Matthews MB, editor. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1986. [Google Scholar]
- 38.Wilson CM, Serrano AE, Wasley A, Bogenschutz MP, Shankar AH, Wirth DF. Science. 1989;244:1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- 39.Foote SJ, Kyle DE, Martin RK, Oduola AM, Forsyth K, Kemp DJ, Cowman AF. Nature. 1990;345:255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- 40.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 41.Pleeter P, Lekostaj JK, Roepe PD. Mol Biochem. Parasitol. 2010;173:158–161. doi: 10.1016/j.molbiopara.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geary TG, Jensen JB, Ginsburg H. Biochem. Pharmacol. 1986;35:3805–3812. doi: 10.1016/0006-2952(86)90668-4. [DOI] [PubMed] [Google Scholar]