Abstract
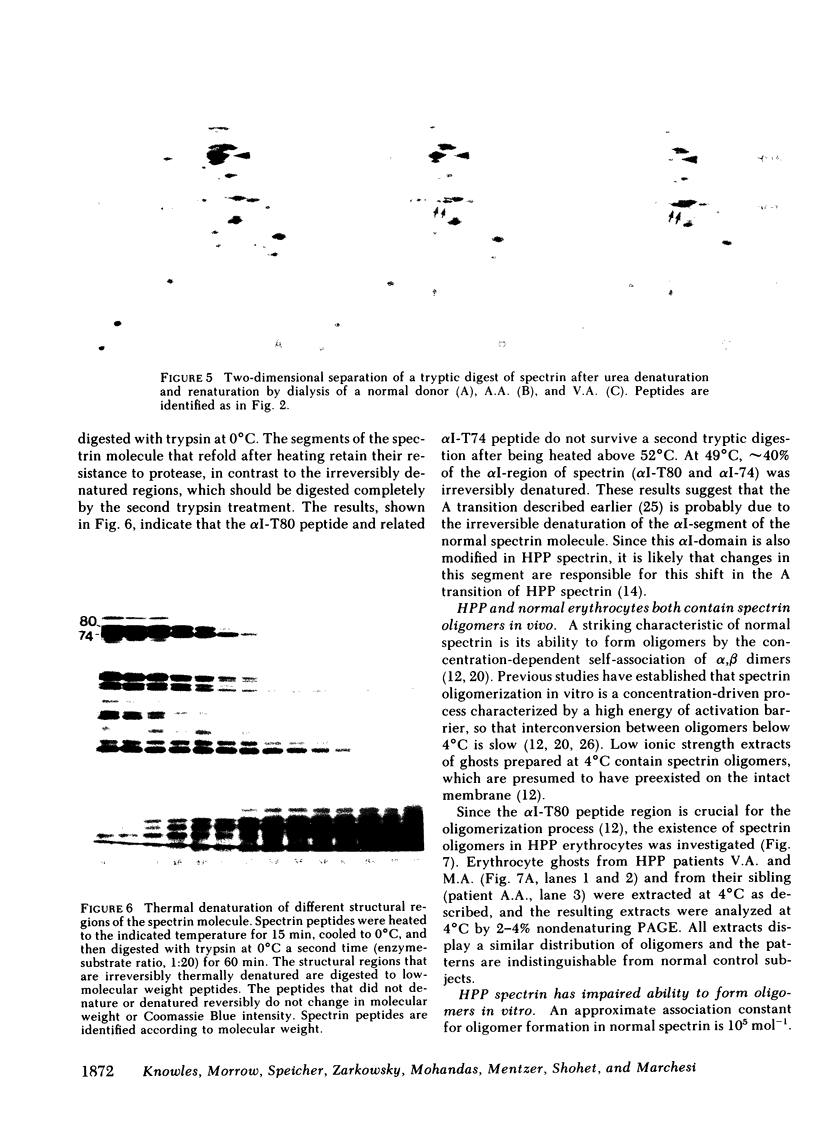
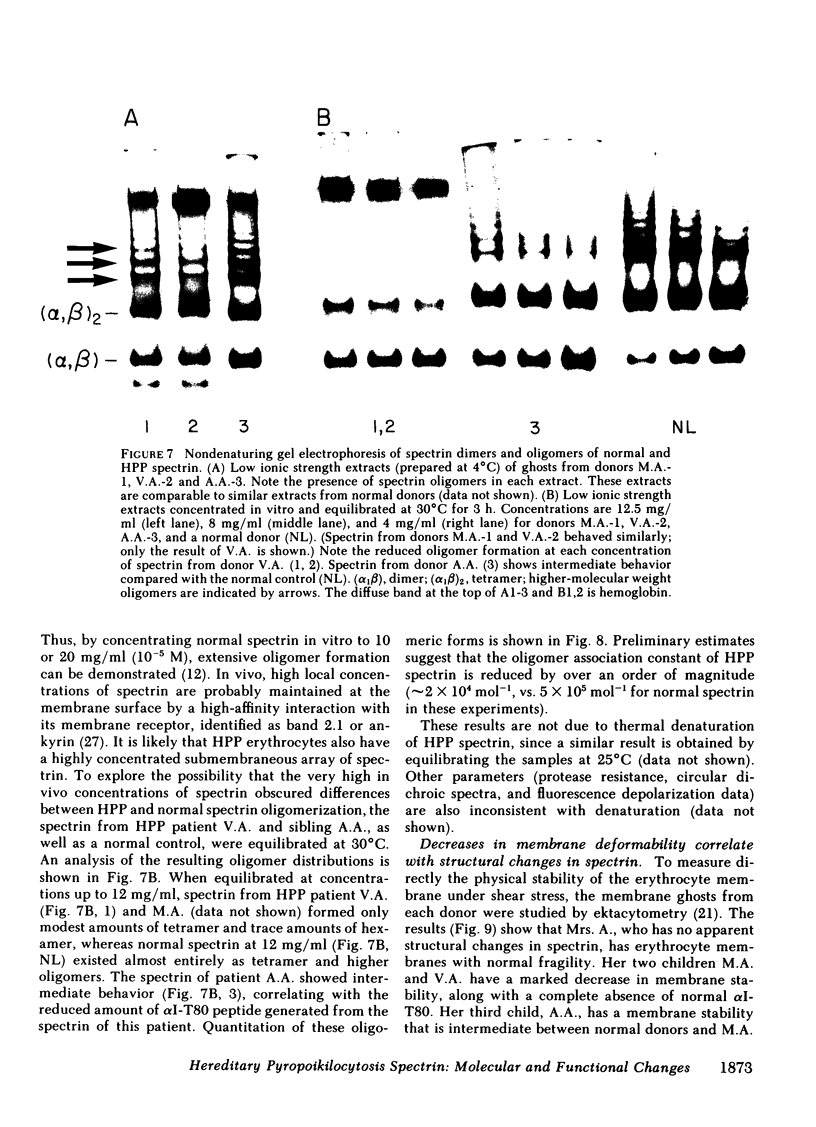
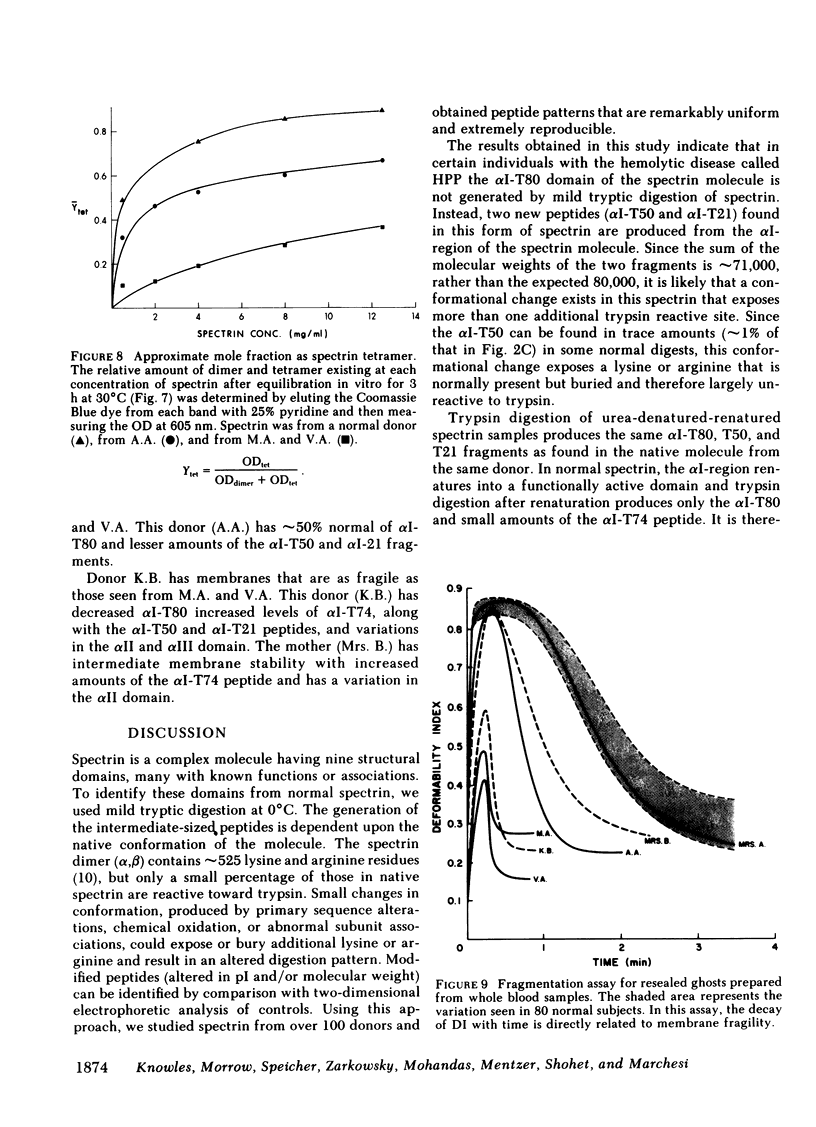
The structural and functional properties of spectrin from normal and hereditary pyropoikilocytosis (HPP) donors from the two unrelated families were studied. The structural domains of the spectrin molecule were generated by mild tryptic digestion and analyzed by two-dimensional electrophoresis (isoelectric focusing; sodium dodecyl sulfate-polyacrylamide gel electrophoresis). The alpha I-T80 peptide (Mr 80,000) is not detectable in two related HPP donors; instead, two new peptides (Mr 50,000 and 21,000) are generated and have been identified as fragments of the normal alpha I-T80. A third sibling has reduced levels of both the normal alpha I-T80 and the two new peptides. A similar analysis of spectrin from another HPP family indicates that their spectrins contain reduced amounts of the alpha I-T80 and the 50,000 and 21,000 fragments of the alpha I domain. The HPP donor also has other structural variations in the alpha I, alpha II, and alpha III domains. The alpha I-T80 domain of normal spectrin has been shown to be an important site for spectrin oligomerization (J. Morrow and V.T. Marchesi. 1981. J. Cell Biol. 88: 463-468), and in vitro assays indicate that HPP spectrin has an impaired ability to oligomerize. Ghost membranes from HPP donors are also more fragile than membranes from normal erythrocytes when measured by ektacytometry. In both the oligomerization and fragility assays, the degree of impairment is correlated with the amount of normal alpha I-T80 present in the spectrin molecule. We believe that a structural alteration in the alpha I-T80 domain perturbs normal in vivo oligomerization of spectrin, producing a marked decrease in erythrocyte stability.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Brandts J. F., Erickson L., Lysko K., Schwartz A. T., Taverna R. D. Calorimetric studies of the structural transitions of the human erythrocyte membrane. The involvement of spectrin in the A transition. Biochemistry. 1977 Jul 26;16(15):3450–3454. doi: 10.1021/bi00634a024. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Spectrin-actin interaction. Phosphorylated and dephosphorylated spectrin tetramer cross-link F-actin. J Biol Chem. 1979 Sep 10;254(17):8620–8627. [PubMed] [Google Scholar]
- Chang K., Williamson J. R., Zarkowsky H. S. Effect of heat on the circular dichroism of spectrin in hereditary pyropoikilocytosis. J Clin Invest. 1979 Jul;64(1):326–328. doi: 10.1172/JCI109456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek J. B., Singer S. J. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979 Jan;16(1):149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- Humphries S. E., Whittall R., Minty A., Buckingham M., Williamson R. There are approximately 20 actin gene in the human genome. Nucleic Acids Res. 1981 Oct 10;9(19):4895–4908. doi: 10.1093/nar/9.19.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Lux S. E., Wolfe L. C. Inherited disorders of the red cell membrane skeleton. Pediatr Clin North Am. 1980 May;27(2):463–486. doi: 10.1016/s0031-3955(16)33862-7. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Spectrin: present status of a putative cyto-skeletal protein of the red cell membrane. J Membr Biol. 1979 Dec 14;51(2):101–131. doi: 10.1007/BF01869164. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Haigh W. B., Jr, Marchesi V. T. Spectrin oligomers: a structural feature of the erythrocyte cytoskeleton. J Supramol Struct Cell Biochem. 1981;17(3):275–287. doi: 10.1002/jsscb.380170308. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palek J., Liu S. C., Liu P. Y., Prchal J., Castleberry R. P. Altered assembly of spectrin in red cell membranes in hereditary pyropoikilocytosis. Blood. 1981 Jan;57(1):130–139. [PubMed] [Google Scholar]
- Ralston G. B. The isolation of aggregates of spectrin from bovine erythrocyte membranes. Aust J Biol Sci. 1975 Jun;28(3):259–266. doi: 10.1071/bi9750259. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P. Integral membrane protein interaction with Triton cytoskeletons of erythrocytes. Biochim Biophys Acta. 1979 Oct 19;557(1):122–134. doi: 10.1016/0005-2736(79)90095-6. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Identification of proteolytically resistant domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5673–5677. doi: 10.1073/pnas.77.10.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S., Mohandas N., Speaker C. B., Shohet S. B. A congenital haemolytic anaemia with thermal sensitivity of the erythrocyte membrane. Br J Haematol. 1975 Apr;29(4):537–543. doi: 10.1111/j.1365-2141.1975.tb02740.x. [DOI] [PubMed] [Google Scholar]