Abstract
The endocervical epithelium is a major reservoir for Chlamydia trachomatis in women, and genital infections are extended in their duration. Epithelial cells act as mucosal sentinels by secreting cytokines and chemokines in response to pathogen challenge and infection. We therefore determined the signature cytokine and chemokine response of primary-like endocervix-derived epithelial cells in response to a common genital serovar (D) of C. trachomatis. For these studies, we used a recently-established polarized, immortalized, endocervical epithelial cell model (polA2EN) that maintains, in vitro, the architectural and functional characteristics of endocervical epithelial cells in vivo including the production of pro-inflammatory cytokines. PolA2EN cells were susceptible to C. trachomatis infection, and chlamydiae in these cells underwent a normal developmental cycle as determined by a one-step growth curve. IL1α protein levels were increased in both apical and basolateral secretions of C. trachomatis infected polA2EN cells, but this response did not occur until 72 hours after infection. Furthermore, protein levels of the pro-inflammatory cytokines and chemokines IL6, TNFα and CXCL8 were not significantly different between C. trachomatis infected polA2EN cells and mock infected cells at any time during the chlamydial developmental cycle up to 120 hours post-infection. Intriguingly, C. trachomatis infection resulted in a significant decrease in the constitutive secretion of T cell chemokines IP10 and RANTES, and this required a productive C. trachomatis infection. Examination of anti-inflammatory cytokines revealed a high constitutive apical secretion of IL1ra from polA2EN cells that was not significantly modulated by C. trachomatis infection. IL-11 was induced by C. trachomatis, although only from the basolateral membrane. These results suggest that C. trachomatis can use evasion strategies to circumvent a robust pro-inflammatory cytokine and chemokine response. These evasion strategies, together with the inherent immune repertoire of endocervical epithelial cells, may aid chlamydiae in establishing, and possibly sustaining, an intracellular niche in microenvironments of the endocervix in vivo.
Keywords: Chlamydia, cytokine, endocervix, epithelial, immune, pro-inflammatory
1. Introduction
Genital serovariants (D-K) of the obligate intracellular bacterium Chlamydia trachomatis are the world's most common sexually transmitted bacterial pathogens, accounting for an estimated 90 million new cases reported annually [1]. C. trachomatis exhibits a tropism for the columnar epithelial cells of the genital mucosae, with the endocervix being the most commonly infected site in women. In a proportion of infected women, organisms also ascend into the endometrium and Fallopian tubes where chronic infection can lead to devastating reproductive consequences, including pelvic inflammatory disease (PID), tubal infertility, and ectopic pregnancy, all of which result from immune mediated damage [1]. The reason why C. trachomatis can cause extended infections, lasting months to years in the face of an immune response [2-6], is not well understood, but does suggest the organism can adapt to, or evade, elements of the local host immune response.
Chlamydiae have a biphasic developmental cycle that begins when non-metabolically active, infectious, elementary bodies (EBs) encounter the apical surface of polarized epithelial cells. Following entry into the host cell, EBs escape lysosomal fusion, and endosomes containing EBs fuse to form the membrane bound vacuole termed an inclusion. EBs differentiate into metabolically active, non-infectious reticulate bodies (RBs) that undergo DNA replication and binary fission. RBs then re-differentiate into EBs that may then escape the host cell through lysis or extrusion mechanisms [7, 8].
Traditional methods for culturing C. trachomatis in vitro utilize either murine fibroblast cell lines or the ectocervix derived cervical carcinoma cell line (HeLa). Recent studies, however, have highlighted the importance of the cell type in which chlamydiae are grown, as cell lines derived from different anatomical sites yield different growth rates and infectious yields [9, 10]. Neither HeLa cells nor murine fibroblast cells accurately represent the target cells in vivo, the polarized columnar epithelial cells, with respect to morphology, expression of innate immune mediators or responsiveness to TLR agonists [11, 12]. Furthermore, innate immune signaling pathways have been altered over the years during the time that HeLa cells have been grown in vitro[13, 14]. Alterations in innate immune pathways are significant, as it has been hypothesized that the epithelial cells, generally believed to be the only sites of chlamydial replication in the female reproductive tract (FRT), are responsible for eliciting and sustaining the inflammatory immune cascade associated with chlamydial disease via the release of intracellular IL1α upon chlamydiae induced host cell lysis [15, 16]. Therefore, traditional cell lines may not be the optimal model systems in which to investigate the innate immune cascade elicited by C. trachomatis infected epithelial cells.
In recent years the orientation of the cells used to culture chlamydiae has been shown to influence chlamydial biology. Columnar epithelial cells, the target cells for chlamydial infection, maintain functionally distinct apical and basolateral membrane domains that are separated by tight junctions. Epithelial cells grown in a polarized orientation contain greater nutrient pools that are important for chlamydial growth, such as tryptophan, than their traditionally-grown submerged cell counterparts grown on plastic surfaces [17]. The use of polarized epithelial cell culture models for chlamydial studies, pioneered by Wyrick, has also revealed differences in chlamydial entry and exit mechanisms, infectious progeny, duration of the developmental cycle, infectivity, duration of the persistent growth state, reactivity to antibiotics, responsiveness to female sex steroid hormones, and innate inflammatory responses (reviewed in [18]). All of these parameters of chlamydial biology may also influence the subsequent innate epithelial immune response to the bacteria as well, although this has not yet been investigated in more primary-like genital epithelial cells.
We recently developed an epithelial cell model derived from human endocervical tissue (A2EN cells). A2EN cells polarize and appropriately express many of the functional proteins of the endocervical epithelium in vivo such as hormone receptors, mucins, anti-microbial peptides, and cytokines [11]. The aim of this study was to determine the characteristics of, and the cytokine response to, C. trachomatis infection in polarized A2EN (polA2EN) cells in order to examine the potential role of the endocervical epithelium in initiating, sustaining and amplifying a pro-inflammatory immune response to this organism.
2. Materials and Methods
2.1 Epithelial Cell Culture
The A2EN human endocervical epithelial cell line was originally generated in our laboratory from primary epithelial cells grown out from an endocervical explant and immortalized using human papilloma virus genes E6 and E7, as recently described [11, 12]. A2EN cells were grown in a phenol red-free serum-free, medium (EpiLife; Cascade Biologics) with a defined growth supplement, and seeded onto 0.4μm transwell inserts under differentiation conditions to induce polarization, as previously described [11]. For comparative studies, primary endocervical epithelial cells were generated from endocervical tissue explants obtained from discarded hysterectomy tissue, as previously described [11, 12]. Collection of hysterectomy tissue was approved by the Louisiana State University Health Sciences Center Institutional Review Board. Primary and A2EN cells were seeded onto transwell membrane inserts, and polarized, as previously described [11].
HeLa 229 cells (CCL2) were obtained from the American Type Culture Collection (ATCC) and were grown in RPMI1640 supplemented with 10% fetal bovine serum.
All epithelial cells were grown and maintained in an atmosphere of 5% CO2 at 37°C.
2.2 Chlamydia trachomatis Infection
PolA2EN or primary endocervical epithelial cells were infected with laboratory adapted biovariants: C. trachomatis serovar D (D/UW3/Cx) or serovar L2/434/Bu that were purified using Opti-Prep Density Gradient Medium (Sigma) [19] and diluted in sucrose-phosphate-glutamic acid buffer (SPG). Using the rocking method for infection, culture medium was removed from cells, and chlamydial inoculum in SPG was overlayed onto cells for 2 hours at 37°C with rocking. SPG containing chlamydial inoculum was then removed, and fresh culture medium added. For centrifugation protocols, chlamydial inoculum in SPG was placed directly into the culture medium, and cultures were centrifuged for 40 minutes at 1825xg at 35°C. Infected cells were then incubated for up to 72 hours at 37°C in culture medium. Cyclohexamide (2μg/mL) was added to the medium for infectivity assays only.
2.3 Inactivation of C. trachomatis Using Ultraviolet Light
Live C. trachomatis serovar D in SPG were placed in 6 well plates (1 mL of stock per well) under a laminar flow biocabinet on a rocker at a distance of 76cm from a 30 Watt ultraviolet light. Chlamydiae were rocked for 10 minutes under the UV light, sonicated using a water bath sonicator for 15 seconds to break up clumps of chlamydial particles, aliquoted, and stored at -80°C. Monolayers of HeLa 229 cells were infected with 10-fold dilutions of UV inactivated chlamydial stocks in SPG for 48 hours, fixed, and immuno-stained for chlamydial LPS to confirm that the UV-inactivated chlamydial particles were not viable and did not form inclusions. PolA2EN cells were exposed to an amount of UV inactivated chlamydiae equivalent to the amount of live bacteria required for a >95% infection rate.
2.4 C. trachomatis Infectivity and One Step Growth Curve Assays
Monolayers of HeLa 229 cells and polA2EN cells were infected with 0.125, 0.25, 0.5, and 1.0μL of C. trachomatis serovar D semi-purified inoculum using the protocol in section 2.2 with cyclohexamide. Infected cells were fixed with 4% paraformaldehyde for 10 minutes at room temperature followed by permeabilization with 0.1% Triton X in PBS for 10 minutes. Fixed cells were then blocked with Background Sniper (Biocare) for 30 minutes at room temperature, and stained with a FITC conjugated anti-chlamydial LPS antibody (Meridian Diagnostics) for 30 minutes at room temperature, followed by washing with PBS. Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Mol. Probes) for 1 minute at room temperature followed by washing with PBS. Membranes from polarized cultures were excised and mounted using ProLong Gold Mounting Media (Mol. Probes). Cell numbers and inclusion numbers were counted in 10 microscopic fields at 400× to obtain percent infectivity values. Using the results from this assay, all subsequent experiments for this manuscript were performed such that cells were infected with an amount of C. trachomatis inoculum that yielded a >95% infection rate to determine the direct effects of chlamydiae on the innate cytokine response in infected cells rather than infected and bystander non-infected cells.
In order to establish C. trachomatis growth curves, polA2EN cells were infected with C. trachomatis serovar D to yield a >95% infection rate. Infected cells were scraped and sonicated for 15 seconds using a water bath sonicator at 0, 12, 24, 36, 48, and 72 hours post infection. Ten-fold dilutions were performed in SPG and used to infect monolayers of HeLa 229 cells as described in section 3.3.2. Infected HeLa 229 cells were fixed 48 hours post-infection and stained with a FITC conjugated anti-chlamydial LPS antibody (Meridian Diagnostics) in order to calculate inclusion forming units (IFU), as previously described [20].
2.5 Transepithelial Electrical Resistance Measurements
A2EN cells were seeded onto transwell membrane inserts as previously described [11]. Five days post-differentiation, cells were infected with C. trachomatis serovar D to yield >95% infection rate. Transepithelial electrical resistance (TEER) measurements were obtained from mock-infected polA2EN cells and C. trachomatis infected polA2EN cells using a voltohmeter (World Precision Instruments) every 24 hours from day 1 post-differentiation to day 9 post differentiation [11].
2.6 Microscopy and Imaging
2.6.1 Immunofluorescence Microscopy
C. trachomatis serovar D-infected polA2EN cells were fixed at 48 hours post-infection and stained using a FITC-conjugated anti-chlamydial LPS antibody (Meridian Diagnostics), and counterstained with DAPI as described in section 3.3.4. Fluorescent images were captured using a Leica DMRXA automated upright epifluorescence microscope (Leica Microsystems, Bannockburn, IL), a Sensicam QE chargecoupled device (Cooke Corp., Auburn Hills, MI), and filter sets optimized for Alexa 488 (exciter HQ480/20, dichroic Q495LP, and emitter HQ510/20m) and 4′,6-diamidino-2-phenylindole (exciter 360/40x, dichroic 400DCLP, and emitter GG420LP). Z-axis plane capture, deconvolution, and analyses were performed with Slidebook™ deconvolution software (Intelligent Imaging Innovations, Denver, CO).
2.6.2 Transmission Electron Microscopy
For ultrastructural studies, C. trachomatis serovar D infected polA2EN cells were fixed in 2% paraformaldehyde/2.5% glutaraldehyde in 200mM phosphate buffer for 1 hour at room temperature, followed by washing with PBS three times [21]. Samples were then processed and imaged for transmission electron microscopy as previously described [21].
2.7 Cytometric Bead Array Assay
HeLa 229 cells were infected with either C. trachomatis serovar D or LGV2 to achieve a >95% infection rate. Supernatants from infected HeLa 229 cell monolayers were collected from both mock-infected and C. trachomatis-infected cells at 24, 36, 48, and 72 hours post-infection and analyzed for IL6 and CXCL8 secretion using a cytometric bead assay (Millipore).
PolA2EN cells were infected with C. trachomatis serovars D and LGV2 to achieve a >95% infection rate. Supernatants from apical and basolateral chambers from both infected and mock-infected cells were collected over time at 24, 36, 48, and 72 hours post-infection and analyzed with a cytometric bead array that initially included (i) the pro-inflammatory cytokines Interleukin (IL)1α, IL1 β, IL6, Tumor Necrosis Factor α (TNFα), IL17, Interferon γ (IFNγ), IL12(p70); (ii) Th2 cytokines IL4, IL5, IL7, IL9, IL10, IL15; (iii) the chemokines Eotaxin, CXCL8, Macrophage Inflammatory Protein (MIP)1α, MIP1β, Interferon Inducible Protein 10 (IP10), Regulated upon Activation, Normal T-cell Expressed (RANTES or CCL5), and Monocyte Chemotactic Protein (MCP)-1, and (iv) the immunoregulatory cytokines IL11, IL13, and IL1 receptor antagonist (IL1ra). Both active and inactive forms of the regulatory cytokines transforming growth factor beta (TGFβ) 1, 2, and 3 were also assayed. Inactive TGFβ was examined by treating the supernatant samples with hydrochloric acid (HCl) followed by neutralization with sodium hydroxide (NaOH) as previously described [22]. Granulocyte Colony Stimulating Factor (G-CSF) and Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) were also measured. All cytokines and chemokines were measured in a either a BioPlex cytometric bead array kit (BioRad) or a Milliplex cytometric bead array kit (Millipore) according to the manufacturer's instructions. Only those cytokines that were detectable by cytometric bead array and are known to be secreted by epithelial cells were chosen for subsequent experiments.
Polarized and non-polarized primary endocervical epithelial cells were infected with C. trachomatis serovar D to achieve a >95% infection rate. Supernatants were collected from mock-infected and C. trachomatis-infected cells at 72 hours post-infection and analyzed with a cytometric bead array that included pro-inflammatory cytokines and chemokines: IL1α, IL1β, IL6, TNFα, CXCL8, MIP1β, IP10, RANTES, G-CSF, and GM-CSF (Millipore).
2.8 Lysate and Supernatant Experiments
Mock-infected and C. trachomatis serovar D-infected polA2EN cells were scraped into culture medium at 24, 48, and 72 hours post-infection. Scraped cells were then sonicated for 30 seconds in a water bath sonicator to release all intracellular proteins. Lysates were then passed through a 0.2μm syringe filter, followed by a 0.1μm syringe filter to remove any chlamydial particles.
Apical and basolateral supernatants from mock-infected and C. trachomatis serovar D-infected polA2EN cells were also collected and filtered using the same protocol described above. Lysates and supernatants were added to non-infected polA2EN cells for 4 hours at 37°C. Lysates and apical supernatants were added to the apical chambers of polA2EN cells, whereas basolateral supernatants were also added to the basolateral chambers of polA2EN cells. Following incubation with lysates or supernatants, polA2EN cells were washed with PBS twice, fresh culture medium was added, and then were incubated for 12 hours at 37°C. Apical and basolateral supernatants were then collected and analyzed with a cytometric bead array that included the pro-inflammatory cytokines IL1α, IL1β, IL6, TNFα, CXCL8, MIP1β, IP10, RANTES, G-CSF, and GM-CSF. Apical and basolateral supernatants from untreated polA2EN cells were used as controls for baseline cytokine secretion. Lysate and supernatant experiments were designed based on previous experiments performed by Rasmussen et al.[15].
2.9 Statistics
For cytometric bead array assays, statistical analyses were completed using Prism software (v4.0; GraphPad, San Diego, CA). One-way analyses of variance (ANOVA) with Bonferroni posttests were employed. A P-value of <0.05 was considered significant.
3. Results
3.1 Polarized A2EN cells are susceptible to C. trachomatis infection
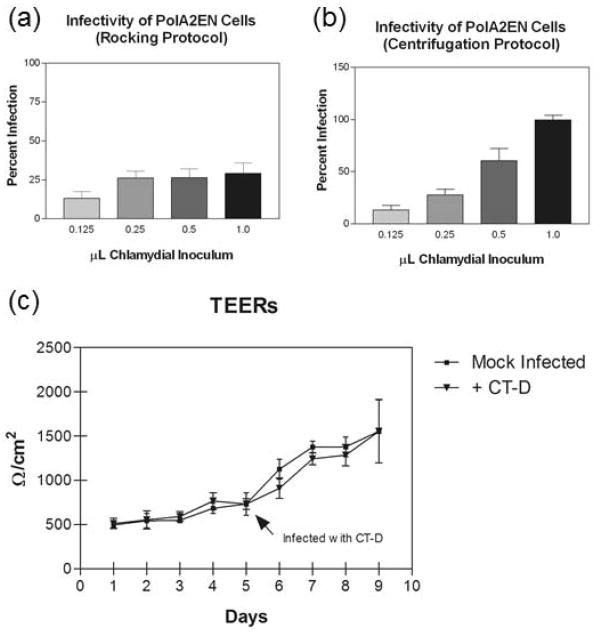
We recently developed a polarized, immortalized, endocervical epithelial cell model (polA2EN) that generates many innate immune mediators including mucin, anti-microbial peptides, cytokines and chemokines [11]. Since constitutively made innate immune mediators might interfere with C. trachomatis infection of polA2EN cells, the first objective of this study was to determine whether or not polA2EN cells could be infected with C. trachomatis. First, we utilized two methods to infect polA2EN cells with varying doses of chlamydial inoculum: the rocking method and the centrifugation method. Using the rocking method, we were only able to achieve approximately a 25% infection rate regardless of the dose used (Figure 1a) possibly because, similar to endocervical epithelial cells in vivo, polA2EN cells generate mucus and/or antimicrobial mediators [11], which may impede chlamydial attachment and entry. Using the centrifugation method, however, a >95% infection rate was consistently achieved (Figure 1b), although a higher volume of chlamydial inoculum was required to yield this infection rate compared to HeLa 229 cells (data not shown). Therefore, for all subsequent experiments in this study, the centrifugation method was employed to achieve a > 95% infection rate in polA2EN cells.
Figure 1. PolA2EN cells are susceptible to C. trachomatis serovar D infection, and chlamydiali infection does not significantly compromise the polarized monolayer.
(a) Using the rocking protocol, infection rates of C. trachomatis in polA2EN cells never exceeded approximately 25% no matter the dose of chlamydial inoculum used. (b) Using the centrifugation protocol for C. trachomatis infection, polA2EN cells were capable of being infected at an infection rate of >95% when 1.0μL chlamydial inoculum was used. No significant cell lysis was observed in infected polA2EN cells. Infection rates shown are mean levels ± the standard deviation (SD) from 3 independent experiments each performed in triplicate. (c) TEER values were not statistically significant between mock infected cells and C. trachomatis infected cells up to 4 days post-infection. Data points represent mean values ±SD pooled from 3 independent experiments each performed in triplicate.
It has been reported that C. trachomatis infection can result in the degradation of epithelial junctional complex proteins, such as nectin-1 [23], which may compromise the integrity of the epithelial cell barrier. We, therefore, verified that the polarized monolayers of A2EN cells were not significantly compromised upon these conditions of chlamydial infection, as this would interfere with our investigation of differential cytokine responses from the apical and basolateral membrane compartments of polA2EN cells. We measured TEERs over a period of 4 days after chlamydial infection, and although the mean TEERs were slightly lower in C. trachomatis infected cells at 24-72 hours post infection compared to mock infected cells, these differences were not statistically significant (Figure 1c). TEER measurements in both mock-infected and C. trachomatis-infected polA2EN cells remained above 1000Ω/cm2, which is the threshold by which we measure polarization [11]. These results suggest that the polarized monolayers of A2EN cells can remain relatively intact up to 4 days after chlamydial infection.
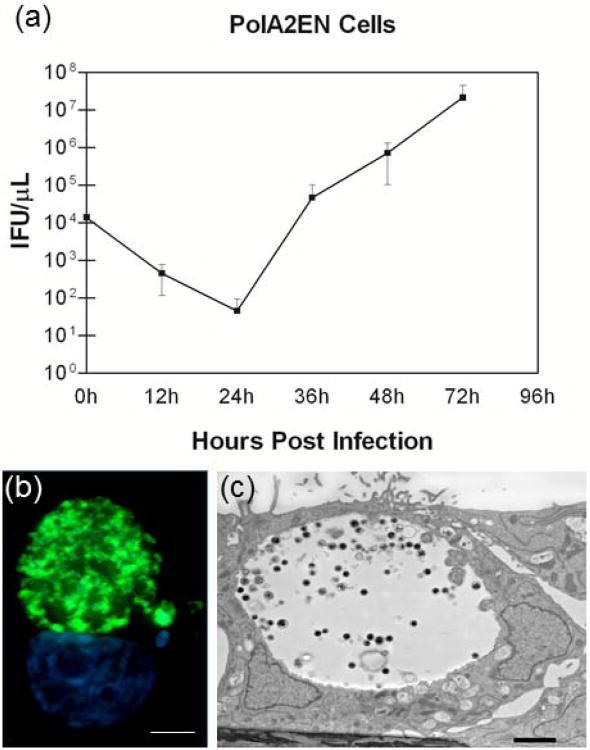
In order to verify that chlamydiae underwent a normal developmental cycle in polA2EN cells, we employed a one-step growth curve technique using C. trachomatis serovar D (Figure 2a). Chlamydiae grown in polA2EN cells exhibited a latency period up to 24 hours post-infection followed by a rapid recrudescence of infectious organisms with titers peaking around 48-72 hours. Although fewer infectious EBs entered polA2EN cells compared to HeLa 229 cells, chlamydiae underwent robust replication that yielded infectious EBs equivalent to numbers of infectious EBs observed in infected HeLa cells (HeLa cell data not shown). These results suggest that the polarized orientation of the A2EN cells creates an intracellular environment that allows for rapid productive replication of chlamydial particles, similar to that reported in polarized endometrial cells [9]. In support of the TEER data, using light microscopy, we observed minimal host cell lysis even after 72 hours in C. trachomatis infected polA2EN cells similar to observations made on C. trachomatis infected trophoblast cells [24]. C. trachomatis infections were also carried out to 96 and 120 hours post-infection in polA2EN cells, and we observed no evidence of significant host cell lysis unlike that observed in infected HeLa cell cultures (data not shown) [7]. These results suggest that the cell type and polarized orientation of the cells may alter the mechanism of chlamydial exit as previously hypothesized by the Wyrick group [25].
Figure 2. C. trachomatis in polA2EN cells exhibits a normal growth pattern and morphology.
(a) IFUs at time 0h post-infection represent chlamydiae that have entered the cells after centrifugation. Values represent mean values ± SD pooled from 3 independent experiments each performed in triplicate. (b) PolA2EN cells were infected with C. trachomatis serovar D, fixed at 72 hours post-infection and immunostained with a FITC conjugated a-LPS antibody (green) and counterstained with DAPI (blue) (1000x magnification). Scale bar represents 5μm. (c) C. trachomatis infected polA2EN cells were fixed at 72 hours post-infection and processed for TEM. Dense particles representing EBs were visible. Scale bar represents 1μm.
Using light microscopy, inclusions were visible by 24 hours post infection in polA2EN cells. Morphological characteristics of chlamydiae at 72 hours post-infection in polA2EN cells are shown using immunostaining against chlamydial LPS, and TEM imaging, in Figure 2b and 2c. Inclusions observed in polA2EN cells contained numerous EB at 72 hours post-infection (Figure 2b, c) and were consistent with healthy chlamydial growth.
3.2 C. trachomatis does not initiate a robust inflammatory cytokine/chemokine response in polA2EN cells
HeLa cells infected with C. trachomatis LGV2 robustly up-regulate the secretion of pro-inflammatory cytokines, including IL6, CXCL8, GROα, GM-CSF, and IL1α [15, 26, 27]. We, first, confirmed these results in HeLa 229 cells using both C. trachomatis serovar D as well as LGV 2. Similar to results found in previous studies [15, 26, 28], we observed a significant up-regulation of both CXCL8 and IL6 by 48 hours following C. trachomatis infection with either serovariant (data not shown). Although we observed a significant up-regulation of pro-inflammatory cytokines in infected HeLa 229 cells, we did not observe the magnitude of up-regulation of pro-inflammatory cytokines (data not shown), particularly CXCL8 (∼23 fold), that Rasmussen et al. observed using C. trachomatis serovar D [15]. Our results were more consistent with results observed by Dessus-Babus et al. who observed a more modest up-regulation of CXCL8 and IL6 upon chlamydial infection of HeLa 229 cells [28].
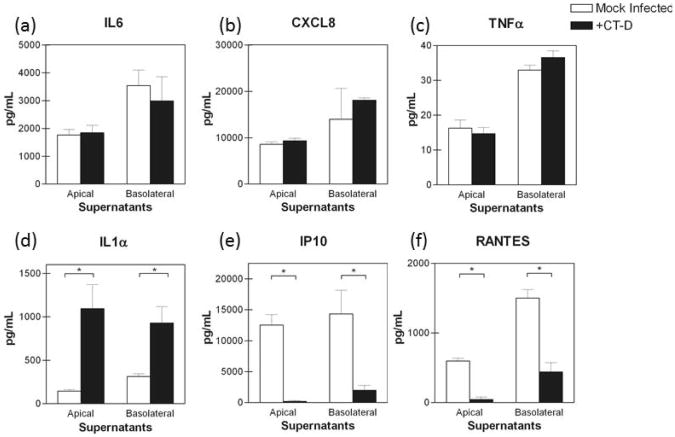
Next, the pro-inflammatory cytokine profiles in C. trachomatis serovar D infected polA2EN cells were determined. PolA2EN cells were infected with C. trachomatis serovar D to yield an infection rate of >95%. Apical and basolateral supernatants from C. trachomatis infected polA2EN cells were collected and subjected to cytometric bead array analyses. We initially assayed for an extended panel of cytokine analytes, which included IL1α, IL1β, IL2, IL4, IL5, IL6, IL7, CXCL8, IL9, IL12(p70), IL13, IL15, IL17, GM-CSF, G-CSF, TNFα, IFNγ, MIP1α, MIP1β, IP10, RANTES, MCP-1, and Eotaxin. Only those cytokines that were secreted by polA2EN cells were chosen for further studies. As described previously, polA2EN cells, similar to other primary-like epithelial cells [29, 30], constitutively secreted CXCL8, IL6, IP10, and RANTES (Figure 3a,b,e,f) as well as G-CSF and GM-CSF (data not shown) [11]. Unlike the results observed in C. trachomatis infected HeLa cells by Rasmussen et al., we observed no significant up-regulation of IL6, CXCL8 or TNFα at any timepoint over the course of the developmental cycle and up to 120 hours post-infection (Figure 3a-c; Supplemental Figure 1a-c). Similar to infected HeLa cells [15], we observed a modest but significant increase in IL1α secretion (Figure 3d, Supplemental Figure 1c) (approximately 4 fold; p<0.001), and this did not occur until 72 hours post-infection. Intriguingly, we consistently found a specific, and highly significant, decrease in the constitutive secretion of IP10 and RANTES by 72 hours post-infection that lasted up to 120 hours post-infection. The latter results suggest that C. trachomatis may actively modulate these T cell chemokines (Figure 3e,f; Supplemental Figure 1e,f; p<0.001). We did not observe a significant modulation in secretion of any other analytes measured upon C. trachomatis infection even when the inoculum dose was increased up to 4 times the amount necessary to achieve >95% infection (representative CXCL8 graph shown in Supplemental Figure 2).
Figure 3. C. trachomatis fails to elicit a robust pro-inflammatory cytokine response and decreases T cell chemokine secretion in polA2EN cells.
PolA2EN cells were infected with C. trachomatis serovar D (>95% infection rate). Apical and basolateral supernatants were subjected to cytometric bead array analyses for (a) IL6, (b) CXCL8, (c) TNFα, (d) IL1α, (e) IP10, and (f) RANTES. Graphs shown represent the 72 hours post-infection; cytokine values represent mean values ± SD. Graphs shown are representative graphs from 3 independent experiments each performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a One-way ANOVA (*p<0.001).
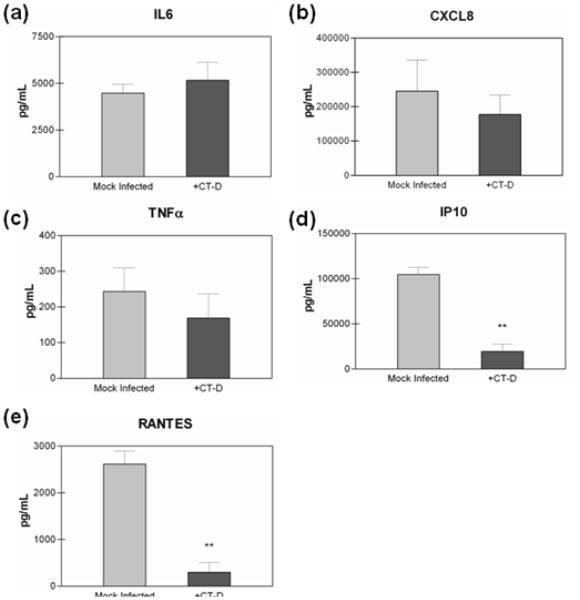
We next investigated whether polarization of the A2EN cells contributed to the lack of pro-inflammatory cytokine secretion upon C. trachomatis infection. We, therefore, infected A2EN cells grown submerged in traditional plastic culture plates at an infection rate of >95%. Similar to results observed in C. trachomatis infected polA2EN cells, infection of non-polarized A2EN cells failed to result in a significant up-regulation in IL6, CXCL8 or TNFα (Figure 4a,b,c). Also similar to results observed in C. trachomatis infected polA2EN cells, IP10 and RANTES were decreased in C. trachomatis infected non-polarized A2EN cells (Figure 4d,e). These results suggest that the polarized orientation of A2EN cells does not alter the pro-inflammatory immune response.
Figure 4. C. trachomatis fails to elicit a pro-inflammatory cytokine response, and decreases T cell chemokines in A2EN cells grown in submerged culture.
A2EN cells grown in submerged culture were infected with C. trachomatis serovar D (>95% infection rate). Supernatants were collected at 72 hours post-infection and subjected to cytometric bead analyses for (a) IL6, (b) CXCL8, (c) TNFα, (d) IP10, and (e) RANTES. Cytokine measurements represent mean values ± SD. Graphs shown are representative graphs from 2 independent experiments each performed in triplicate. Statistical comparisons were performed between mock infected cell and infected cell parameters using a one-way ANOVA (**p<0.001).
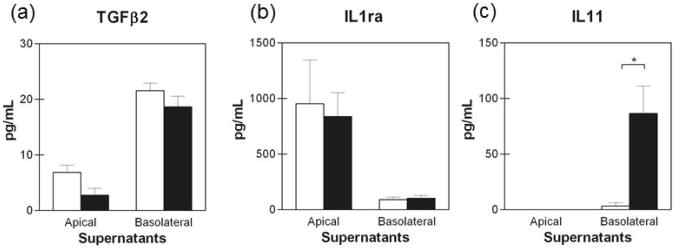
Because no significant pro-inflammatory cytokine response was observed in C. trachomatis serovar D infected polA2EN cells, we determined whether cytokines involved in anti-inflammatory processes might be modulated upon infection. We analyzed TGFβ1, 2, and 3 as well as IL1ra, IL11, IL13, and IL10 secretion upon C. trachomatis infection. PolA2EN cells did not secrete TGFβ 1 or 3 (data not shown). PolA2EN cells also did not secrete detectable levels of IL13, or IL10 (data not shown). TGFβ2 was detectable in polA2EN cell secretions, however it was unaltered upon C. trachomatis infection (Figure 5a). PolA2EN cells secreted high constitutive levels of IL1ra at their apical surface, however secretion was unaltered upon C. trachomatis infection (Figure 5b). Interestingly, the pleiotropic, anti-inflammatory cytokine IL11 was significantly up-regulated only in the basolateral secretions from C. trachomatis infected polA2EN cells (Figure 5c, p<0.001) and was undetectable in apical secretions.
Figure 5. C. trachomatis elicits an IL11 response, but not TGFβ or IL1ra in polA2EN cells.
PolA2EN cells were infected with C. trachomatis serovar D (>95% infection rate). Apical and basolateral supernatants were collected at 72 hours post-infection and subjected to cytometric bead array analyses for (a) TGFβ2, (b) IL1ra, and (c) IL11. Cytokine values represent mean values ± SD pooled from 3 independent experiments each performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a One-way ANOVA (* p<0.001).
We also determined whether the modest pro-inflammatory cytokine secretion in response to C. trachomatis was serovar specific as disseminating biovariants (LGV) and non-disseminating biovariants (D-K) have been shown to elicit different innate immune responses [15, 28]. Similar to what we observed using serovar D, polA2EN cells did not up-regulate IL6 or CXCL8 secretion upon LGV2 infection (data not shown). We also observed a decrease in IP10 and RANTES secretion upon C. trachomatis LGV2 infection (data not shown).
3.3 C. trachomatis infected primary endocervical epithelial cells have a similar cytokine profile to infected polA2EN cells
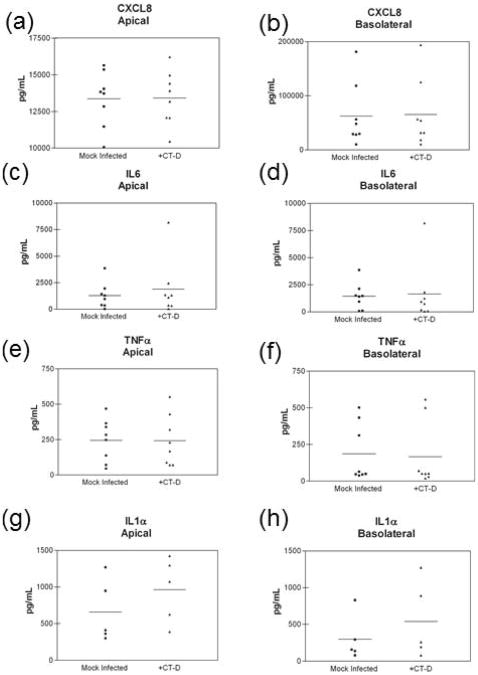
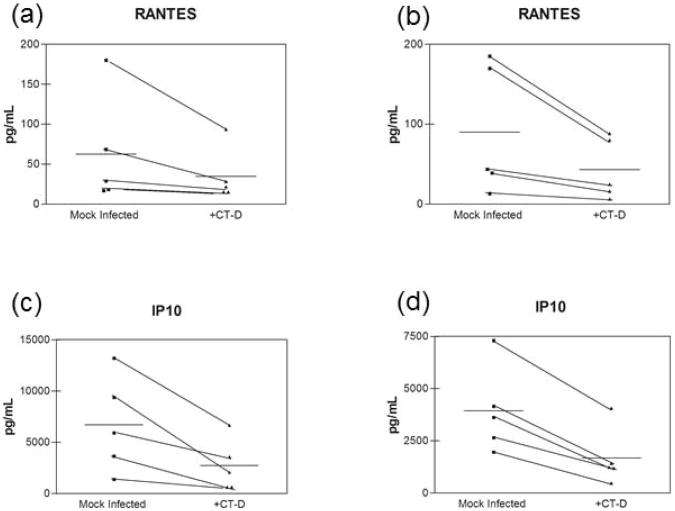
We next investigated whether the modest pro-inflammatory cytokine secretion and the decrease in IP10 and RANTES were an artifact of using an immortalized endocervical epithelial cell model. We therefore utilized polarized primary endocervical epithelial cells and infected them with C. trachzomatis serovar D at >95% infection rate. Polarized primary endocervical epithelial cells did not significantly up-regulate the pro-inflammatory cytokines and chemokines CXCL8, IL6, and TNFα in response to C. trachomatis in either apical or basolateral secretions (Figure 6a-f). IL1α secretion was increased in some patients' cells, while levels in other cells remained unchanged upon C. trachomatis infection (Figure 6g,h). Similar to results observed in polA2EN cells, we also observed a decrease in the T cell chemokines IP10 and RANTES secretion in polarized primary endocervical epithelial cells infected with C. trachomatis (Figure 7a-d). The decrease in IP10 and RANTES secretion we observed in polarized primary endocervical epithelial cells support and validate the results generated in polA2EN cells.
Figure 6. C. trachomatis infected primary endocervical epithelial cells exhibit a similar cytokine pattern to infected polA2EN cells.
Primary endocervical epithelial cells (n=5-8) were infected with C. trachomatis serovar D (>95% infection rate). Apical and basolateral supernatants were collected at 72 hours post-infection and subjected to cytometric bead array analyses for (a,b) CXCL8, (c,d) IL6, (e,f) TNFα, and (g,h) IL1α. Cytokine values represent mean values. Each experiment was performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a Wilcoxon nonparametric matched pairs test.
Figure 7. C. trachomatis infected primary endocervical epithelial cells exhibit a decrease in IP10 and RANTES.
Primary endocervical epithelial cells (n=5) were infected with C. trachomatis serovar D (>95% infection rate). Apical and basolateral supernatants were collected at 72 hours post-infection and subjected to cytometric bead array analyses for (a,b) IP10, and (c,d) RANTES, Cytokine values represent mean values. Overall mean indicated by horizontal line. Each experiment was performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a Wilcoxon nonparametric matched pairs test. Statistical analyses revealed that the decrease in both IP10 and RANTES was approaching significance (p<0.06).
3.4 UV inactivated C. trachomatis does not elicit an IL-1α response, nor significantly decreases the IP10 and RANTES response in polA2EN cells
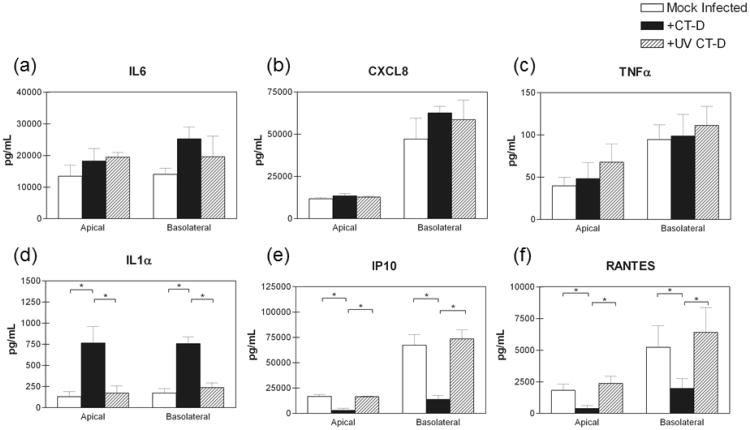
We next investigated whether the modest pro-inflammatory cytokine response and the reduction in T cell chemokine secretion required productive chlamydial infection. We inactivated chlamydiae using ultraviolet light which inhibits active growth of the bacteria, while maintaining the structural integrity of molecules or pattern associated molecular patterns (PAMPs) on the bacteria's surface that may interact with pattern recognition receptors (PRRs). Similar to experiments performed with live, active bacteria, UV inactivated chlamydiae did not elicit a robust CXCL8, IL6, or TNFα response (Figure 8a-c). Interestingly, UV inactivated chlamydiae also did not increase IL1α secretion in polA2EN cells (Figure 8d), suggesting that a productive infection is needed in order to elicit this response similar to what has been previously reported [15, 31]. UV inactivated chlamydiae also did not abrogate IP10 and RANTES secretion in polA2EN cells (Figure 8e,f), also suggesting that a productive chlamydial infection is necessary for the bacteria to cause the decrease in T cell chemokine secretion in polA2EN cells.
Figure 8. UV inactivated chlamydiae do not elicit a pro-inflammatory cytokine response, but also do not decrease IP10 and RANTES secretion from polA2EN cells.
PolA2EN cells were infected with C. trachomatis serovar D (>95% infection rate) or exposed to an equivalent amount of UV inactivated chlamydiae. Mock infected polA2EN cells were utilized to ascertain baseline cytokine levels. Apical and basolateral supernatants were collected at 72 hours post-infection or exposure and subjected to cytometric bead array analyses for (a) IL6, (b) CXCL8, (c) TNFα, (d) IL1α, (e) IP10, and (f) RANTES, Cytokine values represent mean values ± SD from 3 independent experiments each performed in triplicate. Statistical analyses between parameters were performed using a One Way ANOVA (** p<0.001).
3.5 Neither lysates nor supernatants from C. trachomatis infected polA2EN cells stimulate non-infected polA2EN cells to produce pro-inflammatory cytokines
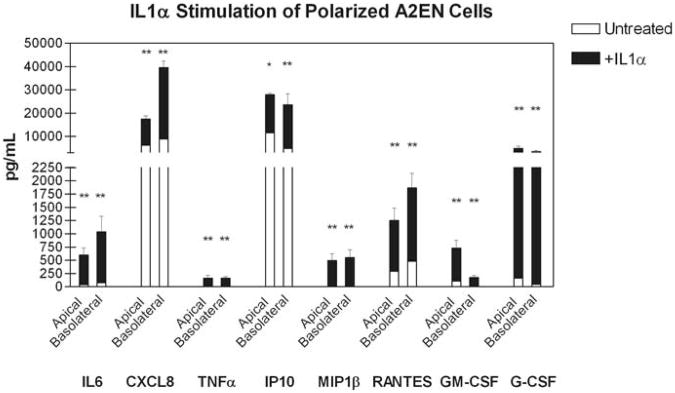
The previously reported robust secretion of CXCL8 following C. trachomatis infection in HeLa cells observed by Rasmussen et al. was suggested to be due to IL1α stimulation of bystander non-infected epithelial cells resulting in the amplification of the CXCL8 response [15]. Therefore, we first investigated whether polA2EN cells could respond to IL1α, as a lack of response to IL1α is a possible explanation for why polarized A2EN cells failed to up-regulate pro-inflammatory cytokines upon chlamydial infection. Figure 9 shows that, upon addition of recombinant IL1α, polarized A2EN cells significantly up-regulated the secretion of IL6, CXCL8, TNFα, IP10, MIP1β, RANTES, GM-CSF, and G-CSF in both apical and basolateral secretions. These results indicate that non-infected polA2EN cells can respond to IL1α to secrete pro-inflammatory cytokines.
Figure 9. PolA2EN cells respond to IL1α stimulation by up-regulating pro-inflammatory cytokines.
PolA2EN cells were exposed to 20ng/mL IL1α for 24 hours. Apical and basolateral supernatants were collected from untreated and treated cells and were subjected to cytometric bead array analyses for IL6, CXCL8, TNFα, IL1α, IP10, and RANTES, GM-CSF, and G-CSF. Cytokine values represent mean values ± SD from 3 independent experiments each performed in triplicate. Statistical analyses between untreated and treated parameters were performed using a One Way ANOVA (* p<0.05, ** p<0.001).
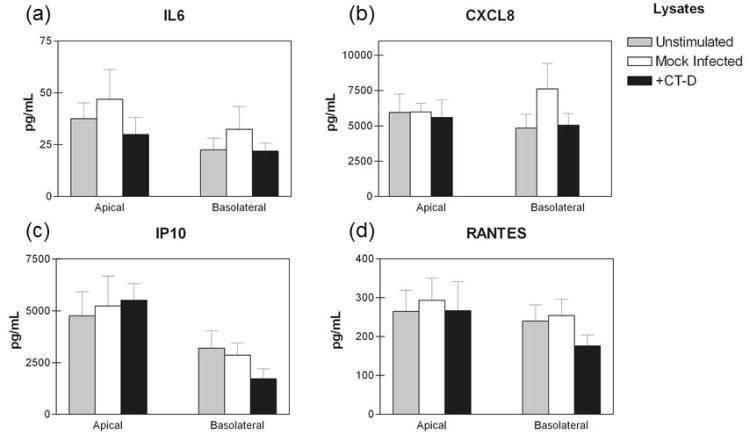
Ampification of the pro-inflammatory cytokine response by C. trachomatis infected HeLa cell cultures was demonstrated to be due to IL1α, which was assumed to be released upon host cell lysis [15]. While we also established that IL1α secretion was significantly increased in C. trachomatis infected polA2EN cells, we did not observe significant epithelial cell lysis up to 120 hours post-infection. We therefore investigated whether lack of cell lysis was responsible for the absence of pro-inflammatory cytokine amplification in infected polA2EN cells. We harvested C. trachomatis infected polA2EN cells at 24, 48, and 72 hours post-infection, sonicated and filtered them to remove chlamydial particles. We then added the filtered lysates to the apical surfaces of non-infected polA2EN cells for 4 hours. Following incubation, cells were washed, and fresh medium was added to both the apical and basolateral chambers for 12 hours. Apical and basolateral secretions were collected and assayed for cytokines and chemokines. The secretion of IL6 and CXCL8 was unaltered between unstimulated, mock-infected lysate and infected lysate treatment conditions (72 hour timepoint shown; 24 and 48 hour timepoints not shown; Figure 10a,b). These results suggest that intracellular components, such as IL1α, released upon host cell lysis during chlamydial infection are unable amplify the pro-inflammatory cytokine response in polA2EN cells. The secretion of IP10 and RANTES was also unaltered between unstimulated, mock-infected lysate and infected lysate treatment conditions (72 hour timepoint shown; 24 and 48 hour timepoints not shown; Figure 10c,d), suggesting that host cell lysis and the components released by infected cells do not down-modulate these T cell chemokines. There were no statistically significant differences between experimental groups for all other analytes measured.
Figure 10. Lysates from C. trachomatis infected polA2EN cells do not amplify the pro-inflammatory cytokine response in non-infected cells.
PolA2EN cells were exposed to filtered lysates from mock infected and C. trachomatis serovar D infected polA2EN cells for 4 hours. Lysates were removed, and fresh medium was incubated with cells for 12 hours. Unstimulated polA2EN cells were utilized as a control. Apical and basolateral supernatants were collected and subjected to cytometric bead array analyses for (a) IL6, (b) CXCL8, (c) IP10, and (d) RANTES. Cytokine values represent mean values ± SD from 3 independent experiments each performed in triplicate. Statistical analyses between parameters were performed using a One Way ANOVA.
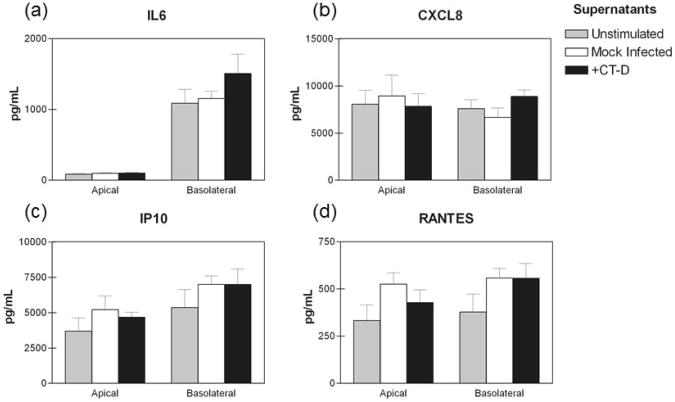
Finally, we determined if apical and basolateral secretions from C. trachomatis infected polA2EN cells could stimulate non-infected cells to modulate cytokine and chemokine secretion. We collected supernatants from C. trachomatis infected polA2EN cells, filtered them to remove chlamydial particles, and added the filtered supernatants to non-infected polA2EN cells for 4 hours. We added apical secretions to apical surfaces, since apical membrane surfaces are likely to be exposed to apical secretions from neighboring cells in vivo. Likewise, we treated basolateral surfaces of polA2EN cells with the collected basolateral supernatants. Similar to the lysate protocol, we removed the supernatants, washed, and incubated the cells for 12 hours with fresh medium. Similar to results observed using C. trachomatis infected cell lysates, there were no statistically significant differences between the experimental groups for IL6, CXCL8, IP10 or RANTES (Figure 11a-d). There were no statistically significant differences for all other analytes measured as well.
Figure 11. Supernatants from C. trachomatis infected polA2EN cells do not amplify the pro-inflammatory cytokine response in non-infected cells.
PolA2EN cells were exposed to filtered supernatants from mock infected and C. trachomatis serovar D infected polA2EN cells for 4 hours. Supernatants were removed, and fresh medium was incubated with cells for 12 hours. Unstimulated polA2EN cells were utilized as a control. Apical and basolateral supernatants were collected and subjected to cytometric bead array analyses for (a) IL6, (b) CXCL8, (c) IP10, and (d) RANTES. Cytokine values represent mean values ± SD from 3 independent experiments each performed in triplicate. Statistical analyses between parameters were performed using a One Way ANOVA.
3.5 Discussion
In order to investigate the endocervical epithelial cytokine response to C. trachomatis, we used a polarized, endocervical epithelial cell model that we could apically challenge, successfully infect, and subsequently differentiate apical and basolateral cytokine responses. We observed that C. trachomatis infection of polA2EN cells significantly increased IL1α in both apical and basolateral secretions, but, unlike other intracellular bacteria that induce a rapid and transient IL1α response [32], the response we observed did not occur until 72 hours post infection. We also found that a >95% C. trachomatis infection of polA2EN cells: (1) failed to up-regulate the secretion of the major pro-inflammatory cytokines CXCL8, IL6 or TNFα, in either apical or basolateral secretions at any time measured; (2) induced secretion of IL11, an anti-inflammatory, tissue repair cytokine, at the basolateral surface of polA2EN cells; and (3) significantly decreased the constitutive apical and basolateral secretion of IP-10 and RANTES in polA2EN cells. Overall, these observations suggest C. trachomatis can evade specific endocervical epithelial cytokine responses, and that these cells could provide a hospitable intracellular niche for genital C. trachomatis infection.
It was not entirely surprising that C. trachomatis failed to robustly up-regulate pro-inflammatory cytokines in polA2EN as numerous studies have documented that this bacteria uses evasion mechanisms to interfere with host immune responses [33-39]. Some of these studies have described chlamydial proteases that are secreted into the host cell cytosol they can manipulate host proteins and signaling pathways [39, 40]. For example, the NFκB pathway may be modulated by several different chlamydial proteins and mechanisms, all of which can interfere with NFκB-mediated gene transcription and regulation [36, 37, 41]. C. trachomatis interference with cytokine signaling pathways may be one explanation as to why chlamydiae failed to robustly upregulate a pro-inflammatory innate response in polA2EN cells, as many of the pro-inflammatory cytokines, such as CXCL8, IL6, TNFα and IL1, are downstream of NFκB activation [42]. Since C. trachomatis infection of polA2EN cells did not cause a decrease in the constitutive secretion of the pro-inflammatory cytokines CXCL8 and IL6, there may be other innate immune signaling pathways involved in the secretion of these cytokines, such as the MAP kinase pathway [26], that are unaltered during infection. It is also unlikely that chlamydial LPS is playing a major role in the endocervical epithlelial cytokine responses, since, TNFα was not upregulated in response to chlamydial exposure unlike what was reported by Ingalls et al. who found modest cytokine responses in CHO cells to be mediated by chlamydial endotoxin [43]. Neither pA2EN cells nor primary endocervical epithelial cells express high levels of the LPS receptors TLR4 or CD14 [11, 12], further suggesting that chlamydial LPS may play a minimal role in eliciting an epithelial cytokine response in the endocervix.
Previous studies have demonstrated that the robust CXCL8 response by C. trachomatis-infected HeLa cell cultures was primarily mediated by IL1α [15, 44]. Although, we observed an increase in IL1α protein levels in C. trachomatis infected polA2EN cells at 72 hours post infection, we did not observe an increase in CXCL8 at any point during the chlamydial developmental cycle and up to 120 hours postinfection. IL1α release upon host cell lysis and stimulation of non-infected bystander epithelial cells was the major mechanism by which the HeLa pro-inflammatory cytokine response was amplified [15]. polA2EN cells infected with C. trachomatis, did not, however, exhibit significant lysis up to 120 hours post-infection. IL1α may be an indicator of cell damage in the absence of cell lysis, however, which may be caused by chlamydiae infection [45]. It is also possible that chlamydial particles exit polA2EN cells by extrusion thus explaining the minimal cell lysis we observed [7]. We, therefore, lysed infected polA2EN cells and investigated whether these lysates, or supernatants could stimulate non-infected polA2EN cells and amplify the CXCL8 response similar to that observed in infected HeLa cells [15]. Neither lysates nor supernatants harvested from C. trachomatis infected polA2EN cells, however, stimulated a pro-inflammatory response in non-infected cells. These results suggest that neither intracellular nor released IL1α, nor any other intracellular factor could amplify the CXCL8 response in non-infected polA2EN cells. Importantly, polA2EN cells and primary endocervical epithelial cells do constitutively secrete a high level of IL1ra from their apical surfaces which HeLa cells do not (data not shown for primary or HeLa cells). IL1ra inhibits the binding of IL1α to its receptor and prevents the subsequent pro-inflammatory immune cascade [46]. The constitutive apical secretion of IL1ra, therefore, may explain why the IL1α that is secreted during C. trachomatis infection is unable to stimulate non-infected cells to amplify the pro-inflammatory cytokine response. PolA2EN cells were capable of responding to exogenous IL1α stimulation, however, it required 20ng/mL, a very high concentration of IL1α, to induce a cytokine response. These results indicate that the high constitutive IL1ra might compete for the IL1 receptor and prevent lower concentrations of IL1α from amplifying the pro-inflammatory cytokine response. Constitutive secretion of IL1ra has been demonstrated in other mucosal cell types and is believed to be an endogenous regulatory mechanism by which unwanted inflammation is controlled in various tissue types [47], and has been negatively associated with pathology in trachoma patients [48-50]. The fact that polA2EN cells constitutively secrete a higher level of IL1ra from the apical membrane compared to the basolateral membrane, suggests that the two membrane compartments could play distinct roles in regulating inflammatory responses. Hence, IL1ra may be constitutively secreted at high concentrations at the apical surface of the endocervical epithelium in order to prevent IL1α from excessively amplifying the epithelial cytokine response and compromising the integrity of this barrier. Since IL1ra is not constitutively secreted, nor upregulated by chlamydial infection at the basolateral membrane, the basal increase in IL1α secretion by infected endocervical epithelial cells could potentially still signal to underlying resident IL-1 Receptor-expressing leukocytes. It is also possible that a sustained secretion of IL1α from the basolateral membrane compartment of infected epithelial cells could contribute to chronic inflammation that may ultimately lead to pathology in some individuals. Our results indicate that further studies on the regulation of IL1α, IL1ra, and IL1 receptor expression are needed to determine how these factors could contribute to the establishment, clearance, chronicity and immunopathology of C. trachomatis infections and also other sexually transmitted diseases at this important site.
The observation that polA2EN cells infected with C. trachomatis fail to elicit a robust epithelial pro-inflammatory cytokine response contrasts with observations made in the murine in vitro model of chlamydial infection using Chlamydia muridarum infection in oviduct-derived epithelial cells [51, 52]. One reason for this discrepancy may be that C. muridarum infection fails to interfere with murine host cell signaling pathways needed to initiate the inflammatory cascade. Christian et al. and others have reported that there are differences between C. muridarum and C. trachomatis with respect to how these species interfere with proteins in innate immune signaling pathways in their respective host cells [36, 41, 53]. Another possible reason for the discrepancy is that the epithelial cells are derived from two distinct anatomical sites, the endocervix and the oviduct, respectively. Epithelial cells in the oviduct, which is a relatively sterile site, may respond more robustly to organisms than cells in the endocervix which routinely encounter microflora from the lower FRT [54, 55]. The hypothesis that there are site specific immune responses in the FRT is indirectly supported by the work of Hvid et al who demonstrated that IL-1 is central to human fallopian tube damage during C. trachomatis infection. This IL1-mediated damage can be inhibited by exogenous IL-1ra, suggesting that this regulatory cytokine is not constitutively made, or upregulated to sufficient concentrations, in the Fallopian tubes [56]. Trophoblast cells infected with C. trachomatis also increases IL1β via Nod1 signaling pathways [24, 57]. Taken together, these studies suggest there may be major differences in Chlamydia's interaction with its target host cells dependent on the species and tissue site. Understanding species and site specific interactions with chlamydial organisms is imperative as we seek to determine the reservoirs of bacteria and mechanisms by which C. trachomatis establishes chronic infection and induces pathology.
In this study, we also demonstrated that C. trachomatis infection up-regulated IL11 in basolateral secretions of polA2EN cells. These results are consistent with observations made by others in HEC-1B endometrial epithelial cells and HeLa cells [28, 58]. IL11 has also been found in ocular secretions from patients who have chronic scarring from trachoma [48]. IL11 is a pleiotropic cytokine that has multiple functions including inhibition of NFκB activation, cytoprotection at mucosal sites, modulation of macrophage phenotypes, promotion of cell survival and differentiation, promotion of tissue repair, and reduction of inflammatory responses ([59]; reviewed in [60, 61]). The role of IL11 in tissue repair is to up-regulate collagen and other connective tissue proteins leading to fibrosis [60]. Our results suggest that the infected endocervical epithelial cells may modulate inflammatory processes associated with chlamydial disease by secreting IL11. The observation that IL11 was only increased in basolateral secretions in C. trachomatis infected polA2EN cells highlights the possibility that the two distinct membrane domains in polarized epithelial cells play different roles in the induction of innate immune responses and tissue repair.
The constitutive secretion of two key T lymphocyte chemokines, IP10 and RANTES, was significantly decreased in secretions from C. trachomatis infected polA2EN cells. IP10 and RANTES are chemokines that bind to CXCR3 and CCR5, respectively, resulting in the recruitment of CXCR3 positive and CCR5 positive leukocytes to the target tissues, which include T cells and NK cells [62, 63]. These two cell types are the major producers of interferon gamma (IFNγ), the key cytokine that can mediate resolution of C. trachomatis infections when infected cells are exposed to sufficiently high concentrations [64-67]. If C. trachomatis can subvert the epithelial cell-mediated secretion of IP10 and RANTES in vivo, this may prevent directed migration of IFNγ secreting T lymphocytes and NK cells into the immediate microenvironment of infected epithelial cells, particularly in psuedoglands of the endocervix [64, 65, 68, 69]. This may be significant as cell-to-cell contact between T cells and epithelial cells is required for T cells to facilitate IFNγ mediated killing of C. trachomatis [70]. The decrease in IP10 and RANTES upon chlamydial infection of endocervical epithelial cells suggests that type I interferon or interferon-stimulated gene (ISG) signaling pathways may also be modulated during C. trachomatis infection. Type I interferons and components of type I interferon signaling pathways were shown to be involved in inflammation and pathology in in vitro and in vivo murine models of Chlamydia [51, 71] as well as in vitro human airway epithelial cell models of C. pneumoniae [72]. Further investigation is needed to determine if C. trachomatis might be interfering with type I interferons, ISGs or other proteins involved in signaling pathways that lead to the downstream transcription, translation, and secretion of IP10 and RANTES, and the functional significance in human endocervical infections.
Overall, we propose that when C. trachomatis survives the innate physical and secreted immune mediators in the lower FRT and endocervix, the endocervical epithelium could provide a hospitable niche for C. trachomatis to establish infection for a number of reasons. First, the delay in IL1α up-regulation combined with a lack in amplification of CXCL8, IL6 and TNFα will likely delay recruitment to, and activation of, leukocytes in an infected endocervix until well after bacteria have established an infection. Second, constitutive, apically-secreted ILra combined with minimal host cell lysis may help protect the integrity of the epithelial cell layer by preventing or regulating the IL1-mediated ampification of a pro-inflammatory response by bystander uninfected cells. Third, IL11 may modulate the phenotype or response of infiltrating leukocytes to reduce a pro-inflammatory response. Finally, the minimal epithelial lysis during productive infection suggests that the bacteria may be able to maintain an intracellular niche in some of the cells it originally infects in addition to infecting new cells. These factors, in combination with the relatively slow in vivo turnover of human endocervical epithelial cells [73], the ability of C. trachomatis to extend the life of infected cells by modulating apoptosis-inducing pathways [74-77], the evasion of IFNγ mediated mechanisms of clearance [20, 53, 78-80], and/or the evasion of key effectors of acquired immunity [34, 35, 81], and the evasion of IFNγ mediated mechanisms of clearance [34, 38], may explain why some infections take significantly extended times to clear. Taken together, these complex factors suggest that foci of infected cells could be maintained in the endocervix even as others are cleared. Though chlamydiae may utilize evasion strategies to circumvent or alter the immune response, our results do not rule out the possibility that infections of endocervical epithelial cells may also induce a significant inflammatory response, as sustained ILα secretion at the basolateral membrane of infected endocervical epithelial cells may overcome evasion mechanisms utilized by chlamydiae and subsequently activate underlying macrophages or dendritic cells. These immune cells are not generally believed to be permissive for productive genital chlamydial infection [82, 83], and therefore may not be subject to active subversive strategies used by the bacteria. Hence, they may activate and amplify pro-inflammatory innate and adaptive immune responses. Whether an ensuing leukocytic infiltrate could clear infection and/or mediate damage and how long this takes is likely dependent upon multiple factors that influence the endocervical micromilieu which is critical for understanding how to prevent chlamydial disease. Furthermore, it is important to investigate the dynamics between the infected host cells and chlamydiae and the ensuing epithelial innate immune response, as long-term survival of bacteria may likely require them to enter into alternative growth forms, which are believed to contribute to chlamydial disease and are more refractory to antibiotic treatment [84, 85].
There are many other confounding factors that influence and likely determine whether an endocervical chlamydial infection clears, sustains, ascends into the endometrium, and/or mediates damage. One may be the initial dose of chlamydial inoculum. If there are high numbers of infectious chlamydial particles in the inoculum, a greater proportion of endocervical epithelial cells will be infected; this may result in an increased concentration of IL1α at the basolateral surface. IL1α, in the absence of an increased IL1ra, may elicit a pro-inflammatory immune response that then may compromise the epithelial barrier. Higher numbers of infectious particles and an increase in IL1α may also change the ratio of the infected cells to non-infected bystander cells, and hence, alter the immune and nutrient milieu in this locale [34, 81, 86]. It is also more likely that upper FRT tissues could be exposed to bacterial particles and become infected when there is a higher initial infectious inoculum. The ratio of dead bacteria to live bacteria may also influence the outcome of chlamydial infection of the endocervix since replicating and non-replicating chlamydiae induce different IL1α and T cell chemokine responses. The presence of a pre-existing inflammatory infiltrate, often observed in normal transformation zone and endocervix tissues, or associated with a coinfection, may also influence the early dynamics, and hence outcome, of infection via the immediate exposure of these leukocytes to bacteria and IL1α [87, 88]. Other co-factors include the presence of female steroid hormones and/or synthetic progestins that may influence acquisition of chlamydial infection [89-92]; relative exposure to TGFβ-rich, immunomodulatory, seminal plasma [93, 94], and vaginal co-infections such as bacterial vaginosis (BV) and trichomoniasis, both of which may provide a source of indole in a tryptophan-depleted microenvironment and therefore aid in the maintenance of a bacterial reservoir in the presence of IFNγ in the endocervix [53, 78-80, 95].
In summary, the data generated in this study provide valuable information on the innate cytokine repertoire of human endocervical epithelial cells and the evasion strategies used by the C. trachomatis to modify some of these innate responses. In vivo exogenous cofactors will also influence this relationship. As we move forward, the polA2EN model will be valuable in establishing the role of, and mechanism by which, these factors influence bacterial growth, survival, and subsequent modulation of the innate immune response in the endocervical epithelium. Generation of similar endometrial and Fallopian tube epithelial cell models will also allow us to examine site specific differences in these responses. Together, these studies may enlighten us to why and how human C. trachomatis infections can be so extended.
Supplementary Material
Supplementary Figure 1. The kinetics of cytokine responses over the course of the chlamydial developmental cycle. PolA2EN cells were infected with C. trachomatis serovar D (>95% infection rate). Apical (A) and basolateral (B) supernatants were collected at 24, 36, 48, and 72 hours post-infection and subjected to cytometric bead array analyses for (a) IL6, (b) CXCL8, (c) TNFα, (d) IL1α, (e) IP10, and (f) RANTES. Cytokine values represent mean values ± SD. Graphs shown are representative graphs from 3 independent experiments each performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a One-way ANOVA (*p<0.001).
Supplementary Figure 2. CXCL8 levels in C. trachomatis infected polA2EN cells do not change with increasing inoculum. PolA2EN cells were infected with C. trachomatis serovar D using an inoculum, that achieves a >95% infection rate (1) as well as two-fold (2), three-fold (3), and 4-fold (4) this initial dose. Apical and basolateral supernatants were collected at 72 hours post-infection and were subjected to cytometric bead analyses. CXCL8 cytokine measurements shown. Cytokine values represent mean values ± SD. Graph shown is a representative graph from 3 independent experiments each performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a One-way ANOVA.
Highlights for Buckner et al. Cytokine.
We infected a novel polarized endocervical epithelial model with C. trachomatis
C. trachomatis infection failed to yield a robust pro-inflammatory cytokine response
Constitutive RANTES and IP10 were decreased by C. trachomatis infection
C. trachomatis infected primary cells yielded similar cytokine responses Results suggest
C. trachomatis uses evasive strategies to survive in the endocervix
Acknowledgments
This study was supported by NIH grant AI087899. The authors thank Dr. Wandy Beatty for EM imaging and Drs. Chris McGowin, Danny Schust and Priscilla Wyrick for critical review of the manuscript.
Abbreviations
- polA2EN
polarized endocervical epithelial cells
- SPG
sucrose phosphate glutamic acid buffer
- IFU
inclusion forming units
- FRT
female reproductive tract
- PID
pelvic inflammatory disease
- EBs
elementary bodies
- RBs
reticulate bodies
- DAPI
4′, 6-diamidino-2-phenylindole
- TEER
transepithelial electrical resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5(2):149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 2.Golden MR, Schillinger JA, Markowitz L, St Louis ME. Duration of untreated genital infections with Chlamydia trachomatis: a review of the literature. Sex Transm Dis. 2000;27(6):329–37. doi: 10.1097/00007435-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Joyner JL, Douglas JM, Jr, Foster M, Judson FN. Persistence of Chlamydia trachomatis infection detected by polymerase chain reaction in untreated patients. Sex Transm Dis. 2002;29(4):196–200. doi: 10.1097/00007435-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, Franceschi S, Ronderos M, Munoz N, van den Brule AJ. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis. 2005;191(6):907–16. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 5.Morre SA, van den Brule AJ, Rozendaal L, Boeke AJ, Voorhorst FJ, de Blok S, Meijer CJ. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int J STD AIDS. 2002;1(Suppl 2):12–8. doi: 10.1258/095646202762226092. [DOI] [PubMed] [Google Scholar]
- 6.Parks KS, Dixon PB, Richey CM, Hook EW., 3rd Spontaneous clearance of Chlamydia trachomatis infection in untreated patients. Sex Transm Dis. 1997;24(4):229–35. doi: 10.1097/00007435-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci U S A. 2007;104(27):11430–5. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55(1):143–90. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dessus-Babus S, Moore CG, Whittimore JD, Wyrick PB. Comparison of Chlamydia trachomatis serovar L2 growth in polarized genital epithelial cells grown in three-dimensional culture with non-polarized cells. Microbes Infect. 2008;10(5):563–70. doi: 10.1016/j.micinf.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyrick PB, Gerbig DG, Jr, Knight ST, Raulston JE. Accelerated development of genital Chlamydia trachomatis serovar E in McCoy cells grown on microcarrier beads. Microb Pathog. 1996;20(1):31–40. doi: 10.1006/mpat.1996.0003. [DOI] [PubMed] [Google Scholar]
- 11.Buckner LR, Schust DJ, Ding J, Nagamatsu T, Beatty W, Chang TL, Greene SJ, Lewis ME, Ruiz B, Holman SL, Spagnuolo RA, Pyles RB, Quayle AJ. Innate immune mediator profiles and their regulation in a novel polarized immortalized epithelial cell model derived from human endocervix. J Reprod Immunol. 2011;92(1-2):8–20. doi: 10.1016/j.jri.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, 2nd, Chesson R, Spagnuolo RA, Pyles RB. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59(3):212–24. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 13.Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22(1):50–8. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- 14.Landry J, Pyl PT, Rausch T, Tekkedil MM, Stuetz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, Gagneur J, Korbel JO, Huber W, Steinmetz LM. The genomic and transcriptomic landscape of a HeLa cell line, G3. 2013 doi: 10.1534/g3.113.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, Fierer J, Stephens RS, Kagnoff MF. Secretion of pro-inflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99(1):77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11(1):44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 17.Kane CD, Vena RM, Ouellette SP, Byrne GI. Intracellular tryptophan pool sizes may account for differences in gamma interferon-mediated inhibition and persistence of chlamydial growth in polarized and nonpolarized cells. Infect Immun. 1999;67(4):1666–71. doi: 10.1128/iai.67.4.1666-1671.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyrick P. Polarized Epithelial Cell Culture for Chlamydia trachomatis. In: Wyrick P, Bavoil P, editors. Chlamydia Genomics and Pathogenesis. Horizon Bioscience; Norfolk, UK: 2006. pp. 323–338. [Google Scholar]
- 19.Frohlich K, Hua Z, Wang J, Shen L. Isolation of Chlamydia trachomatis and membrane vesicles derived from host and bacteria. J Microbiol Methods. 2012;91(2):222–30. doi: 10.1016/j.mimet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belland RJ, Nelson DE, Virok D, Crane DD, Hogan D, Sturdevant D, Beatty WL, Caldwell HD. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc Natl Acad Sci U S A. 2003;100(26):15971–6. doi: 10.1073/pnas.2535394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J Cell Sci. 2006;119(Pt 2):350–9. doi: 10.1242/jcs.02733. [DOI] [PubMed] [Google Scholar]
- 22.Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58(5):1217–25. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Schoborg RV. The host adherens junction molecule nectin-1 is degraded by chlamydial protease-like activity factor (CPAF) in Chlamydia trachomatis-infected genital epithelial cells. Microbes Infect. 2009;11(1):12–9. doi: 10.1016/j.micinf.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 24.de la Torre E, Mulla MJ, Yu AG, Lee SJ, Kavathas PB, Abrahams VM. Chlamydia trachomatis infection modulates trophoblast cytokine/chemokine production. J Immunol. 2009;182(6):3735–45. doi: 10.4049/jimmunol.0800764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles DK, Whittimore JD, LaRue RW, Raulston JE, Wyrick PB. Ultrastructural analysis of chlamydial antigen-containing vesicles everting from the Chlamydia trachomatis inclusion. Microbes Infect. 2006;8(6):1579–91. doi: 10.1016/j.micinf.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz KR, Stephens RS. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect Immun. 2007;75(12):5924–9. doi: 10.1128/IAI.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mpiga P, Mansour S, Morisset R, Beaulieu R, Ravaoarinoro M. Sustained interleukin-6 and interleukin-8 expression following infection with Chlamydia trachomatis serovar L2 in a HeLa/THP-1 cell co-culture model. Scand J Immunol. 2006;63(3):199–207. doi: 10.1111/j.1365-3083.2006.01734.x. [DOI] [PubMed] [Google Scholar]
- 28.Dessus-Babus S, Darville TL, Cuozzo FP, Ferguson K, Wyrick PB. Differences in innate immune responses (in vitro) to HeLa cells infected with nondisseminating serovar E and disseminating serovar L2 of Chlamydia trachomatis. Infect Immun. 2002;70(6):3234–48. doi: 10.1128/IAI.70.6.3234-3248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20(6):1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 30.Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60(2):508–14. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 31.O'Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem. 2006;281(3):1652–9. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- 32.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61(11):4569–74. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci U S A. 1993;90(9):3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibana JA, Schust DJ, Sugimoto J, Nagamatsu T, Greene SJ, Quayle AJ. Chlamydia trachomatis immune evasion via downregulation of MHC class I surface expression involves direct and indirect mechanisms. Infect Dis Obstet Gynecol. 2011;2011:420905. doi: 10.1155/2011/420905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawana K, Quayle AJ, Ficarra M, Ibana JA, Shen L, Kawana Y, Yang H, Marrero L, Yavagal S, Greene SJ, Zhang YX, Pyles RB, Blumberg RS, Schust DJ. CD1d degradation in Chlamydia trachomatis-infected epithelial cells is the result of both cellular and chlamydial proteasomal activity. J Biol Chem. 2007;282(10):7368–75. doi: 10.1074/jbc.M610754200. [DOI] [PubMed] [Google Scholar]
- 36.Lad sp, Li J, da Silva Correia J, Pan Q, Gadwal S, Ulevitch RJ, Li E. Cleavage of p65/RelA of the NF-kappaB pathway by Chlamydia. Proc Natl Acad Sci U S A. 2007;104(8):2933–8. doi: 10.1073/pnas.0608393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Negrate G, Krieg A, Faustin B, Loeffler M, Godzik A, Krajewski S, Reed JC. ChlaDub1 of Chlamydia trachomatis suppresses NF-kappaB activation and inhibits IkappaBalpha ubiquitination and degradation. Cell Microbiol. 2008;10(9):1879–92. doi: 10.1111/j.1462-5822.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhong G, Fan T, Liu L. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J Exp Med. 1999;189(12):1931–8. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong G, Liu L, Fan T, Fan P, Ji H. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in Chlamydia-infected cells. J Exp Med. 2000;191(9):1525–34. doi: 10.1084/jem.191.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Frohlich KM, Buckner L, Quayle AJ, Luo M, Feng X, Beatty W, Hua Z, Rao X, Lewis ME, Sorrells K, Santiago K, Zhong G, Shen L. Altered protein secretion of Chlamydia trachomatis in persistently infected human endocervical epithelial cells. Microbiology. 2011;157(Pt 10):2759–71. doi: 10.1099/mic.0.044917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christian J, Vier J, Paschen SA, Hacker G. Cleavage of the NF-kappaB family protein p65/RelA by the chlamydial protease-like activity factor (CPAF) impairs pro-inflammatory signaling in cells infected with Chlamydiae. J Biol Chem. 2010;285(53):41320–7. doi: 10.1074/jbc.M110.152280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–55. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 43.Ingalls RR, Rice PA, Qureshi N, Takayama K, Lin JS, Golenbock DT. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63(8):3125–30. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun. 2008;76(3):942–51. doi: 10.1128/IAI.01313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012;2012:315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallegua DS, Weisman MH. Potential therapeutic uses of interleukin 1 receptor antagonists in human diseases. Ann Rheum Dis. 2002;61(11):960–7. doi: 10.1136/ard.61.11.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis. 2000;59(Suppl 1):i60–4. doi: 10.1136/ard.59.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skwor TA, Atik B, Kandel RP, Adhikari HK, Sharma B, Dean D. Role of secreted conjunctival mucosal cytokine and chemokine proteins in different stages of trachomatous disease. PLoS Negl Trop Dis. 2008;2(7):e264. doi: 10.1371/journal.pntd.0000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrier S, Kherratia B, Deschaumes C, Ughetto S, Kemeny JL, Baudet-Pommel M, Sauvezie B. IL-1ra and IL-1 production in human oral mucosal epithelial cells in culture: differential modulation by TGF-beta1 and IL-4. Clin Exp Immunol. 2002;127(1):53–9. doi: 10.1046/j.1365-2249.2002.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levine SJ, Wu T, Shelhamer JH. Extracellular release of the type I intracellular IL-1 receptor antagonist from human airway epithelial cells: differential effects of IL-4, IL-13, IFN-gamma, and corticosteroids. J Immunol. 1997;158(12):5949–57. [PubMed] [Google Scholar]
- 51.Derbigny WA, Hong SC, Kerr MS, Temkit M, Johnson RM. Chlamydia muridarum infection elicits a beta interferon response in murine oviduct epithelial cells dependent on interferon regulatory factor 3 and TRIF. Infect Immun. 2007;75(3):1280–90. doi: 10.1128/IAI.01525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RM. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect Immun. 2004;72(7):3951–60. doi: 10.1128/IAI.72.7.3951-3960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson DE, Virok DP, Wood H, Roshick C, Johnson RM, Whitmire WM, Crane DD, Steele-Mortimer O, Kari L, McClarty G, Caldwell HD. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc Natl Acad Sci U S A. 2005;102(30):10658–63. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57(1-2):61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 55.Wra CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53(2):65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 56.Hvid M, Baczynska A, Deleuran B, Fedder J, Knudsen HJ, Christiansen G, Birkelund S. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 2007;9(12):2795–803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 57.Kavathas PB, Boeras CM, Mulla MJ, Abrahams VM. Nod1, but not the ASC inflammasome, contributes to induction of IL-1beta secretion in human trophoblasts after sensing of Chlamydia trachomatis. Mucosal Immunol. 2013;6(2):235–43. doi: 10.1038/mi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis CH, Wyrick PB. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect Immun. 1997;65(7):2914–24. doi: 10.1128/iai.65.7.2914-2924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein W, Tromm A, Griga T, Fricke H, Folwaczny C, Hocke M, Eitner K, Marx M, Duerig N, Epplen JT. A polymorphism in the IL11 gene is associated with ulcerative colitis. Genes Immun. 2002;3(8):494–6. doi: 10.1038/sj.gene.6363897. [DOI] [PubMed] [Google Scholar]
- 60.Dorner AJ, Goldman SJ, Keith JC., Jr Interleukin-11. BioDrugs. 1997;8(6):418–29. doi: 10.2165/00063030-199708060-00002. [DOI] [PubMed] [Google Scholar]
- 61.Putoczki T, Ernst M. More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J Leukoc Biol. 2010;88(6):1109–17. doi: 10.1189/jlb.0410226. [DOI] [PubMed] [Google Scholar]
- 62.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 6. New York, NY: Garland Science Publishing; 2005. [Google Scholar]
- 63.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101(4):746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramsey KH, Rank RG. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59(3):925–31. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rank RG, Soderberg LS, Barron AL. Chronic chlamydial genital infection in congenially athymic nude mice. Infect Immun. 1985;48(3):847–9. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62(9):3705–11. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Role of tryptophan in gamma interferon-mediated chlamydial persistence. Ann N Y Acad Sci. 1994;730:304–6. doi: 10.1111/j.1749-6632.1994.tb44274.x. [DOI] [PubMed] [Google Scholar]
- 68.Tseng CT, Rank RG. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66(12):5867–75. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rank RG, Bowlin AK, Kelly KA. Characterization of lymphocyte response in the female genital tract during ascending Chlamydial genital infection in the guinea pig model. Infect Immun. 2000;68(9):5293–8. doi: 10.1128/iai.68.9.5293-5298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Igietseme JU, Wyrick PB, Goyeau D, Rank RG. An in vitro model for immune control of chlamydial growth in polarized epithelial cells. Infect Immun. 1994;62(8):3528–35. doi: 10.1128/iai.62.8.3528-3535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prantner D, Sikes JD, Hennings L, Savenka AV, Basnakian AG, Nagarajan UM. Interferon regulatory transcription factor 3 protects mice from uterine horn pathology during Chlamydia muridarum genital infection. Infect Immun. 2011;79(10):3922–33. doi: 10.1128/IAI.00140-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf K, Fields KA. Chlamydia pneumoniae impairs the innate immune response in infected epithelial cells by targeting TRAF3. J Immunol. 2013;190(4):1695–701. doi: 10.4049/jimmunol.1202443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hiersche HD, Nagl W. Regeneration of secretory epithelium in the human endocervix. Arch Gynecol. 1980;229(2):83–90. doi: 10.1007/BF02109947. [DOI] [PubMed] [Google Scholar]
- 74.Rodel J, Grosse C, Yu H, Wolf K, Otto GP, Liebler-Tenorio E, Forsbach-Birk V, Straube E. Persistent Chlamydia trachomatis infection of HeLa cells mediates apoptosis resistance through a Chlamydia protease-like activity factor-independent mechanism and induces high mobility group box 1 release. Infect Immun. 2012;80(1):195–205. doi: 10.1128/IAI.05619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma M, Machuy N, Bohme L, Karunakaran K, Maurer AP, Meyer TF, Rudel T. HIF-1alpha is involved in mediating apoptosis resistance to Chlamydia trachomatis-infected cells. Cell Microbiol. 2011;13(10):1573–85. doi: 10.1111/j.1462-5822.2011.01642.x. [DOI] [PubMed] [Google Scholar]
- 76.Ying S, Christian JG, Paschen SA, Hacker G. Chlamydia trachomatis can protect host cells against apoptosis in the absence of cellular Inhibitor of Apoptosis Proteins and Mcl-1. Microbes Infect. 2008;10(1):97–101. doi: 10.1016/j.micinf.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Ying S, Pettengill M, Ojcius DM, Hacker G. Host-Cell Survival and Death During Chlamydia Infection. Curr Immunol Rev. 2007;3(1):31–40. doi: 10.2174/157339507779802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73(10):6407–18. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fehlner-Gardiner C, Roshick C, Carlson JH, Hughes S, Belland RJ, Caldwell HD, McClarty G. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J Biol Chem. 2002;277(30):26893–903. doi: 10.1074/jbc.M203937200. [DOI] [PubMed] [Google Scholar]
- 80.McClarty G, Caldwell HD, Nelson DE. Chlamydial interferon gamma immune evasion influences infection tropism. Curr Opin Microbiol. 2007;10(1):47–51. doi: 10.1016/j.mib.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Ibana JA, Myers L, Porretta C, Lewis M, Taylor SN, Martin DH, Quayle AJ. The major CD8 T cell effector memory subset in the normal and Chlamydia trachomatis-infected human endocervix is low in perforin. BMC Immunol. 2012;13:66. doi: 10.1186/1471-2172-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koehler L, Nettelnbreker E, Hudson AP, Ott N, Gerard HC, Branigan PJ, Schumacher HR, Drommer W, Zeidler H. Ultrastructural and molecular analyses of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. Microb Pathog. 1997;22(3):133–42. doi: 10.1006/mpat.1996.0103. [DOI] [PubMed] [Google Scholar]
- 83.Yong EC, Chi EY, Kuo CC. Differential antimicrobial activity of human mononuclear phagocytes against the human biovars of Chlamydia trachomatis. J Immunol. 1987;139(4):1297–302. [PubMed] [Google Scholar]
- 84.Ibana JA, Belland RJ, Zea AH, Schust DJ, Nagamatsu T, AbdelRahman YM, Tate DJ, Beatty WL, Aiyar AA, Quayle AJ. Inhibition of indoleamine 2,3-dioxygenase activity by levo-1-methyl tryptophan blocks gamma interferon-induced Chlamydia trachomatis persistence in human epithelial cells. Infect Immun. 2011;79(11):4425–37. doi: 10.1128/IAI.05659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reveneau N, Crane DD, Fischer E, Caldwell HD. Bactericidal activity of first-choice antibiotics against gamma interferon-induced persistent infection of human epithelial cells by Chlamydia trachomatis. Antimicrob Agents Chemother. 2005;49(5):1787–93. doi: 10.1128/AAC.49.5.1787-1793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ibana JA, Aiyar A, Quayle AJ, Schust DJ. Modulation of MICA on the surface of Chlamydia trachomatis-infected endocervical epithelial cells promotes NK cell-mediated killing. FEMS Immunol Med Microbiol. 2012;65(1):32–42. doi: 10.1111/j.1574-695X.2012.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38(5):350–9. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 88.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73(6):1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 89.Guseva NV, Dessus-Babus SC, Whittimore JD, Moore CG, Wyrick PB. Characterization of estrogen-responsive epithelial cell lines and their infectivity by genital Chlamydia trachomatis. Microbes Infect. 2005;7(15):1469–81. doi: 10.1016/j.micinf.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Guseva NV, Knight ST, Whittimore JD, Wyrick PB. Primary cultures of female swine genital epithelial cells in vitro: a new approach for the study of hormonal modulation of Chlamydia infection. Infect Immun. 2003;71(8):4700–10. doi: 10.1128/IAI.71.8.4700-4710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maslow AS, Davis CH, Choong J, Wyrick PB. Estrogen enhances attachment of Chlamydia trachomatis to human endometrial epithelial cells in vitro. Am J Obstet Gynecol. 1988;159(4):1006–14. doi: 10.1016/s0002-9378(88)80189-3. [DOI] [PubMed] [Google Scholar]
- 92.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. AIDS. 2012;26(3):375–80. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 93.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 94.Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57(1-2):109–28. doi: 10.1016/s0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 95.Morrison RP. New insights into a persistent problem -- chlamydial infections. J Clin Invest. 2003;111(11):1647–9. doi: 10.1172/JCI18770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The kinetics of cytokine responses over the course of the chlamydial developmental cycle. PolA2EN cells were infected with C. trachomatis serovar D (>95% infection rate). Apical (A) and basolateral (B) supernatants were collected at 24, 36, 48, and 72 hours post-infection and subjected to cytometric bead array analyses for (a) IL6, (b) CXCL8, (c) TNFα, (d) IL1α, (e) IP10, and (f) RANTES. Cytokine values represent mean values ± SD. Graphs shown are representative graphs from 3 independent experiments each performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a One-way ANOVA (*p<0.001).
Supplementary Figure 2. CXCL8 levels in C. trachomatis infected polA2EN cells do not change with increasing inoculum. PolA2EN cells were infected with C. trachomatis serovar D using an inoculum, that achieves a >95% infection rate (1) as well as two-fold (2), three-fold (3), and 4-fold (4) this initial dose. Apical and basolateral supernatants were collected at 72 hours post-infection and were subjected to cytometric bead analyses. CXCL8 cytokine measurements shown. Cytokine values represent mean values ± SD. Graph shown is a representative graph from 3 independent experiments each performed in triplicate. Statistical analyses between mock infected and C. trachomatis infected parameters were performed using a One-way ANOVA.