Abstract
The zebrafish (Danio rerio) has become a very important animal model in biomedical research. In contrast to other models, such as mice, there has been relatively little documentation or control of subclinical disease in zebrafish research facilities. Several infectious and non-infectious conditions are consistently detected by histopathology in apparently healthy D. rerio. The most commonly observed infectious agent in zebrafish is Pseudoloma neurophilia, which is a microsporidian organism that targets the central nervous system, peripheral nerves, and occasionally other tissues. Mycobacteriosis, caused by M. chelonae and other species, is also a frequent finding. Less commonly encountered agents include Pseudocapillaria tomentosa, which can cause extensive proliferative enteritis, and a myxozoan (Myxidium sp.) that inhabits the urinary tract, but appears to cause few if any pathological changes. Non-infectious diseases that are often clinically unapparent in zebrafish include hepatic megalocytosis, bile and pancreatic ductal proliferation, and neoplasms of the ultimobranchial gland, gastrointestinal tract, and testis. To date there is little information on the degree to which these conditions may impact research in subclinically affected fish, but there is reason to believe that they should be considered as potentially significant causes of non-protocol variation in experiments. Therefore, it is imperative that research facilities monitor their stocks for the presence of these occult diseases, and be aware of their existence when interpreting study results. Furthermore, for underlying disease conditions that cannot be readily eradicated, it is essential to determine the physiological and immunological changes that they elicit in zebrafish. Understanding the cause, modes of transmission, and distribution of the pathogens would provide useful information for the development of control and prevention strategies.
Introduction
The benefit of using aquatic models as part of an integrative approach to improve human health is being realized by the scientific community. The use of aquatic animals as models in biomedical research has dramatically increased in the last decade, largely led by the exploitation of the zebrafish (Danio rerio) model (Lieschke and Currie 2007). The ZFIN web site (http://zfin.org) lists 687 laboratories that employ zebrafish. The field was initially led by investigations in developmental genetics in which experimental end points involved primarily embryos or larval fish. Adult zebrafish are now used extensively as models throughout the biomedical research arena, including areas such as infectious disease susceptibility and immune system function (Prouty et al. 2003; Trede et al. 2004; Phelps and Neely 2005), aging (Gerhard 2007), toxicology (Truong et al. 2011; Matthew et al. 2009), and oncology (Liu and Leach 2011; Coel et al. 2011). However, investigators may not be fully aware of the potential impact that underlying disease may have on their research when post-larval zebrafish are used in medium or long term experiments. For example, it is not difficult to imagine that subclincial infections of the nervous system might have untoward effects on zebrafish studies that have behavioral or psychological endpoints, as in some recently published work (Sisson et al. 2007).
Subclinical infections are by far the most commonly diagnosed histopathological changes observed in zebrafish (http://zebrafish.org/zirc/health/index.php). The following are common infections or lesions found in apparently healthy zebrafish. The two most common infections of zebrafish in research laboratories are microsporidiosis, caused by Pseudoloma neurophilia, and mycobacteriosis (http://zebrafish.org/zirc/health/index.php). Less frequently observed, but still a significant problem, are intestinal infections with the nematode Pseudocapillaria tomentosa. A myxozoan (Myxidium sp.) is found in the common mesospheric ducts, and does not appear to be associated with clinical disease. Additional common histopathologically-evident conditions that may or may not have an infectious etiology include egg-associated inflammation (EAIF) in reproductively mature females and two categories of liver lesions. Liver lesions, such as hepatic megalocytosis are frequently seen in sentinel fish. Added to that, a few types of neoplasms are regularly seen in normal appearing fish, and these include tumors of the ultimobranchial glands and intestinal carcinomas (Spitsbergen et al. 2011).
Compared to experiments involving laboratory rodents, for example, there is far less known about effects that subclinical disease might have on zebrafish research models. It is well recognized that chronic subclinical infections can compromise mouse studies; therefore, to set the stage for the present discussion, we will present a few examples of subclinical disease effects in laboratory mice. As reviewed by Lipman and Perkins (2003), potential subclinical disease agents in mice include: mouse hepatitis virus (MHV), Sendai virus, LCMV, mouse parvovirus (MPV), Citrobacter rodentium, Clostridium piliforme, helicobacter, and Pseudomonas spp. MHV is the most common virus in laboratory mice and is well known to interfere with research by altering the physiology, and since MHV infects the lymphatic tissue it has long lasting effects on the immune system of both immunocompetent and immunocompromised mice. Other examples of possible confounding effects caused by MHV infections in mice include: involution and apoptosis of the thymus; altered macrophage activity; altered hepatic enzymes activity; reduced liver regeneration; and hematological changes (Lipman and Perkins, 2002). In contrast to the broad spectrum of disease caused by MHV, other agents may specifically target a single organ and alter its function. For example, Citrobacter rodentium is the etiologic agent of transmissible murine colonic hyperplasia. These bacterial infections alter the latency period for tumor induction and have cytokinetic effects on the colonic mucosal epithelium. Citrobacter rodentium may also alter the immune system by inhibiting antigen-specific cytotoxic T-cell activity (Lipman and Perkins 2002).
The following is a review of some of the most common lesions and infections that have been observed in zebrafish. Listed are both documented effects and our interpretation of potential effects on zebrafish in a research setting.
Pseudoloma neurophilia
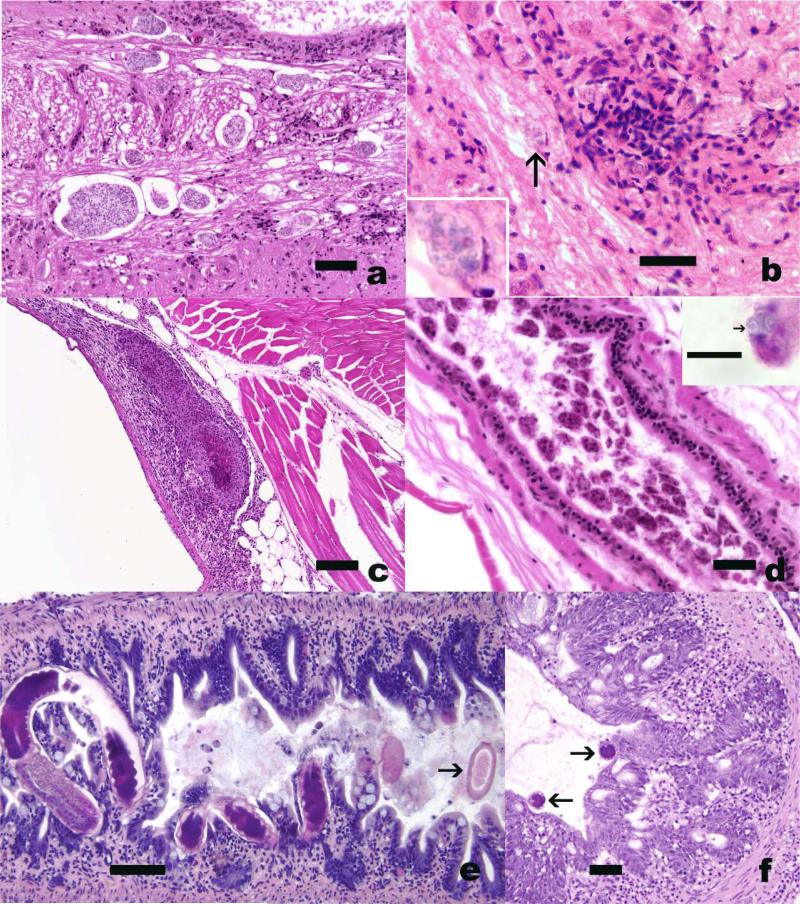
This microsporidium has been diagnosed in zebrafish from greater than half of the facilities that have submitted cases to the Zebrafish International Resource Center (ZIRC, University of Oregon, Eugene, OR, USA) diagnostic service (http://zebrafish.org/zirc/health/index.php). Clinical and macroscopic changes associated with this infection include emaciation, skeletal deformities, and lethargy (Matthews et al. 2001; Harper and Lawrence 2011; Murray et al. 2011). As the species name implies, the organism's primary site of infection is the hind brain, spinal cord and nerve root ganglia (Figure 1). Fish with clinical disease often exhibit chronic myositis in which individual spores are found with special stains in these regions of inflammation and muscle degeneration (Ramsay et al. 2009; Peterson et al. 2011; Sanders et al. 2012). In other instances, skeletal muscle lesions may be limited to patchy areas of atrophy in the epaxial musculature; the lack of inflammation in such areas suggests that these lesions represent denervation atrophy caused by microsporidian infection of spinal nerves. The presence of chronic, asymmetrical muscle lesions can result in grossly-apparent curvature of the spinal axis. Although an intense localized inflammatory response consisting of glial cell aggregates may be present in neural tissues where spores have been released, intact P. neurophilia plasmodia located within the spinal cord and brain are typically not associated with inflammation, and the plasmodia may instead act as slow-growing, space-occupying lesions that are tolerated by the host to a point. Microsporidian infections of the central nervous system (Fig. 1), and occasionally skeletal muscle, are frequently seen in apparently healthy fish submitted to the ZIRC diagnostic service (http://zebrafish.org/zirc/health/index.php).
Figure 1.
Pathogens in zebrafish with subclinical infections. H&E. A) Pseudoloma neurophilia in hind brain. Numerous aggregates of spores (arrows) with minimal tissue reaction. Bar = 50 μm B) Gliosis associated with a small nest of spores of P. neurophilia. Bar = 20 um Arrow = high magnification inset showing spores. C) Granuloma in swim bladder wall associated with Mycobacterium infection. D) Plasmodia of Myxidium sp. in the lumen of the common mesonephoric duct. Bar = 25 μm. Insert shows spore with characteristic polar capsules (arrow) at opposing end of oval spore. Bar = 10 μm. E, F) Pseudocapillaria tomentosa infections of the intestine. Bars = 50 μm. E) Sagittal section, showing adult worms penetrating epithelium. Arrow = free egg with bipolar plug. F) Cross sections of worms (arrow) associated with epithelial hyperplasia.
Documented Effects
In some respects, the microsporidium Pseudoloma neurophilia of zebrafish is analogous to Encephalitozoon cuniculi of laboratory rabbits and rodents (Künzel and Joachim 2010). Both can infect neural tissues, and infections are often asymptomatic in healthy animals. Zebrafish with clinical microsporidiosis are emaciated and may exhibit skeletal deformities (Matthews et al. 2001; Harper and Lawrence 2011), but many infected fish are asymptomatic and the infection is often seen in sentinel fish (http://zebrafish.org/zirc/health/index.php). Ramsay et al. (2009) conducted a large, long term in vivo study to evaluate the effects of the P. neurophilia on growth and fecundity in experimentally infected zebrafish, some of which were exposed to stressors. Infections were correlated with reduced growth, and were more pronounced in the TL strain of zebrafish as compared to the AB strain. Control TL fish weighed 27% more than experimentally-infected TL fish held under identical conditions. In another experiment conducted with AB fish, the severity of infection was demonstrated to correlate negatively with fecundity when fish were subjected to stress. The latter finding is particularly important, as egg production is a central requirement for laboratories that study developmental genetics. However, the detection of subclinical infections may not be a high priority in facilities that rear zebrafish embryos, for example, because the adult fish are used as broodstock and not test subjects. Ramsay et al. (2009) demonstrated that even these types of facilities should be cognizant of the impact that undetected disease can have in their fish stocks. As is often the case in chronic parasitic infections, it is difficult to determine the degree to which mortality is caused directly by P. neurophilia infections. However, we have seen less than 0.3% annual mortality in post-larval zebrafish at the Oregon State University SPF facility that has eliminated this parasite (Kent et al. 2011). Overall, fish in this laboratory appear healthy, are fecund, and do not exhibit emaciation or scoliotic changes that have been associated with the microsporidian infections.
Potential Effects
This microsporidium infects the central nervous system, and thus would certainly be a concern in any research projects that involved the assessment of neural function in adult fish. It is reasonable to assume that space occupying or inflammatory lesions in the brain, spinal cord, meninges, or skeletal muscle could profoundly affect behavior, which is an emerging field of zebrafish research (Sisson et al. 2006). In support of these assumptions, there is abundant literature in which asymptomatic parasitic infections of the central nervous system, especially those involving Toxoplasma gondii in both humans and mice (Flegr 2007), have been associated with behavioral alterations. Other impacts of P. neurophilia would likely occur in experiments with growth and fecundity endpoints. Larval zebrafish are quite susceptible to P. neurophilia infections (Ferguson et al. 2007), and hence the infection could compromise developmental neurotoxicity testing and musculoskeletal development studies.
Microsporidia are well recognized for their ability to cause disease in immunocompromised hosts, although there were fewer than 10 reports of such infections in humans prior to the onset of the acquired immune deficiency syndrome (AIDS) pandemic. Now several microsporidian species are responsible for gastrointestinal infections in AIDS patients (Didier 2005). Likewise, among laboratory animals, Encephalitozoon cuniculi is usually only a problem in rabbits and rodents that are immune-compromised (Künzel and Joachim 2010). Zebrafish are now used extensively in tissue transplant studies, in which the immune system is intentionally incapacitated by gamma irradiation (Traver et al. 2004), and latent microsporidian infections would likely become clinical in these fish or in other studies that involve immunlogically challenged zebrafish.
Mycobacterium spp.
Although frequently not identified to the species level, mycobacteriosis has been diagnosed at many facilities (http://zebrafish.org/zirc/health/index.php; Whipps et al. 2012). When species identifications are made by culture or PCR directly from tissues, zebrafish mycobacteria most often turn out to be M. chelonae. (Kent et al. 2004; Murray et al. 2011; Whipps et al. 2008, 2011). The Sinhhuber Aquatic Research Laboratory at Oregon State University has established a specific pathogen-free (SPF) zebrafish facility in which P. neurophilia has been eradicated, but mycobacteriosis is occasionally diagnosed by histopathology in clinically normal sentinel fish or 1 year old retired brood stock at this facility (Kent et al. 2011). This demonstrates that opportunistic Mycobacterium spp. are ubiquitous in zebrafish facilities, even those with pathogen control.
A wide variety of chronic inflammatory lesions are observed in clinically normal zebrafish with underlying Mycobacterium spp. infections, and common targets include abdominal organs such as the kidney, spleen, ovary, and swim bladder (diffuse, chronic aerocystitis) (Kent et al. 2004; Watral and Kent 2007; Whipps et al. 2008). The extent, distribution, and microscopic appearance of the inflammatory response can vary considerably, and is presumably dependent on the species and strain of the mycobacterium, the level of exposure, the chronicity of the particular infection, the immune status of the host, and potentially, undetermined environmental factors. Lesions range from discrete spherical to ovoid granulomas with or without necrotic centers (Figure 1), to diffuse aggregates or sheets of macrophages. The macrophages themselves are often epithelioid (cells with abundant, dense cytoplasm and enlarged, open-faced nuclei), or they may evolve into flattened, fibroblast-like cells as the granulomas become senescent. Suspected infections with acid fast bacilli such as Mycobacterium or Nocardia spp. are commonly confirmed via the use of Ziehl-Neelsen staining or other specialized histologic procedures.
Documented Effects
Mycobacterium haemophilium and M. marinum can cause severe disease in zebrafish (Watral and Kent 2007; Whipps et al 2007), although most cases are associated with less pathogenic species, such as M. chelonae (Astrosky et al 2000; Watral and Kent 2007 Whipps et al. 2008). Based on ZIRC diagnostic service data and some of our other studies, it is not uncommon to find granulomatous lesions associated with acid fast bacilli in multiple anatomic sites throughout clinically normal fish (e.g., sentinels sacrificed for the purpose of disease monitoring). In older fish from our P. neurophilia SPF facility, we detected an approximately 4% prevalence of mycobacterial infection by histology (Kent et al. 2011), and we have seen infection frequencies as high as 50% in presumably normal appearing sentinel fish from other facilities (Whipps et al. 2008; Murray et al. 2011). It is also recognized that the histopathologic diagnosis of mycobacteriosis through the use of one or more of the various acid-fast stains can yield false negative results (Fukunaga et al. 2002), which has prompted the development of molecular methods for the detection, confirmation and speciation of mycobacterial infections (Whipps et al. 2011).
Zebrafish mycobacteria are not usuually identified to the species level, because the disease is most often diagnosed by histopathology, and the submission of specimens in formalin-based fixatives precludes culturing the organisms. However, a survey conducted at ZIRC that employed culture or molecular techniques for species determination revealed that many asymptomatic fish were infected with M. chelonae (Whipps et al. 2008). In addition, experimental infections with M. chelonae by intrapertioneal injection yielded varying levels of infection, but minimal or no clinical disease and mortality (Watral and Kent 2007; Ramsay et al. 2009). Replication of these studies using the same strain of M. chelonae produced similar results (Peterson, T., Oregon State University, pers. comm.).
Little information exists in the literature on the impacts of asymptomatic mycobacterial infections on the outcomes of zebrafish studies. When Capps et al. (2004) investigated the formation of macrophage aggregates in zebrafish following exposure to perchlorate, the histopathological examination of some experimental groups was terminated due to underlying mycobacterial infections. We are not aware of other examples in zebrafish research in which confounding effects of mycobacteriosis have been documented. However, Broussard et al. (2009) demonstrated that Japanese medaka (Oryzias latipes) with M. marinum infections develop more liver tumors than controls when exposed to benzo-a-pyrene. Additionally, there are several reports of toxicology or carcinogenicity studies that featured medaka, guppies (Poecilia reticulata) or fathead minnows (Pimephales promelas), in which the interpretation of neoplastic or toxicopathic changes was compromised by a high prevalence of inflammatory lesions due to Mycobacterium spp. infections (Abner et al. 1994).
From a risk assessment standpoint, it is reasonable to assume that many may question the applicability of studies in which mycobacteriosis appears to be a confounding factor for the interpretation of experimental results. Because some pathogenic mycobacteria are capable of causing localized or systemic disease in humans (especially in immune-compromised individuals), one final consideration concerns the recognition that occult mycobacterial contamination of experimental aquatic systems may serve as potential reservoirs for zoonotic disease transmission. It should be noted that all Mycobacterium spp. that have been demonstrated to cause disease in zebrafish (Whipps et al. 2012) are considered to be potential human pathogens.
Potential Effects
Zebrafish are currently employed as models for a wide variety of bacterial and viral diseases, for both humans and aquacultures fishes (Phelps and Neely 2005; Petrie-Hanson et al. 2007; Crim and Riley 2011). Occult infections caused by Mycobacterium spp. could directly compromise studies of unrelated infectious diseases, and certainly, undetected infections with mycobacteria such as M. chelonae would represent a confounding factor for research involving experimental infections of other Mycobacterium spp. (Prouty et al. 2003). In some instances, mycobacteria-induced inflammatory lesions may be severe in fish that do not exhibit obvious clinical signs. That degree of chronic inflammation could have profound effects on genomic, proteinomic, or metabolomic studies designed to investigate inflammation-mediated pathways. Tumor necrosis factor alpha (TNFα), nuclear factor kappa beta (NFκβ) and other inflammatory mediators are key regulators of normal development and homeostasis for many tissues including those of the central nervous system, endocrine and, cardiovascular systems, and of bone, fat, hemopoietic and lymphoid tissues (Boersma and Meffert 2008; Chaisson et al. 2004; Doherty 2007; Hernandez-Gutierrez et al. 2006). TNFα is also a potent endogenous mutagen that acts via the production of reactive oxygen radicals (Yan et al. 2006). Accordingly, alterations in the balance of such inflammatory mediators by mycobacteria or other agents that evoke chronic inflammation could disrupt normal tissue development and homeostasis, and thereby radically alter study results.
Pseudocapillaria tomentosa
This capillarid nematode is found in the proximal intestinal tract, and localized reactions to the presence and migration of this nematode can include florid mucosal hyperplasia and marked segmental inflammation (enteritis) (Kent et al. 2002). Affected fish are often clinically symptomatic, and exhibit profound wasting that is presumably due to an inability to absorb sufficient nutrients. It is likely that progression of the disease may be more insidious in lightly infected fish or in early infections, and asymptomatic fish have been seen at the ZIRC diagnostic service (http://zebrafish.org/zirc/health/index.php). The diagnosis of pseudocapillarid infections is typically not difficult, as the cross-sections of partially or wholly embedded worms are readily visualized in the mucosal epithelium, and the gut lumen may contain the double-operculated, barrel-shaped eggs (Figure 1). Conversely, chronically infected fish may present with severe atrophy of the proximal intestinal mucosa and few or no remaining parasites.
Potential Effects
Proliferative enteritis caused by P. tomentosa infections has been observed in concert with intestinal neoplasia in zebrafish, and a causal relationship has been postulated (Kent et al. 2002; Spitsbergen et al. 2012). Consequently, the possibility exists that parasite-induced tumors might be attributed incorrectly to experimental chemical exposure or other oncogenic etiologies. Additionally, in studies where the nematodes are responsible for inflammatory, hyperplastic and/or atrophic lesions in the gut, it is likely that adverse impacts on nutrient absorption would affect experimental endpoints related to metabolism, electrolyte balance, growth, or ability to thrive.
Myxidium sp.
Plasmodial stages of a myxozoan (Myxidium sp.) are frequently seen in large numbers in the common mesonephric ducts of zebrafish (Figure 1). They are invariably confined to the lumen or attached to the surface of epithelial cells, and have not been associated with significant histopathological changes or clinical disease. The microscopic appearance of these myxozoans in tissue sections is rather unusual, because the plasmodia tend to vary considerably in size and shape, and because the ragged contours and heterogeneous coloration of the parasites bears some resemblance to necrotic cellular debris. The species identity of this parasite has not been confirmed, but spore morphology, with polar capsules at opposing ends of the spore, indicate that it is a member of the genus Myxidium (Figure 1).
Potential Effects
Infections by this myxozoan in the common mesospheric ducts and occasionally renal tubules have consistently been associated with few histological changes. Admittedly, it has not yet been established if the relationship between these organisms and the host is one of parasitism or commensalism (mutualism being considered unlikely). However, given that the plasmodial stages often fill the lumens of affected renal structures, it is possible that their presence in heavily infected fish might have some influence on urine flow and osmoregulation.
Egg Associated Inflammation and Fibroplasia (EAIF)
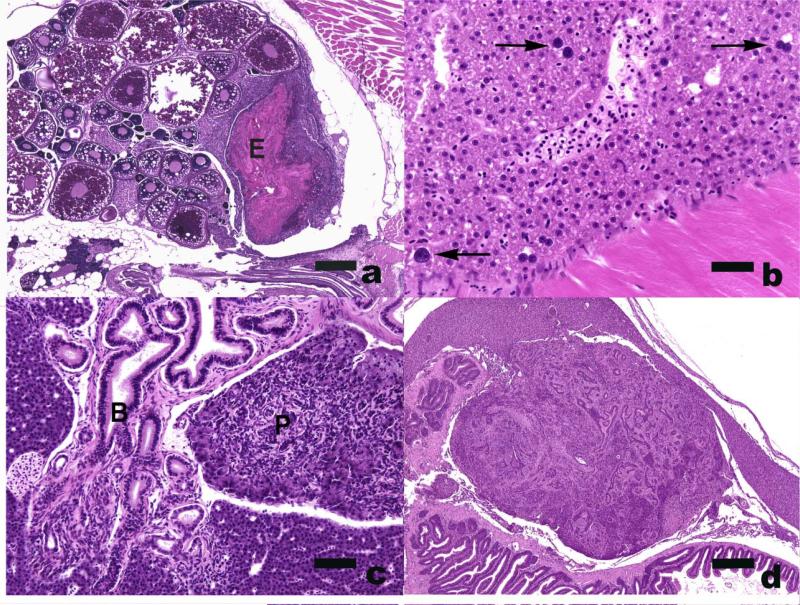
This condition has been seen at many institutes and there is strong, albeit anecdotal, evidence to suggest that EAIF is caused by egg retention in fish that have not had the opportunity to spawn frequently. Although ovaries with this condition may be infected with mycobacteria (Whipps et al 2008), many cases are not associated with readily detectable infectious agents (Rossteuscher et al. 2008). In one zebrafish study, increased atresia (degeneration) of mature eggs was statistically correlated with the occurrence of egg debris (Rossteucher et al. 2008). The authors of that investigation concluded that the associated inflammatory changes could be caused either by primary inflammation followed by increased atresia, or conversely, by a secondary inflammatory response to the presence of egg debris. Degeneration of eggs with concurrent chronic inflammation is a common occurrence in many fishes that do not spawn on a regular basis. Zebrafish in advanced stages of EAIF exhibit swollen abdomens, but mild forms of the condition may be subclinical. In histologic sections stained with hematoxylin and eosin, EAIF presents as a large, irregularly-shaped, disorganized coagulum of heterogeneous pink and blue material that is frequently located near the caudal aspect of an ovary that may or may not contain excessive numbers of follicles (Figure 2). The pink material typically represents yolk derived from atretic oocytes, and the yolk may be combined in a layered fashion with folded chorionic (vitelline) membranes. Often located at the periphery of the yolk material, or occasionally scattered throughout, is a dusting of finely granular blue (basophilic) material that consists of lysed inflammatory cells, necrotic cellular debris, and occasionally, visible bacterial colonies. The yolk coagulum may be surrounded by an intense granulomatous inflammatory reaction that features epithelioid macrophages and/or mutinucleated giant cells.
Figure 2.
Lesions in asymptomatic zebrafish. H&E A) Eggs associated inflammation and fibroplasia. Note large accumulation of eosinophilic yolk debris (E). Bar = 250 μm B) Hepatic megalocytosis. Arrows = enlarged, hyperchromatic hepatocyte nuclei. Bar = 25 μm. C) Bile (B) and pancreatic (P) duct hyperplasia. Bar = 50 um D) pancreatic ductular carcinoma. Bar = 250 μm.
Documented Effects
Retention of eggs has been reported to reduce future viability in several fish species, including zebrafish (Hisoaka and Firlit 1962; Skinner and Watt 2007). This is clearly a concern to research areas such as developmental genetics, which rely on zebrafish to consistently produce large numbers of eggs.
Potential Effects
Zebrafish are one of three small aquarium species (the other two are medaka and fathead minnow) that are most commonly used to investigate disruption of the vertebrate reproductive system by exogenous chemicals that have hormonal activity (Segner 2009; Scholz and Mayer 2008). As oocyte (follicular) atresia is a common non-specific effect of several classes of endocrine disrupting compounds (EDC), is understandable that interpretation of EDC study results might be confounded by spontaneous occurrences of chronic oophoritis, which is a hallmark of EAIF. Furthermore, because it is likely that egg production would be reduced in females with significant EAIF, at least some of which may not have obvious clinical signs (i.e., abdominal swelling), there is the potential that EAIF may negatively affect performance in multigenerational studies, and bias the outcomes of experiments that have fertility/fecundity endpoints. Indeed, we have seen females with large rafts of eosiphilic debris in the oviducts that did not have swollen abdomens.
Liver Lesions
We frequently observe hepatic megalocytosis and/or bile duct lesions in sentinel fish. Megalocytosis is characterized by hepatocytes that have both enlarged nuclei and excessive amounts of cytoplasm (Figure 2). Megalocytic hepatocytes, which tend to be randomly distributed in affected zebrafish livers, often have multiple (e.g., two or three) nuclei, consistent with the notion that they represent cells that have failed to divide mitotically. The nuclei of such cells may be round or slightly irregular in outline, and tend to be darker than those of neighboring hepatocytes due to amplified chromatin material. Severe hepatic megalocytosis was found in zebrafish in a new facility, and was presumed to be associated with a toxicant from new plastics, glues, etc. Affected fish were morbibund in that particular case (Kent et al. 2011). In contrast, we have seen many instances of this condition in well established facilities, and such fish usually do not present with clinical signs (http://zebrafish.org/zirc/health/index.php). Spitsbergen and Kent (2003) noted that hepatic megalocytosis was particularly common in recirculating systems that used fluidized sand filters.
Hepatic megalocytosis and other similar liver changes in fish can be caused by both natural toxins (e.g., cyanobacteria in water, pyrollizidine alkaloids from terrestrial plants) (Andersen et al. 1993; Hendricks et al. 1981) or a variety of anthropogenic agents (Myers et al. 2003). The cause of the hepatic megalocytosis in zebrafish has not been determined. Although fish exposed to chemical carcinogens may develop hepatic megatocytosis, there is no firm evidence as yet to indicate that affected hepatocytes are precursors of hepatocellular neoplasms. Enlarged hepatocytes with polypoid nuclei can persists for many months in livers of fish that have recovered from an acute toxicity event (Kent 1990). Therefore, the observation of this lesion without other concurrent toxic changes may indicate that the fish was exposed to a hepatoxicant at an earlier life stage.
Bile duct proliferation in the liver is often noted in normal-appearing fish, and seems to be particularly common in some lines of TL zebrafish (Figure 2). Lesions are not always confined to the biliary tree, as the exocrine pancreatic ducts are also periodically affected, and in some cases the origin of the ductular tissue, i.e., biliary or pancreatic, may not be clear. The condition is typically characterized by a locally extensive proliferation of multiple, variably-sized, ductular profiles that may be intra-hepatic, extra-hepatic, or both. Each duct is lined by a single layer of columnar epithelium that features densely basophilic, elongated, basilar nuclei. The proliferating ducts are frequently surrounded by variable amounts of scirrous fibroblastic tissue that is populated by low to moderate numbers of mononuclear inflammatory cells. Distinguishing proliferations that are merely hyperplastic (potentially reversible due to a lack of permanent genetic alterations in the cells) as compared to those that are truly neoplastic (autonomously growing benign or malignant tumors) based solely on morphologic characteristics can be a challenge, as the pattern of duct formation may be a bit haphazard and extensive in some cases. On the other hand, large masses of disorganized ductular tissue that have abundant cellular atypica are readily diagnosed as carcinomas (Figure 2).
Potential Effects
Regardless of the cause of these commonly observed liver lesions (e.g., hepatic megalocytosis), it is obvious that the presence of lesions or unidentified hepatoxicants would profoundly affect any toxicological study involving liver morphology or function. Although genetic predisposition may be the primary determinant for bile duct proliferation in zebrafish, in a toxicological bioassay it is conceivable that type of lesion could be ascribed inadvertently to some form of experimental treatment.
Neoplasia
Neoplasms are not uncommon in zebrafish, particularly in older individuals. Neoplasms common in zebrafish are reviewed by Spitsbergen et al. (2012). Spindle cell sarcomas (malignant nerve sheath tumors) often present as macroscopic masses in the flanks or eyes of fish, and male fish with spermacytic seminomas often have swollen abdomens. Ultimobranchial gland tumors and intestinal carcinomas (see Spitsbergen et al. 2012) are two of the most common neoplasms found in histological examinations of apparently healthy sentinel fish.
Potential Effects
It is remarkable how extensive gastrointestinal tumors may be in sentinel zebrafish that do not have obvious signs of clinical disease. Given the advanced nature of such lesions, it is likely they would impact the general health of affected fish, including metrics such as growth, fecundity, and susceptibility to infectious diseases. Clearly “background” neoplasms would certainly confound carcinogenicity studies, and would likely compromise nutritional experiments in which growth is an endpoint.
The ultimobranchial gland normally secretes calcitonin, the role of which is to decrease plasma calcium; however, to the knowledge of the authors, the functionality of ultimobranchial gland tumors and potential effects of these neoplasms on calcium homeostasis in zebrafish has not yet been investigated.
Although there has been little well-established scientific evidence to suggest that EDCs are capable of inducing gonadal germ cell neoplasms, it is possible that the spontaneous occurrence of spermatocytic seminomas in one or more EDC-exposed zebrafish might be misinterpreted as a de facto effect of EDC exposure.
Conclusions and Recommendations
In all likelihood, the negative effects of subclinical disease are drastically underreported. There are several plausible reasons for this. First, many experiments do not incorporate histopathologic endpoints; and in such studies there would be little opportunity to identify conditions that may only be evident at the microscopic level. Second, a study is often not published when bacterial or parasitic infection of the test subjects has contributed to the failure of the experiment, either by adversely affecting the study performance, or by hampering the interpretation of the experimental results. Facility administrators may also be loathe to publish such findings, because they may be perceived as public admissions of underlying husbandry problems. A third probable reason why asymptomatic disease is underreported is because manuscript authors and journal reviewers often consider these types of conditions to be incidental to the primary focus of the study, and therefore not worthy of publication. Whatever the explanation, underreporting is one overall explanation why the existence and potential consequences of subclinical diseases may be underappreciated by investigators.
Reduction or elimination of the infectious diseases and other disorders described heretofore would certainly improve the zebrafish model. Basic research that elucidates the etiology of idiopathic conditions, such as hepatic megalocytosis, may also be of benefit. In order to better control pathogens, it is vital to understand aspects of infectivity such as modes of transmission, host range, and viability in containment systems. Additionally, the development of diagnostic tests and treatments are needed to create effective strategies for avoiding or treating these infections. One strategic approach is the establishment of SPF zebrafish, which has already been accomplished for P. neurophilia (Kent et al. 2011). Fortunately, the zebrafish life cycle, like those of other egg-laying teleosts, is particularly amenable to the development of SPF stocks. In contrast to mammals, embryos can easily be separated from the parents as unhatched eggs. Eggs in turn can be surface-disinfected and hatched in sterile water, which is already a common practice in the zebrafish community. Lastly, as several hundred eggs are laid at each spawning, a statistically relevant subsample can be lethally screened from each spawn. One obstacle to controlling or eradicating subclinical disease has been a general lack of enthusiasm for this goal among research facilities that have not experienced significant mortality events. This may be due to a lack of awareness regarding the existence of these conditions, or in some cases, to the perception that occult disease has little or no impact on research findings. Therefore, studies designed to directly and quantitatively evaluate the effects of subclinical disorders on commonly employed metrics, such as physiological, immunological, microanatomical, or growth endpoints, are warranted. The availability of this type of information would not only be of value to investigators, as well the recognition of quantifiable effects should provide facility administrators with further incentive for eliminating these conditions.
Contributor Information
Michael L. Kent, Department of Microbiology, 220 Nash Hall, Oregon State University, Corvallis, Oregon, 977331. Michael.Kent@oregonstate.edu.
Claudia Harper, DVM, Boston, MA harpermead@gmail.com.
Jeffrey C. Wolf, DVM, Dipl. ACVP Experimental Pathology Laboratories, Inc. 45600 Terminal Drive, Sterling, VA, 20166 USA Tel: 703-471-7060 Ext 242 Fax: 703-471-8447 jwolf@epl-inc.com
References
- Abner SR, Frazier CL, Scheibe JS, Krol RA, Overstreet RM, Walker WW, Hawkins WE. Chronic inflammatory lesions in two small fish species, medaka (Oryzias latipes) and guppy (Poecilia reticulata), used in carcinogenesis bioassays. In: Stolen JS, Fletcher TC, editors. Modulators of Fish Immune Responses: Models for Environmental Toxicology, Biomarkers, and Immunostimulators. SOS Publications; Fair Haven, NJ, USA: 1994. pp. 219–233. [Google Scholar]
- Andersen RJ, Luu HA, Chen DZX, Holmes CFB, Kent ML, Le Blanc M, Taylor FJR, Williams DE. Chemical and biological evidence links microcystin to salmon ‘netpen liver disease’. Toxicon. 1993;31:1315–1323. doi: 10.1016/0041-0101(93)90404-7. [DOI] [PubMed] [Google Scholar]
- Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp Med. 2000;50:666–72. [PubMed] [Google Scholar]
- Boersma MC, Meffert MK. Novel roles for the NF-kappaB signaling pathway in regulating neuronal function. Sci. Signal. 2008;1(6):pe7. doi: 10.1126/stke.16pe7. [DOI] [PubMed] [Google Scholar]
- Broussard GW, Norris MB, Schwindt AR, Fournie JW, Winn RN, Kent ML, Ennis DG. Chronic Mycobacterium marinum infection acts as a tumor promoter in Japanese medaka. Comp. Biochem. Physiol–Part C (Toxicol. Pharmacol.) 2009;149:152–160. doi: 10.1016/j.cbpc.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capps T, Mukhi S, Rinchard JJ, Theodorakis CW, Blazer VS, Patino R. Exposure to perchlorate induces formation of macrophage aggregates in the trunk kidney of zebrafish and mosquito fish. J. Aquat. Animal Health. 2004;16:145–151. [Google Scholar]
- Chaisson ML, Branstetter DG, Derry JM, Armstrong AP, Tometsko ME, Takeda K, Akira S, Dougall WC. Osteoclast differentiation is impaired in the absence of inhibitor of kappa B kinase alpha. J Biol Chem. 2004;279:54841–54848. doi: 10.1074/jbc.M406392200. [DOI] [PubMed] [Google Scholar]
- Craig JC, et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–517. doi: 10.1038/nature09806. eol and 21 co-authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim M, Riley L. Status of viral diseases in zebrafish. ILAR J. 2011;(this issue) doi: 10.1093/ilar.53.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES. Microsporidiosis: An emerging and opportunistic infection in humans and animals. Acta Tropica. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Doherty GH. Developmental switch in the effects of TNFalpha on ventral midbrain dopaminergic neurons. Neurosci. Res. 2007;57:296–305. doi: 10.1016/j.neures.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Gerhard GS. Small laboratory fish as models for aging research. Ageing Res Rev. 2007;6:64–72. doi: 10.1016/j.arr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish Microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Dis Aquat Org. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Flegr J. Effects of Toxoplasma on human behavior. Schizophr Bull. 2007;33:757–760. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H, Murakami T, Gondo T, Sugi K, Ishihara T. Sensitivity of acid-fast staining for Mycobacterium tuberculosis in formalin-fixed tissue. Am J Respir Crit Care Med. 2002;166:994–997. doi: 10.1164/rccm.2111028. [DOI] [PubMed] [Google Scholar]
- Hendricks JD, Sinnhuber RO, Henderson MC, Buhler DR. Liver and kidney pathology in rainbow trout (Salmo gairdneri) exposed to dietary pyrrolizidine (Senecio) alkaloids. Exper Mol Pathol. 1981;35:170–183. doi: 10.1016/0014-4800(81)90057-5. [DOI] [PubMed] [Google Scholar]
- Hernández-Gutierrez I, García-Peláez, Zentella-Dehesa A, Ramos-Kuri M, Hernández-Franco P, Hernández-Sánchez F, Rojas E. NF-kappaB signaling blockade by Bay 11-7085 during early cardiac morphogenesis induces alterations of the outflow tract in chicken heart. Apoptosis. 2006;11:1101–1109. doi: 10.1007/s10495-006-6984-z. [DOI] [PubMed] [Google Scholar]
- Hisaoka KK, Firlit CF. Ovarian cycle and egg production in the zebrafish, Brachydanio rerio. Copeia. 1962;1962:788–792. [Google Scholar]
- Kent ML. Netpen liver disease (NLD) of salmonid fishes reared in sea water: species susceptibility, recovery, and probable cause. Dis Aquat Org. 1990;8:21–28. [Google Scholar]
- Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med. 2002;52:354–588. [PubMed] [Google Scholar]
- Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, Tanguay RL. Development and maintenance of a specific pathogen free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis. Aquat. Org. 2011 doi: 10.3354/dao02333. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Matthews JL, Bishop-Stewart JK, Whipps CM, Watral V, Poort M, Bermudez LE. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp. Biochem. Physiol–Part C (Toxicol. Pharmacol.) 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Künzel F, Joachim A. Encephalitozoonosis in rabbits. Parasitol Res. 2010;106:299–309. doi: 10.1007/s00436-009-1679-3. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nature Rev Gen. 2007;5:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lipman NS, Perkins SE. Factors that may influence animal research. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine. 2nd ed. Academic Press; Orlando FL: 2002. pp. 1143–1184. chapter 29. [Google Scholar]
- Matthews JL, Brown AMV, Larison K, Bishop-Stewart JK, Rogers P, Kent ML. Pseudoloma neurophilia n.g., n.sp., a new genus and species of Microsporidia from the central nervous system of the zebrafish (Danio rerio). J Euk Microbiol. 2001;48:229–235. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Mathew LK, Simonich MT, Tanguay RL. AHR-dependent misregulation of Wnt signaling disrupts tissue regeneration. Biochem Pharmacol. 2009;77:498–4950. doi: 10.1016/j.bcp.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Bauer J, Tallen A, Matthews JL, Westerfield M, Varga ZM. Characterization and management of asymptomatic Mycobacterium infections at the Zebrafish International Resource Center. J Am Asso Lab Sci. 2011;50:675–679. [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, Bauer J, Varga ZM, Westerfield M. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comp Med. 2011;61:322–329. [PMC free article] [PubMed] [Google Scholar]
- Myers MS, Johnson LL, Collier TK. Establishing the causal relationship between polycyclic aromatic hydrocarbon (PAH) exposure and hepatic neoplasms and neoplasia-related liver lesions in English sole (Pleuronectes vetulus) Human Ecoll Risk Assess. 2003;9:67–94. [Google Scholar]
- Peterson TS, Spitsbergen JM, Feist SW, Kent ML. The Luna stain, an improved selective stain for detection of microsporidian spores in histologic sections. Dis Aquat Org. 2011 doi: 10.3354/dao02346. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie-Hanson L, Romano CL, Mackey RB, Khosravi P, Hohn CM, Boyle CR. Evaluation of zebrafish Danio rerio as a model for Enteric Septicemia of Catfish (ESC). J Aquat Anim Health. 2007;19:151–158. doi: 10.1577/H06-026.1. [DOI] [PubMed] [Google Scholar]
- Phelps HA, Neely MN. Evolution of the zebrafish model: From development to immunity and infectious disease. Zebrafish. 2005;2:87–103. doi: 10.1089/zeb.2005.2.87. [DOI] [PubMed] [Google Scholar]
- Prouty MG, Correa NE, Barker LP, Jagadeeswaran P, Klose KE. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS. 2003;225:177–182. doi: 10.1016/S0378-1097(03)00446-4. [DOI] [PubMed] [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). J Fish Dis. 2009;32:931–941. doi: 10.1111/j.1365-2761.2009.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia (Microsporidia) infections in zebrafish (Danio rerio): Effects of stress on survival, growth and reproduction. Dis Aquat Org. 2009;88:69–84. doi: 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossteuscher S, Schmidt-Posthaus H, Schäfers C, Teigeler T, Segner H. Background pathology of the ovary in a laboratory population of zebrafish Danio rerio. Dis Aquat Org. 2008;79:169–172. doi: 10.3354/dao01893. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Watral V, Kent ML. Microsporidiosis in zebrafish research facilities. ILAR J. 2012;(This Issue) doi: 10.1093/ilar.53.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz S, Mayer I. Molecular biomarkers of endocrine disruption in small model fish. Mol Cell Endocrinol. 2008;293:57–70. doi: 10.1016/j.mce.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Segner H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:187–195. doi: 10.1016/j.cbpc.2008.10.099. [DOI] [PubMed] [Google Scholar]
- Shu Liu S, Leach SD. Zebrafish models for cancer. Ann Rev Pathol Mech Dis. 2011;6:71–93. doi: 10.1146/annurev-pathol-011110-130330. [DOI] [PubMed] [Google Scholar]
- Sisson M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: zebra fish as an upcoming model system. Lab Anim. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- Skinner AMJ, Watt PJ. Strategic egg allocation in the zebra fish, Danio rerio. Behav Ecol. 2007;18:905–909. [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research – advantages and current limitations. Toxicol Pathol. 2003;31(suppl.):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Buhler DR, Peteson TS. Neoplasia in zebrafish reseach colonies. ILAR. 2012;((this issue)) doi: 10.1093/ilar.53.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver DA, Winzeler HM, Stern EA, Mayhall DM, Langenau JL, Look Kutok AT, Zon LI. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104:1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- Trede NS, Langenau M, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaulation of embyrotoxicity using the zebrafish model. Methods Mol Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comp Biochem Physiol –Part C (Toxicol. Pharmacol.) 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [Special Issues for Proceed. NCRR Aquat. Animal Models Human Dis. Conf. 30 Oct – 2 Nov, 2005, Athens, Georgia]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in zebrafish research colonies. ILAR J. 2012;(this issue) doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol Lett. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish (Danio rerio). Dis Aquat. Org. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]