Abstract
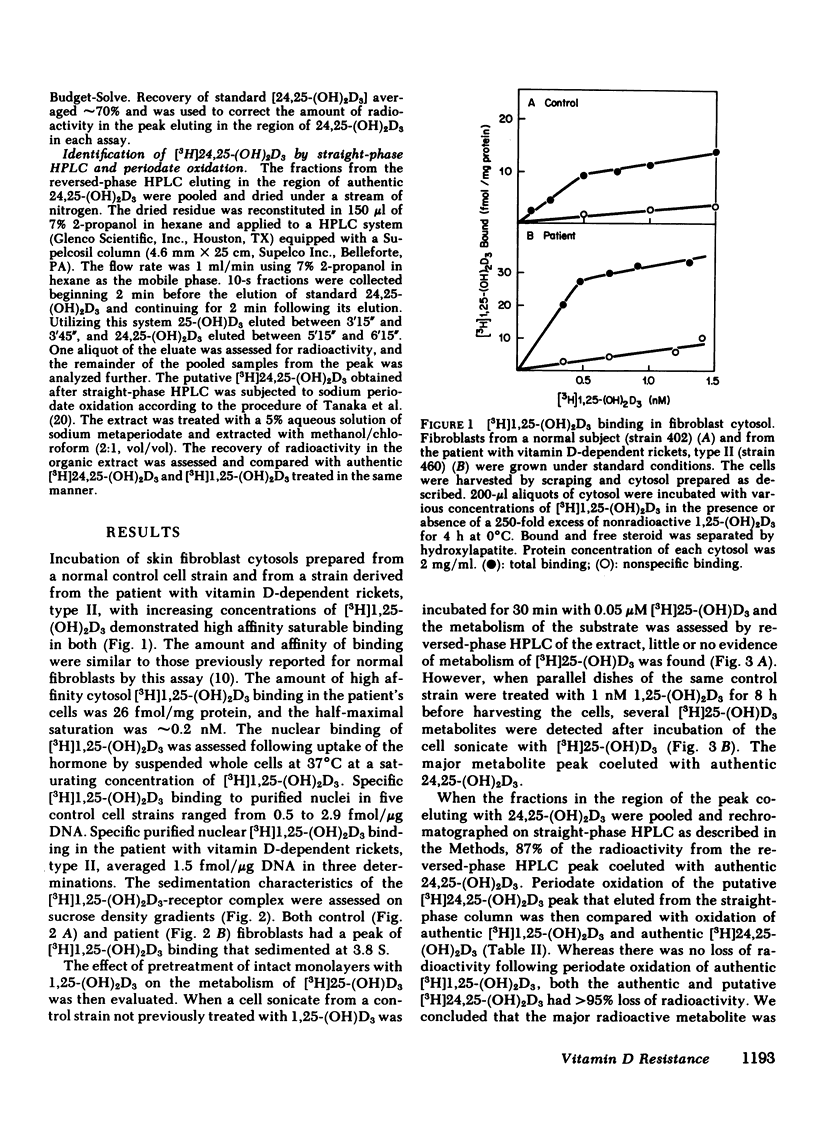
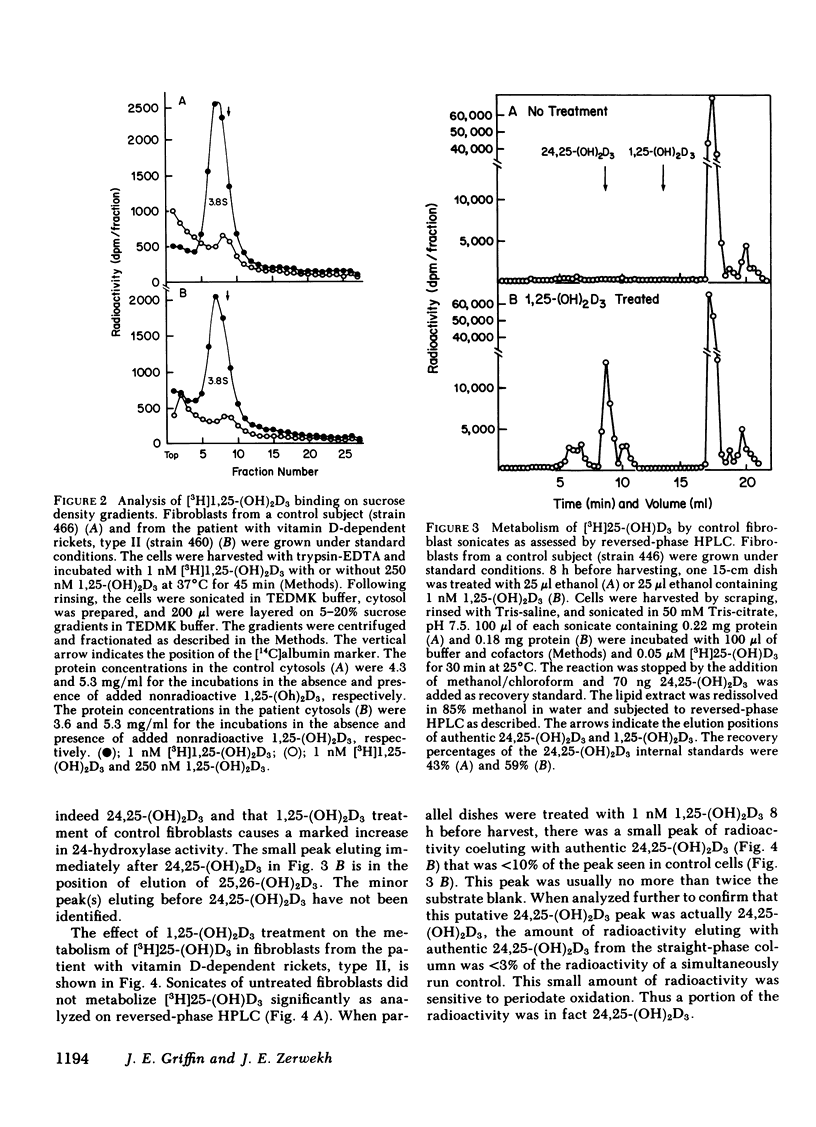
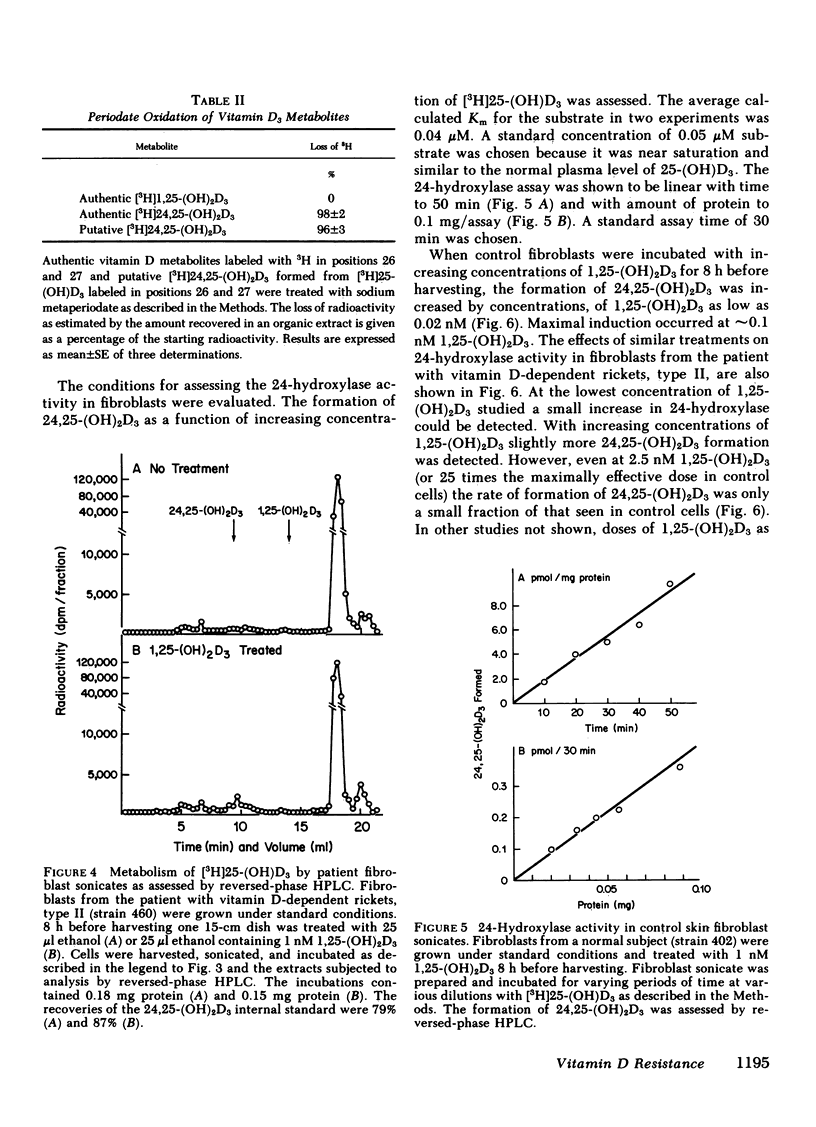
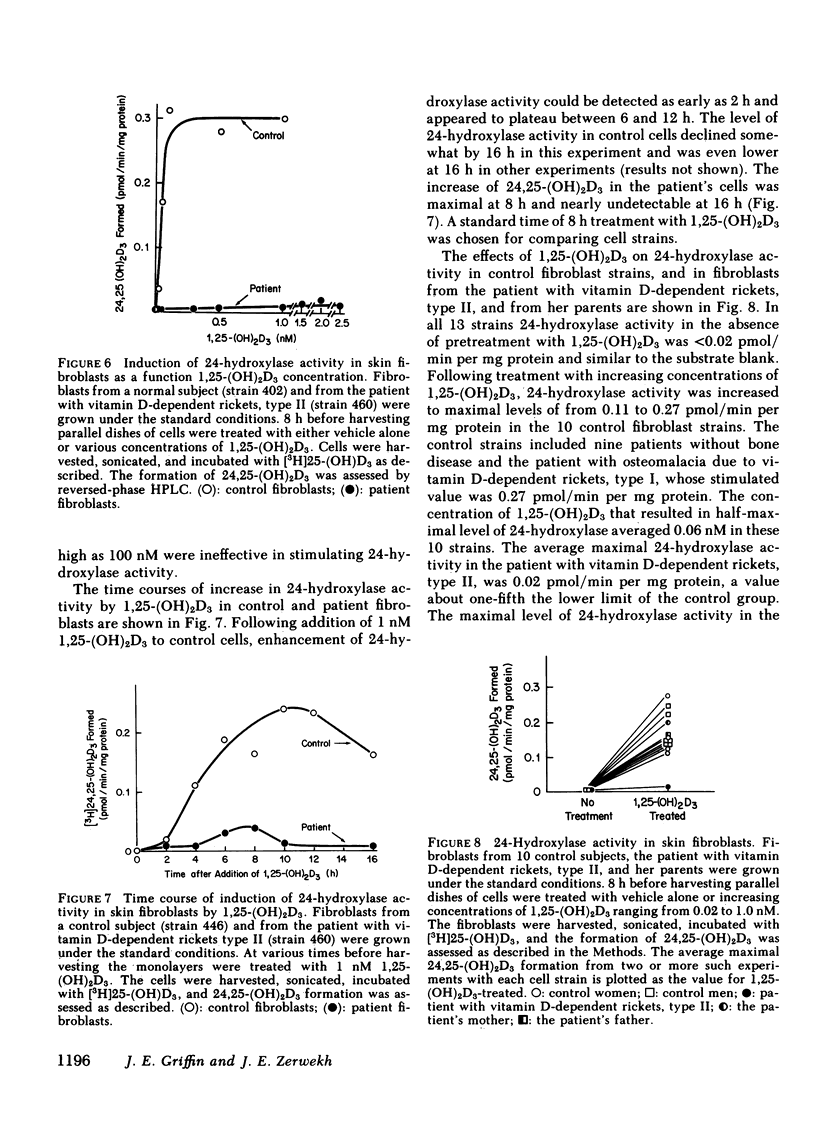
We describe studies of the molecular defect in 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] action in cultured skin fibroblasts from a patient previously reported to have vitamin D-dependent rickets, type II. Binding of [3H]1,25-(OH)2D3 in fibroblast cytosol was normal with a Bmax (amount of high affinity binding) of 26 fmol/mg protein and a half-maximal saturation of 0.2 nM. Nuclear binding of [3H]1,25-(OH)2D3 following whole cell uptake was 1.5 fmol/micrograms DNA in patient fibroblasts compared with a range of 0.5-2.9 fmol/micrograms DNA in five control strains. The size of the [3H]1,25-(OH)2D3-receptor complex on sucrose density gradients, 3.8 S, was the same as in normal cells. This patient, therefore, appeared to have a receptor-positive form of resistance to 1,25-(OH)2D3. To document resistance to 1,25-(OH)2D3 in the fibroblasts we developed a method for detection of 1,25-(OH)2D3 action in normal skin fibroblasts. Following treatment of normal cell monolayers with 1,25-(OH)2D3 there was more than a 20-fold increase of 25-hydroxy-vitamin D-24-hydroxylase (24-hydroxylase) activity. Treatment of 10 control cell strains with 1,25-(OH)2D3 for 8 h increased the formation of 24,25-dihydroxy-vitamin D3 from 25-hydroxyvitamin D3 in cell sonicates from less than 0.02 to 0.11-0.27 pmol/min per mg protein. When cells from the patient with vitamin D-dependent rickets, type II were treated with 1,25-(OH)2D3 in a similar manner, maximal 24-hydroxylase activity was only 0.02 pmol/min per mg protein, less than a fifth the lower limit of normal. 24-Hydroxylase activity in fibroblasts from the parents of the patient increased normally following treatment with 1,25-(OH)2D3. We conclude that impaired induction of 24-hydroxylase in the presence of normal receptor binding is evidence for postreceptor resistance to the action of 1,25-(OH)2D3.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein J. A., Meyer W. J., 3rd, Jones H. W., Jr, Migeon C. J. Androgen insensitivity in man: evidence for genetic heterogeneity. Proc Natl Acad Sci U S A. 1976 Mar;73(3):891–894. doi: 10.1073/pnas.73.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beer S., Tieder M., Kohelet D., Liberman O. A., Vure E., Bar-Joseph G., Gabizon D., Borochowitz Z. U., Varon M., Modai D. Vitamin D resistant rickets with alopecia: a form of end organ resistance to 1,25 dihydroxy vitamin D. Clin Endocrinol (Oxf) 1981 Apr;14(4):395–402. doi: 10.1111/j.1365-2265.1981.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Diploid and haploid states of the glucocorticoid receptor gene of mouse lymphoid cell lines. Cell. 1977 Jun;11(2):423–430. doi: 10.1016/0092-8674(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F., Huet M. Glucorticoid resistance in murine lymphoma and thymoma lines. Cancer Res. 1978 Nov;38(11 Pt 2):4279–4284. [PubMed] [Google Scholar]
- Brooks M. H., Bell N. H., Love L., Stern P. H., Orfei E., Queener S. F., Hamstra A. J., DeLuca H. F. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978 May 4;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Maes M., Rothwell S. W., Migeon C. J. Human complete androgen insensitivity with normal dihydrotestosterone receptor binding capacity in cultured genital skin fibroblasts: evidence for a qualitative abnormality of the receptor. J Clin Endocrinol Metab. 1982 Jul;55(1):61–69. doi: 10.1210/jcem-55-1-61. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Vingerhoeds A., Brandon D., Eil C., Pugeat M., DeVroede M., Loriaux D. L., Lipsett M. B. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982 Jun;69(6):1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier M. E., Griffin J. E., Wilson J. D. Intranuclear binding of [3H]dihydrotestosterone by cultured human fibroblasts. Endocrinology. 1978 Oct;103(4):1499–1505. doi: 10.1210/endo-103-4-1499. [DOI] [PubMed] [Google Scholar]
- Colston K., Feldman D. 1,25-Dihydroxyvitamin D3 receptors and functions in cultured pig kidney cells (LLC PK1). Regulation of 24,25-dihydroxyvitamin D3 production. J Biol Chem. 1982 Mar 10;257(5):2504–2508. [PubMed] [Google Scholar]
- Eil C., Liberman U. A., Rosen J. F., Marx S. J. A cellular defect in hereditary vitamin-D-dependent rickets type II: defective nuclear uptake of 1,25-dihydroxyvitamin D in cultured skin fibroblasts. N Engl J Med. 1981 Jun 25;304(26):1588–1591. doi: 10.1056/NEJM198106253042608. [DOI] [PubMed] [Google Scholar]
- Eil C., Marx S. J. Nuclear uptake of 1,25-dihydroxy[3H]cholecalciferol in dispersed fibroblasts cultured from normal human skin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2562–2566. doi: 10.1073/pnas.78.4.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H., Kiyoki M., Kawashima K., Naruchi T., Hashimoto Y. Vitamin D3 metabolites and PTH synergistically stimulate bone formation of chick embryonic femur in vitro. Nature. 1980 Jul 17;286(5770):262–264. doi: 10.1038/286262a0. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Cone C., Hirst M., Shani S., Benderli A., Hochberg Z. Vitamin D resistant rickets with alopecia: cultured skin fibroblasts exhibit defective cytoplasmic receptors and unresponsiveness to 1,25(OH)2D3. J Clin Endocrinol Metab. 1982 Nov;55(5):1020–1022. doi: 10.1210/jcem-55-5-1020. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Hirst M., Colston K., Karasek M., Cone C. Demonstration of 1,25-dihydroxyvitamin D3 receptors in human skin biopsies. J Clin Endocrinol Metab. 1980 Dec;51(6):1463–1465. doi: 10.1210/jcem-51-6-1463. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Durrant J. L. Qualitative receptor defects in families with androgen resistance: failure of stabilization of the fibroblast cytosol androgen receptor. J Clin Endocrinol Metab. 1982 Sep;55(3):465–474. doi: 10.1210/jcem-55-3-465. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Punyashthiti K., Wilson J. D. Dihydrotestosterone binding by cultured human fibroblasts. Comparison of cells from control subjects and from patients with hereditary male pseudohermaphroditism due to androgen resistance. J Clin Invest. 1976 May;57(5):1342–1351. doi: 10.1172/JCI108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Haussler M. R. Characterization of the metabolites of vitamin D 3 in the chick. Steroids. 1972 Nov;20(5):639–650. doi: 10.1016/0039-128x(72)90021-9. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Metabolism of 25-hydroxy-vitamin D3 by primary cultures of chick kidney cells. Biochem Biophys Res Commun. 1977 Jan 24;74(2):768–774. doi: 10.1016/0006-291x(77)90368-0. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Vitamin D: two dihydroxylated metabolites are required for normal chicken egg hatchability. Science. 1978 Sep 1;201(4358):835–837. doi: 10.1126/science.684411. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Taylor A. N., Norman A. W. Response of chick parathyroid glands to the vitamin D metabolites, 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol. J Nutr. 1977 Oct;107(10):1918–1926. doi: 10.1093/jn/107.10.1918. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Turner R. T., Sherrard D. J., Baylink D. J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981 Aug 10;256(15):7738–7740. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liberman U. A., Eil C., Marx S. J. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest. 1983 Feb;71(2):192–200. doi: 10.1172/JCI110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman U. A., Samuel R., Halabe A., Kauli R., Edelstein S., Weisman Y., Papapoulos S. E., Clemens T. L., Fraher L. J., O'Riordan J. L. End-organ resistance to 1,25-dihydroxycholecalciferol. Lancet. 1980 Mar 8;1(8167):504–506. doi: 10.1016/s0140-6736(80)92763-4. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Spiegel A. M., Brown E. M., Gardner D. G., Downs R. W., Jr, Attie M., Hamstra A. J., DeLuca H. F. A familial syndrome of decrease in sensitivity to 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 1978 Dec;47(6):1303–1310. doi: 10.1210/jcem-47-6-1303. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Goodwin D., Noff D., Edelstein S. 24, 25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature. 1978 Nov 30;276(5687):517–519. doi: 10.1038/276517a0. [DOI] [PubMed] [Google Scholar]
- Pavlovitch J. H., Cournot-Witmer G., Bourdeau A., Balsan S., Fischer J. A., Heynen G. Suppressive effects of 24,25-dihydroxycholecalciferol on bone resorption induced by acute bilateral nephrectomy in rats. J Clin Invest. 1981 Sep;68(3):803–810. doi: 10.1172/JCI110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. F., Fleischman A. R., Finberg L., Hamstra A., DeLuca H. F. Rickets with alopecia: an inborn error of vitamin D metabolism. J Pediatr. 1979 May;94(5):729–735. doi: 10.1016/s0022-3476(79)80139-0. [DOI] [PubMed] [Google Scholar]
- Tahaka Y., Lorenc R. S., DeLuca H. F. The role of 1,25-dihydroxyvitamin D3 and parathyroid hormone in the regulation of chick renal 25-hydroxyvitamin D3-24-hydroxylase. Arch Biochem Biophys. 1975 Dec;171(2):521–526. doi: 10.1016/0003-9861(75)90061-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F., Kobayashi Y., Taguchi T., Ikekawa N., Morisaki M. Biological activity of 24,24-difluoro-25-hydroxyvitamin D3. Effect of blocking of 24-hydroxylation on the functions of vitamin D. J Biol Chem. 1979 Aug 10;254(15):7163–7167. [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974 Mar;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Schnoes H. K., Smith C. M., DeLuca H. F. 1,25,26-trihydroxyvitamin D3: isolation, identification, and biological activity. Arch Biochem Biophys. 1981 Aug;210(1):104–109. doi: 10.1016/0003-9861(81)90169-7. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Mawer E. B., Wallace J. E., St John J., Cochran M., Russell R. G., Kanis J. A. The absence of 24,25-dihydroxycholecalciferol in anephric patients. Clin Sci Mol Med Suppl. 1978 Dec;55(6):541–547. doi: 10.1042/cs0550541. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Matsuo N., Cho H., Kumagai M., Yasaka A., Suda T., Orimo H., Shiraki M. An unusual form of vitamin D-dependent rickets in a child: alopecia and marked end-organ hyposensitivity to biologically active vitamin D. J Clin Endocrinol Metab. 1980 Oct;51(4):685–690. doi: 10.1210/jcem-51-4-685. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Norman A. W. An hydroxylapatite batch assay for the quantitation of 1alpha,25-dihydroxyvitamin D3-receptor complexes. Anal Biochem. 1979 Jan 15;92(2):314–323. doi: 10.1016/0003-2697(79)90664-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Gehring U., Stampfer M. R., Sibley C. H. Genetic approaches to steroid hormone action. Recent Prog Horm Res. 1976;32:3–32. doi: 10.1016/b978-0-12-571132-6.50008-7. [DOI] [PubMed] [Google Scholar]
- Zerwekh J. E., Glass K., Jowsey J., Pak C. Y. An unique form of osteomalacia associated with end organ refractoriness to 1,25-dihydroxyvitamin D and apparent defective synthesis of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 1979 Aug;49(2):171–175. doi: 10.1210/jcem-49-2-171. [DOI] [PubMed] [Google Scholar]


