Abstract
Using gene-expression data from over 6,000 breast cancer patients, we report herein that high CD73 expression is associated with a poor prognosis in triple-negative breast cancers (TNBC). Because anthracycline-based chemotherapy regimens are standard treatment for TNBC, we investigated the relationship between CD73 and anthracycline efficacy. In TNBC patients treated with anthracycline-only preoperative chemotherapy, high CD73 gene expression was significantly associated with a lower rate of pathological complete response or the disappearance of invasive tumor at surgery. Using mouse models of breast cancer, we demonstrated that CD73 overexpression in tumor cells conferred chemoresistance to doxorubicin, a commonly used anthracycline, by suppressing adaptive antitumor immune responses via activation of A2A adenosine receptors. Targeted blockade of CD73 enhanced doxorubicin-mediated antitumor immune responses and significantly prolonged the survival of mice with established metastatic breast cancer. Taken together, our data suggest that CD73 constitutes a therapeutic target in TNBC.
Keywords: ectonucleotidase, immunogenic cell death, immunotherapy
Triple-negative breast cancer (TNBC), as defined by the absence of estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) expression, accounts for 15–20% of all breast cancers (1). Compared with other breast cancer subtypes, TNBC is characterized by a worse prognosis and increased risk of metastasis to vital organs (1). There are currently no known molecular targets for this subgroup of breast cancer patients and there seem to be few therapeutic options on the horizon, especially given that the development of poly (ADP ribose) polymerase and EGFR inhibitors have been thus far disappointing (2). Hence, the treatment of TNBC is a significant challenge in today’s clinical practice and the identification of therapeutic targets an area of urgent clinical need (3).
Cytotoxic chemotherapy, particularly anthracycline-based regimens, therefore remains the mainstay of treatment for TNBC today. Although TNBC is associated with a poor prognosis, some patients seem to respond well to anthracycline-based chemotherapy, reflecting a significant degree of molecular heterogeneity within this subgroup (3–5). Unfortunately, the molecular mechanisms underlying this heterogeneity and its relationship to treatment response are still poorly understood. Recently, new light has been shed on the mechanism-of-action of anthracyclines, which may help elucidate new mechanisms of chemoresistance. Accumulating data suggest that anthracyclines mediate their anticancer activity not only by direct cytotoxic effects but also through activation of adaptive antitumor immune responses (6) by inducing a type of tumor cell death that is “immunogenic” (7–10). In mice, chemotherapy with anthracyclines requires IFN-γ–producing CD8+ T cells for optimal activity. This finding is further supported by correlative clinical studies that report that high intratumoral levels of IFN-γ and CD8+ T cells are associated with better clinical responses to anthracycline-based chemotherapy regimens (7, 11).
To trigger antitumor immune responses, anthracyclines require extracellular release of ATP by tumor cells, a process induced by autophagy (8). Extracellular ATP activates P2X7 receptors on dendritic cells, which initiates a cascade of events that ultimately culminates in the generation of IFN-γ–producing CD8+ T cells. Because of its proinflammatory effects, extracellular levels of ATP are tightly regulated. Extracellular levels of ATP are essentially controlled by membrane-bound ecto-nucleotidases and kinases (12). Hydrolysis of ATP to ADP is catalyzed by CD39 (ENTPD1), and CD73 (NT5E) catalyses the hydrolysis of AMP to adenosine, a potent immunosuppressor (13). Although CD39 activity is negatively regulated by the actions of NDP kinase and adenylate kinase, the activity of CD73 is irreversible, which places CD73 at a crucial checkpoint in the conversion of ATP to adenosine. By increasing extracellular levels of adenosine, CD73 further suppresses immune responses, essentially via activation of high affinity A2A adenosine receptors (14, 15).
CD73 is overexpressed in various types of cancer (16–19). We and others have recently demonstrated that CD73 expression favors tumor growth by suppressing antitumor functions of CD8+ T cells (20–24). We further established the proof-of-concept that targeted blockade of CD73 with a monoclonal antibody (mAb) can delay tumor growth in mice (20, 22). Nevertheless, whether CD73 constitutes a valid cancer target remains to be shown. Herein, we investigated the clinical relevance of CD73 expression in breast cancer patients. Using gene-expression analysis from over 6,000 breast cancer cases, we found that high CD73 expression is significantly associated with a poor prognosis, particularly in TNBC. Our analysis also revealed that CD73 expression in TNBC patients is associated with an increased resistance to doxorubicin (DOX), a commonly used anthracycline chemotherapy. Using mouse models and human TNBC cell lines, we demonstrate that DOX treatment induces CD73 expression on tumor cells, which promotes DOX resistance by suppressing IFN-γ–producing CD8+ T cells via the activation of A2A adenosine receptors. Our study thus reveals a unique mechanism of chemoresistance mediated by the accumulation of extracellular adenosine and identifies CD73 as a previously unexplored therapeutic target for TNBC.
Results
To assess the clinical importance of CD73 in breast cancer, we performed gene-expression profile analysis of 44 publically available microarray datasets comprising over 6,000 breast cancer patients (Table S1). Using a robust and previously validated classification model, patients were assigned to three main breast cancer molecular subtypes: Luminal (ER+/HER2−), HER2-overexpressing (HER2+), and TNBC (Fig. S1) (25, 26). We first assessed whether high CD73 expression was associated with a particular molecular subtype of breast cancer. Our analysis revealed that CD73 gene expression was significantly, albeit modestly, highest in TNBC (P < 0.001) (Fig. S2). We also found that CD73 expression was negatively correlated with the gene encoding the estrogen receptor (ESR1) and a ESR1 gene module, and positively correlated with a well-known invasion/metastasis-associated gene plasminogen activator urokinase (PLAU) and PLAU gene module (Fig. 1A) (26, 27). CD73 positively correlated with PLAU irrespective of subtype (Fig. S3).
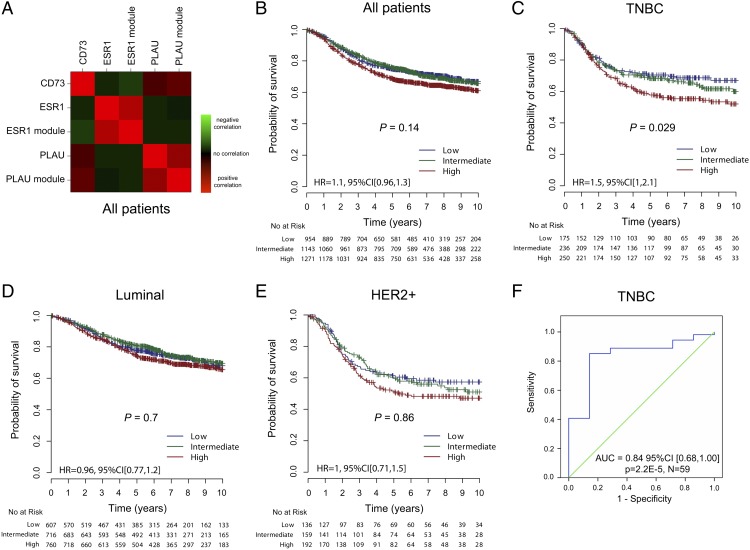
Fig. 1.
High CD73 gene expression is associated with poor prognosis in the TNBC subtype. We collected gene-expression profiles of 44 publically available microarray datasets of breast cancer patients (n = 6,209). Patients were assigned to the three main molecular subtypes using the SCM (Fig. S1). (A) Correlation (Spearman ρ) heatmap of CD73 expression with ESR1 (single gene and gene module) and PLAU (invasion gene and gene module). Red indicates positive correlation, with green indicating an inverse correlation and black indicating no correlation (n = 6,209). From our compendium of datasets we retrieved all of the patients with survival data available. We assessed the prognostic value of tertiles of CD73 gene expression in: (B) all patients (n = 3,368), (C) TNBC (n = 661), (D) Luminal (n = 2,083), (E) HER2+ (n = 487) breast cancer subtypes. Significance (P values) of differences in survival between patients groups defined by tertiles of CD73 expression is estimated by log-rank test. (F) ROC curve of CD73 expression as a continuous variable for predicting the pCR of 59 ER− (IHC) HER2− (FISH) patients in the TOP trial (27). The null hypothesis is that AUC = 0.5 and is depicted by the diagonal line. The upper line indicates the performance of CD73. High CD73 levels are associated with poor response to preoperative epirubicin. Area under the ROC curve = 0.84 (95% CI 0.68, 1.00), P = 0.00002.
We next assessed whether CD73 gene expression was correlated with survival in patients with relapse data available (Fig. 1B). We collected the gene-expression profiles of 44 publicly available microarray datasets involving 6,209 breast cancer patient samples. As shown in Fig. 1C, CD73 gene expression was significantly associated with a worse prognosis in TNBC patients (n = 661, P = 0.029), but not in patients with Luminal (Fig. 1D) (n = 2,083, P = 0.7) or HER2+ (Fig. 1E) (n = 487, P = 0.86) breast cancer. Notably, in a multivariate analysis, CD73 as a continuous variable was significantly independent of lymph node involvement, tumor size, and age as a predictor of adverse clinical outcome in TNBC patients [hazard ratio 1.34, 95% confidence interval (CI) (1.03–1.74), P = 0.029]. Therefore, the association between higher CD73 expression and poor outcomes in TNBC is linear.
Because CD73 is immunosuppressive, we hypothesized that CD73 expression might be associated with resistance to anthracycline therapy in TNBC. To test our hypothesis, we analyzed CD73 gene expression in human TNBC biopsies taken at diagnosis from a clinical trial of preoperative treatment with single agent epirubicin, a commonly used anthracycline (27). We evaluated the association between CD73 gene expression and pathologic complete responses (pCR), defined as the absence of invasive tumor at surgery after chemotherapy, an accepted surrogate of breast cancer survival (28). In 59 TNBC patients treated with four cycles of epirubicin-only preoperative chemotherapy, low CD73 gene expression was significantly associated with an increased pCR rate [AUC = 0.84, 95% CI (0.68, 1.00), P = 0.00002, Fig. 1F]. This data further supported the prognostic data above (Fig. 1C) where the majority of patients had received an anthracycline as part of their adjuvant chemotherapy.
Therefore, our analysis suggested that CD73 may promote anthracycline resistance. We next investigated the effect of CD73 overexpression, CD73 gene-silencing, and CD73 inhibition on anthracycline activity. In vitro, CD73 overexpression in AT-3 mouse breast cancer cells (Fig. S4A), CD73 gene-silencing in human MDA-MB-231 breast cancer cells (Fig. S4B), and CD73 inhibition in 4T1.2 mouse breast cancer cells (Fig. S4C) had no effect on DOX activity. Consistent with these results, CD73 gene-silencing in MDA-MB-231 cells had no effect on Bcl2 or p-glycoprotein expression (Fig. S4D).
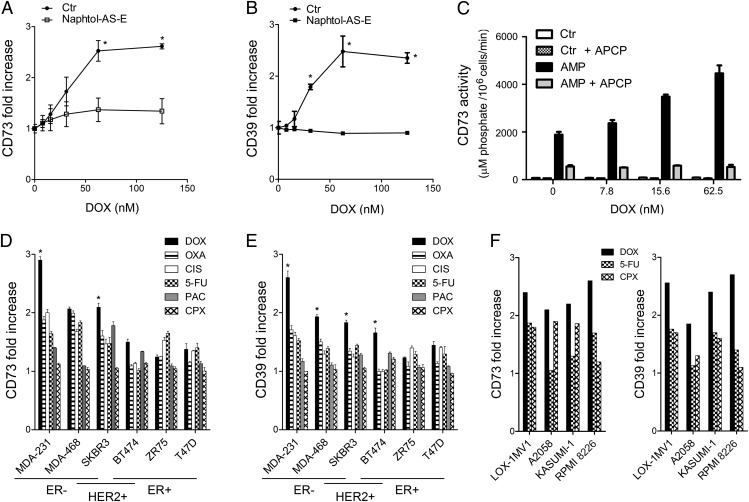
We next investigated whether DOX treatment could regulate CD73 expression in breast cancer cells. We found that DOX treatment significantly up-regulated CD73 expression on MDA-MB-231 breast cancer cells in vitro (Fig. 2A) and in vivo (Fig. S5). DOX also up-regulated CD39 expression (Fig. 2B), thus providing cells with the ectonucleotidase potential to hydrolyze extracellular ATP—released upon anthracycline treatment (8)—to adenosine. DOX-mediated up-regulation of CD73 and CD39 was inhibited by naphtol-AS-E, suggesting a cyclic AMP-responsive element binding-dependent mechanism (Fig. 2 A and B). Consistent with these results, DOX treatment significantly increased the production of extracellular adenosine by MDA-MB-231 cells, and this was inhibited by the selective CD73 inhibitor α,β-methylene ADP (APCP) (P < 0.05) (Fig. 2C). We investigated whether CD73 and CD39 up-regulation was specific for DOX using various chemotherapeutic drugs, and if it was specific to TNBC using a panel of human breast cancer lines of distinct subtypes (Fig. S4E). Among tested chemotherapeutic drugs [i.e., DOX, oxaliplatin, cisplatin, 5-fluorouracil (5-FU), paclitaxel (PAC), and cyclophosphamide], DOX induced the greatest up-regulation of CD73 and CD39 expression (Fig. 2 D and E). Oxaliplatin, cisplatin, and 5-FU were also associated with CD73 and CD39 up-regulation, although not to the extent of DOX. Among different breast cancer lines, CD73 and CD39 up-regulation was most significant in TNBC and HER2+ER− cells (Fig. 2 D and E and Fig. S6). Interestingly, DOX also up-regulated CD73 and CD39 expression in human melanoma (LOX-1MV1 and A2058) and leukemia (Kasumi-1 and RPMI-8226) cells, suggesting a more general effect (Fig. 2F and Fig. S7). In addition, chemotherapy-induced CD73 and CD39 up-regulation were positively correlated, suggesting a united mechanism (Fig. S8A). CD73 up-regulation was also positively correlated with endogenous CD73 levels, but CD39 up-regulation was not correlated with endogenous CD39 levels (Fig. S8B).
Fig. 2.
DOX up-regulates CD73 and CD39 expression on tumor cells. (A) MDA-MB-231 cells were treated for 48 h with DOX at increasing doses with or without the cyclic AMP-responsive element binding inhibitor naphtol-AS-E (10 nM). Cell-surface CD73 or (B) CD39 up-regulation (relative to untreated cells) was then measured by flow cytometry. Means ± SEs of triplicate are shown (*P < 0.05 by Mann–Whitney test). (C) MDA-MB-231 cells were treated with DOX at increasing doses for 48 h and CD73 activity, following addition of AMP (250 µM) with or without APCP (100 µM), was measured using a Malachite green phosphate detection kit. Means ± SEs of triplicate are shown (*P < 0.05 by Mann–Whitney test). Results are representative of two individual experiments. (D) CD73 and (E) CD39 up-regulation (maximum fold increase relative to untreated cells ± SEs) in response to DOX, oxaliplatin (OXA), cisplatin (CIS), 5-FU, PAC, and cyclophosphamide (CPX) in human breast cancer cells (*P < 0.05 comparing DOX to each drug). (F) CD73 and CD39 up-regulation in response to DOX, 5-FU, and CPX in human melanoma (LOX-1MV1, A2058) and leukemia (KASUMI-1 and RPMI-8226) cells.
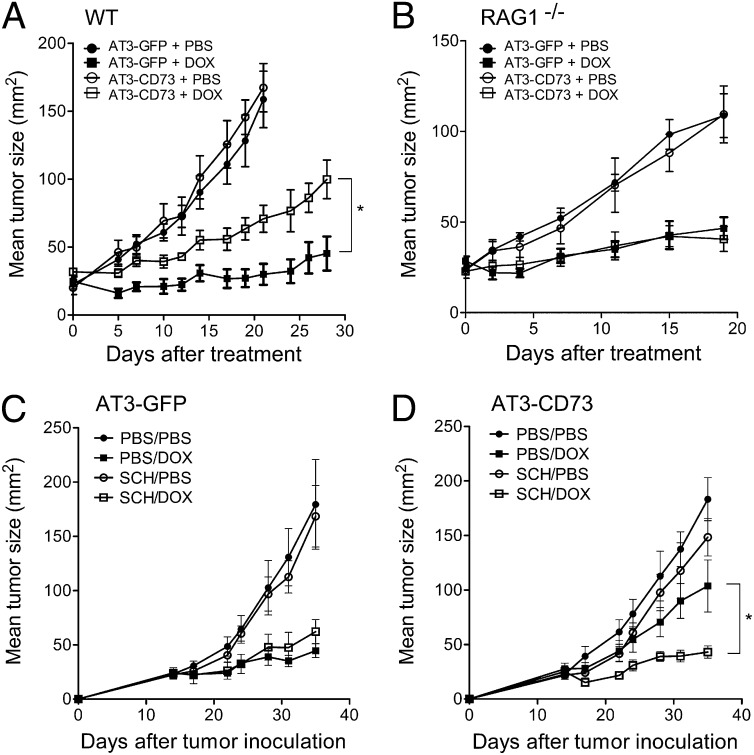
We next investigated the effect of CD73 expression on DOX activity in vivo. We found that CD73 overexpression in AT-3 tumors conferred chemoresistance to DOX in immunocompetent mice (P < 0.05) (Fig. 3A). In contrast, CD73 overexpression in AT-3 tumors had no effect on DOX activity in recombination activating gene (RAG)1−/− mice, which lack adaptive immunity (Fig. 3B). We next assessed whether CD73-mediated chemoresistance could be overcome by blocking extracellular adenosine signaling. Extracellular adenosine has been shown to inhibit adaptive antitumor immunity via A2A adenosine receptors (14). We thus hypothesized that targeted blockade of A2A adenosine receptors could rescue DOX sensitivity in CD73-expressing tumors. Accordingly, treatment of chemoresistant CD73-expressing tumors with the A2A antagonist SCH58261 could rescue DOX activity in immunocompetent mice (P < 0.05) (Fig. 3 C and D).
Fig. 3.
CD73 promotes DOX resistance in vivo via A2A adenosine receptor. (A) Wild-type and (B) RAG1−/− C57BL/6 mice were injected subcutaneously with 5 × 105 AT3-CD73 or AT3-GFP tumors cells and treated when tumors reached 25 mm2 with an intratumoral injection of DOX (1 mM in 50 µL PBS) or PBS. Means ± SEs of five to seven mice per group are shown (*P < 0.05 by Mann–Whitney test). (C) Wild-type C57BL/6 mice were injected subcutaneously with 5 × 105 AT3-GFP or (D) AT3-CD73 tumors cells and were treated at day 14 once with DOX (1 mM in 50 µL PBS, intratumorily) and PBS or SCH58261 (1 mg/kg, i.p) twice weekly. Means ± SEs of four to five mice per group are shown (*P < 0.05 by Mann–Whitney test).
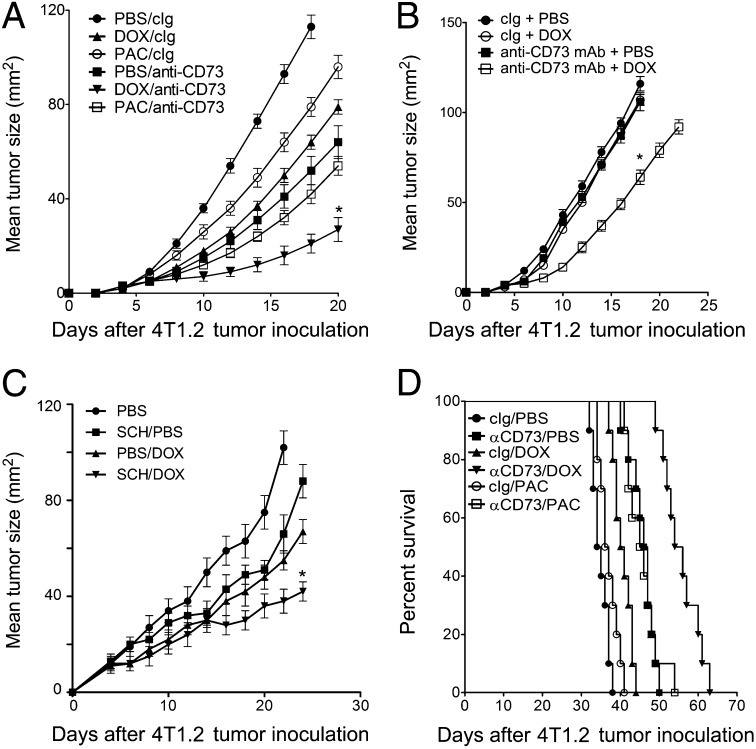
We next investigated whether anti-CD73 mAb therapy could potentiate DOX activity against breast tumors that constitutively express high levels of CD73. We and others have previously demonstrated that anti-CD73 mAb therapy can stimulate adaptive antitumor immunity (20–23). We found that targeted blockade of CD73 with an anti-CD73 mAb significantly enhanced DOX activity against 4T1.2 tumors (Fig. 4A). In contrast to its synergistic effect when used in combination with DOX, anti-CD73 mAb therapy failed to synergize with PAC (Fig. 4A). Consistent with our previous results (Fig. 3), treatment of 4T1.2 tumors with anti-CD73 mAb and DOX was more effective in immunocompetent vs immunodeficient mice (Fig. 4B). We next investigated whether targeted blockade of A2A adenosine receptor could recapitulate the therapeutic effect of anti-CD73 mAb against 4T1.2 tumors. As shown in Fig. 4C, treatment of 4T1.2 tumors with the A2A antagonist SCH58261 significantly enhanced DOX activity (Fig. 4C).
Fig. 4.
Targeted blockade of CD73 enhances DOX activity in vivo. (A) Wild-type and (B) SCID BALB/c mice were injected subcutaneously with 2 × 105 4T1.2 tumor cells and treated on day 3 and day 10 with PBS, DOX (2 mg/kg, i.v.) or PAC (10 mg/kg, i.p.), or on days 3, 7, 10, and 14 with anti-CD73 mAb (100 µg, i.p., clone TY/23) or control Ig (100 µg, i.p., clone MAC4). Means ± SEs of five mice per group are shown (*P < 0.05 by Mann–Whitney test, compared with monotherapy). A representative of two experiments is shown. (C) BALB/c mice were injected subcutaneously with 2 × 105 4T1.2 tumor cells and treated on day 3 and day 10 with PBS or DOX (2 mg/kg, i.v.) or SCH58261 (1 mg/kg, i.p., twice weekly). Means ± SEs of five mice per group are shown (*P < 0.05 by Mann–Whitney test, compared with monotherapy). (D). Fifty-thousand 4T1.2 tumor cells were inoculated into the mammary fat-pad of wild-type BALB/c mice (n = 8 per group), primary tumors were surgically removed on day 25 and mice were treated with DOX (2 mg/kg, i.v.) or PAC (10 mg/kg, i.p.) on day 28 and 35 or anti-CD73 mAb (100 µg, i.p., clone TY/23) on days 28, 32, 36, and 40. Significance of differences in survival between groups is estimated by log-rank (Mantel–Cox) test (P < 0.0001).
We further assessed the therapeutic effect of anti-CD73 mAb therapy in combination with DOX on established metastatic breast cancer. For this purpose, syngeneic immunocompetent mice were injected in the mammary fat pad with metastatic 4T1.2 cells and primary tumors were surgically removed at day 25. Consistent with previous studies (29), tumor-bearing mice undergoing surgery alone, with subsequent control treatment, died of metastatic disease by day 40 (median survival of 36.5 d) (Fig. 4D). Postsurgery chemotherapy with DOX had only a modest effect on survival (median survival of 40 d) (Fig. 4D). In contrast, combined chemotherapy with DOX and anti-CD73 mAb therapy prolonged by ∼50% the survival of mice compared with DOX monotherapy (median survival of 61 d) (Fig. 4D).
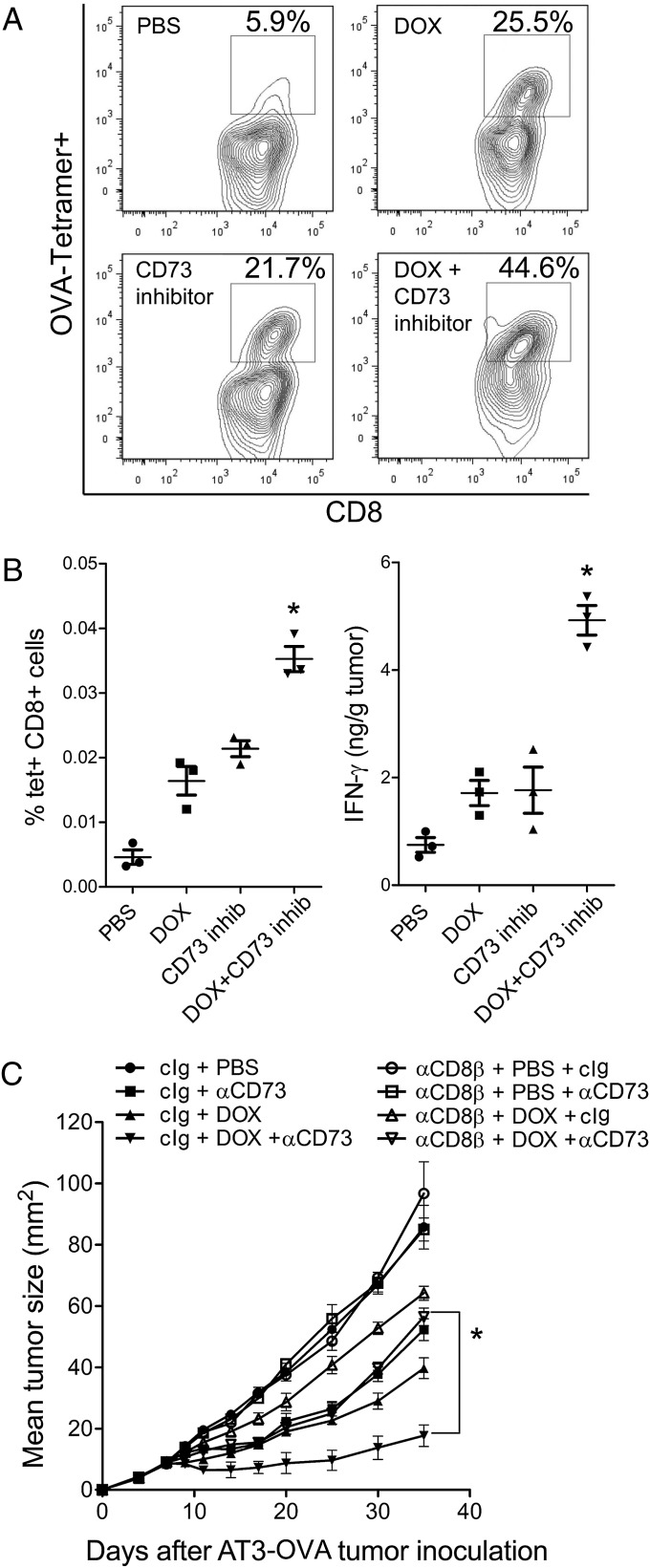
Because chemotherapy with anthracyclines requires priming of IFN-γ–producing CD8+ T cells for optimal activity, we investigated whether targeted blockade of CD73 enhanced antitumor CD8+ T-cell responses triggered by DOX. Using ovalbumin-expressing AT-3 tumors (AT3-OVA), we found that targeted blockade of CD73 significantly increased the frequency of tumor-specific CD8+ T cells (Fig. 5A) and the levels of IFN-γ (Fig. 5B) in tumors. Further in support of a critical role for CD8+ T cells, the improved response to DOX following anti-CD73 mAb therapy was abolished in mice depleted of CD8β+ T cells (Fig. 5C).
Fig. 5.
CD73 blockade in combination with DOX enhances adaptive antitumor immune responses. Wild-type C57BL/6 mice were injected subcutaneously with 106 AT3-OVA tumors cells and treated when tumors reached 25 mm2 with an intratumoral injection of DOX (1 mM) or the CD73 inhibitor APCP (400 µg/mouse) in 50 µL PBS. Tumors were harvested 4 d posttreatment and analyzed for the presence of (A and B) OVA-specific CD8+ T cells and (B) IFN-γ levels. Symbols represent individual mice, means ± SE are shown (*P < 0.05 by Mann–Whitney test, compared with monotherapy). A representative of two experiments is shown. (C) Wild-type C57BL/6 mice were injected subcutaneously with 106 AT3-OVA tumors cells and treated at day 7 with an intratumoral injection of DOX (1 mM in 50 µL PBS) or anti-CD73 mAb (100 µg, i.p., clone TY/23) or control Ig (100 µg, i.p., clone MAC4) twice weekly from day 7. Where indicated, mice were depleted of CD8+ T cells using anti-CD8β mAb (20 μg, i.p., clone 53–5.8 weekly from day 0). Means ± SEs of five mice per group are shown (*P < 0.05 by Mann–Whitney test). A representative of two experiments is shown.
Discussion
In this study, we demonstrated the importance of CD73 expression in the survival of TNBC patients and the effect of CD73-mediated immunosuppression on the promotion of anthracycline resistance. Our study supports the concept that CD73 and its downstream effector A2A adenosine receptor can be targeted to improve clinical outcomes in TNBC, in particular by enhancing anthracycline activity. TNBC remains a challenging disease with the poorest prognosis of all of the breast cancer subtypes. Importantly, there are currently no known molecular targets for this subgroup of breast cancer patients. The identification of new targets could potentially have a major impact on the disease.
Although CD73 expression has been documented in various types of cancer, including breast cancer, correlative analysis to clinical outcome has been limited (30). Herein we show that CD73 expression in breast cancer is higher in the TNBC subtype and that TNBC patients with high CD73 expression have a higher risk of distant metastases, the main cause of death from breast cancer. For decades, anthracycline-based chemotherapy has been and still remains the standard-of-care treatment for TNBC, although there have been some attempts to move away from anthracycline-based regimens. We thus investigated whether high CD73 expression before treatment could affect treatment response to anthracyclines, as our clinical analysis suggested that CD73 was associated with resistance to anthracycline treatment. We therefore explored the relationship between CD73 and DOX activity in vitro and in immunocompetent mouse models of breast cancer. First, we observed that CD73 expression had no effect on the intrinsic sensitivity of breast tumor cells to DOX. Because anthracyclines belong to a class of chemotherapeutic agents characterized by their ability to promote antitumor immune responses, we investigated whether CD73-mediated immunosuppression could suppress DOX activity in immunocompetent mice. Our data revealed that CD73-mediated immunosuppression suppresses the therapeutic activity of DOX in vivo.
Anthracyclines like DOX rely on activation of anti-tumor CD8+ T cells for efficacy, a phenomenon dependent on extracellular accumulation of ATP. We demonstrated that CD73 expression in breast tumors suppressed antitumor immune response via A2A adenosine receptor. Furthermore, CD73 inhibition potentiated the CD8-dependent anti-tumor immune response following DOX treatment. In a mouse model of established metastatic breast cancer, we showed that postsurgery DOX alone had minimal impact on survival, and combining DOX with anti-CD73 mAb therapy prolonged mouse survival by over 50%. Intriguingly, we observed that DOX treatment, and to some extent oxaliplatin, cisplatin, and 5-FU, significantly up-regulated CD39 and CD73 ectonucleotidase expression on human breast cancer, melanoma, and leukemia cells. Hence, some chemotherapeutic drugs are endowed with the potential to up-regulate the enzymatic machinery that converts proinflammatory extracellular ATP to immunosuppressive adenosine. This finding suggests that a counterbalancing process to chemotherapy-induced immunogenic cell death could be chemotherapy-induced accumulation of extracellular adenosine.
Our study provides strong evidence that immunosuppression mediated by CD73 suppresses anthracycline efficacy and this could contribute to the poorer outcomes observed in some patients with TNBC, especially those with high levels at diagnosis (pretreatment). We note that, although CD73 inhibition seems to act synergistically with anthracyclines, we have not definitively proven that this effect is specific to anthrayclines and it is quite possible that this effect may also occur with other chemotherapeutic agents. Nevertheless, anthracyclines are usually standard treatment for these patients. Interestingly, we observed that anti-CD73 therapy failed to potentiate chemotherapy with a commonly used taxane, PAC. Taking these data together, we propose that CD73 and its downstream effector A2A adenosine receptor could represent unique therapeutic targets in this subgroup of breast cancer patients currently characterized by a relative paucity of targeted treatment options.
Materials and Methods
Cell Lines, Antibodies, and Chemicals.
AT-3 and 4T1.2 mouse breast tumor cell lines have been previously described (7, 21, 22). For CD73 overexpression, AT-3 cells were transduced with retroviral vectors coexpressing mouse CD73 cDNA (provided by Linda H. Thompson, Oklahoma Medical Research Foundation, Oklahoma City, OK) and GFP or GFP only, and FACS sorted based on GFP expression. For OVA expression, AT-3 cells were transduced with retroviral vectors expressing chicken OVA cDNA. For CD73 gene-silencing, MDA-MB-231 cells were transduced with lentiviral vectors produced from 293FT cells transfected with human CD73 shRNA (TRCN0000048755) or control GFP shRNA plasmids from Open Biosystems and selected for 7 d in 1 µg/mL puromycin-supplemented media. CD73/CD39 expression was assessed using phycoerythrin (PE)-conjugated anti-mouse CD73 mAb (clone TY/23; BD Bioscience), PE-conjugated anti-human CD73 mAb (clone AD2; BD Bioscience), and APC-conjugated anti-human CD39 mAb (clone TU66; BD Bioscience). Purified anti-mouse CD73 mAb (clone TY/23; provided by Linda H. Thompson, Oklahoma Medical Research Foundation, Oklahoma City, OK), anti-CD8β mAb (clone 53–5.8) and control Ig (clone MAC4) were produced in house. APC-conjugated anti-mouse CD8 (53-6.7) was purchased from BD Bioscience and PE-conjugated MHC (class I/SIINFEKL tetramers was purchased from Andrew Brooks (University of Melbourne, Parkville, VIC, Australia). APCP and naphtol-AS-E were purchased from Sigma (Sigma-Aldrich). Chemotherapeutic drugs were obtained from the pharmacy of the Centre Hospitalier de l’Université de Montréal or Peter MacCallum Cancer Centre.
Cell Viability Assays.
For colorimetric assays, 104 AT3-GFP and AT3-CD73 cells per well, 104 MDA-MB-231 shGFP and shCD73 cells per well, or 5 × 103 4T1.2 cells per well were cultured in 96-well plates with or without DOX for 3 or 4 d, as indicated. Cell viability was measured using CellTiter Assay (Promega).
CD73 Activity.
MDA-MB-231 cells were plated at 7.5 × 103 cells per well in a 96-well plate in complete DMEM. After 24 h, cells were treated with DOX at increasing doses. After 48 h, cell media was removed and cells were washed twice with prewarmed phosphate-free buffer (2 mM MgCl2, 125 mM NaCl, 1 mM KCl, 10 mM glucose, 10 mM Hepes pH 7.2, diluted in ddH2O). APCP (100 µM final; Sigma) diluted in phosphate-free buffer or phosphate-free buffer alone were added to the cells and incubated for 10 min at 37 °C. AMP (250 µM final; Sigma) diluted in phosphate-free buffer or phosphate-free buffer alone were then added and incubated for 1 min at 37 °C. Phosphate concentrations resulting from AMP hydrolysis were measured using the malachite green phosphate detection kit (R&D Systems) following the manufacturer’s instructions.
Experimental Tumor Models.
C57BL/6 wild-type, C57BL/6 RAG1−/−, BALB/c wild-type, and BALB/c SCID mice were bred and maintained at the Peter MacCallum Cancer Centre or purchased from The Jackson Laboratory and maintained at the Centre de Recherche du Centre Hospitalier de l'Université de Montréal. All experiments were carried out in accordance with guidelines set out by the respective Animal Experimental Ethics Committee. Where indicated, mice were treated with DOX (2 mg/kg, i.v. in PBS or 1 mM in 50 μL PBS intratumoral), PAC (10 mg/kg, i.p.), anti-CD73 mAb TY/23 (100 μg, i.p), control Ig MAC4 (100 μg, i.p), APCP (20 mg/kg, i.v. or 400 μg/mouse intratumorally), SCH58261 (twice weekly injections of 1 mg/kg; Sigma) or anti-CD8β mAb clone 53–5.8 (20 μg, i.p). For survival experiments, 5 × 104 4T1.2 tumor cells were inoculated into the fourth mammary fat-pad of BALB/c mice. On day 25, mice were anesthetized with methoxyflurane, the primary tumor was surgically removed, the wound was closed with surgical clips and treatments commenced 3 d later.
Intratumoral Lymphocytes and IFN-γ.
Established AT3-OVA tumors (5 mm × 5 mm) were treated with an intratumoral injection of DOX (1 mM) and/or APCP (400 µg/mouse) in 50 µL PBS. For tumor-infiltrating lymphocyte analysis, tumors were excised 4 d posttreatment, minced with scissors, and incubated 1 h at 37 °C in PBS containing collagenase type 4 (Worthington Biochemical) and DNase I (Roche). Tumor cell suspensions were passed through a 70-μm cell strainer, washed twice in PBS, and resuspended in PBS 2% serum for flow cytometric analysis. Anti-CD16/32 mAb (clone 2.4G2) was used to block Fc receptors. Flow cytometry was performed on a LSR II (BD Bioscience) and analyzed using the software program FlowJo. For intratumoral IFN-γ analysis, tumors were excised 4 d posttreatment, minced with scissors, and homogenized 2 mL PBS. Tumor homogenates were centrifuged at 10,000 × g for 5 min at 4 °C and IFN-γ levels were measured by ELISA (eBioscience).
Supplementary Material
Acknowledgments
The authors thank Kathleen Spring, Bruno G. Leclerc, Isabelle Cousineau, Martin Turcotte, David Allard, Guillaume Chouinard, Francis Rodier, Guillaume Cardin, Nicole McLaughlin, Qerime Mundrea, and Ben Venville for technical support. This work was supported in part by Susan G. Komen for the Cure (IIR12221504); the National Health and Medical Research Council of Australia (1013667, 1007902); the Victorian Cancer Agency (EOI09_71); the Canadian Institutes of Health Research; the Cancer Research Society of Canada; a National Health and Medical Research Council early career Fellowship and Fonds J. C. Heuson (to S.L.); a National Health and Medical Research Council Career Development Award 2 award (to P.K.D.); National Health and Medical Research Council Australia Fellowship 628623 (to M.J.S.); and the Famille Jean-Guy Sabourin Research Chair of University of Montreal (J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222251110/-/DCSupplemental.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, et al. TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30(21):2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irshad S, Ellis P, Tutt A. Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr Opin Oncol. 2011;23(6):566–577. doi: 10.1097/CCO.0b013e32834bf8ae. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2011;16(Suppl 1):71–78. doi: 10.1634/theoncologist.2011-S1-71. [DOI] [PubMed] [Google Scholar]
- 5.Metzger-Filho O, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30(15):1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: Immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11(3):215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 7.Mattarollo SR, et al. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71(14):4809–4820. doi: 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- 8.Michaud M, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208(3):491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 11.West NR, et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13(6):R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: A potent suppressor of antitumor immune responses. Trends Immunol. 2012;33(5):231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 14.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 16.Bavaresco L, et al. The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem. 2008;319(1–2):61–68. doi: 10.1007/s11010-008-9877-3. [DOI] [PubMed] [Google Scholar]
- 17.Sadej R, Spychala J, Skladanowski AC. Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16(3):213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 18.Serra S, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118(23):6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spychala J, et al. Role of estrogen receptor in the regulation of ecto-5′-nucleotidase and adenosine in breast cancer. Clin Cancer Res. 2004;10(2):708–717. doi: 10.1158/1078-0432.ccr-0811-03. [DOI] [PubMed] [Google Scholar]
- 20.Stagg J, et al. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72(9):2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 21.Stagg J, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71(8):2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 22.Stagg J, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107(4):1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121(6):2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yegutkin GG, et al. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41(5):1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 25.Haibe-Kains B, et al. A three-gene model to robustly identify breast cancer molecular subtypes. J Natl Cancer Inst. 2012;104(4):311–325. doi: 10.1093/jnci/djr545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desmedt C, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14(16):5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 27.Desmedt C, et al. Multifactorial approach to predicting resistance to anthracyclines. J Clin Oncol. 2011;29(12):1578–1586. doi: 10.1200/JCO.2010.31.2231. [DOI] [PubMed] [Google Scholar]
- 28.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;2001(30):96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 29.Kershaw MH, et al. Gene-engineered T cells as a superior adjuvant therapy for metastatic cancer. J Immunol. 2004;173(3):2143–2150. doi: 10.4049/jimmunol.173.3.2143. [DOI] [PubMed] [Google Scholar]
- 30.Lo Nigro C, et al. NT5E CpG island methylation is a favourable breast cancer biomarker. Br J Cancer. 2012;107(1):75–83. doi: 10.1038/bjc.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.