Abstract
Literacy for blind people requires learning Braille. Along with others, we have shown that reading Braille activates visual cortex. This includes striate cortex (V1), i.e., banks of calcarine sulcus, and several higher visual areas in lingual, fusiform, cuneus, lateral occipital, inferior temporal, and middle temporal gyri. The spatial extent and magnitude of magnetic resonance (MR) signals in visual cortex is greatest for those who became blind early in life. Individuals who lost sight as adults, and subsequently learned Braille, still exhibited activity in some of the same visual cortex regions, especially V1. These findings suggest these visual cortex regions become adapted to processing tactile information and that this cross-modal neural change might support Braille literacy. Here we tested the alternative hypothesis that these regions directly respond to linguistic aspects of a task. Accordingly, language task performance by blind persons should activate the same visual cortex regions regardless of input modality. Specifically, visual cortex activity in blind people ought to arise during a language task involving heard words. Eight early blind, six late blind, and eight sighted subjects were studied using functional magnetic resonance imaging (fMRI) during covert generation of verbs to heard nouns. The control task was passive listening to indecipherable sounds (reverse words) matched to the nouns in sound intensity, duration, and spectral content. Functional responses were analyzed at the level of individual subjects using methods based on the general linear model and at the group level, using voxel based ANOVA and t-test analyses. Blind and sighted subjects showed comparable activation of language areas in left inferior frontal, dorsolateral prefrontal, and left posterior superior temporal gyri. The main distinction was bilateral, left dominant activation of the same visual cortex regions previously noted with Braille reading in all blind subjects. The spatial extent and magnitude of responses was greatest on the left in early blind individuals. Responses in the late blind group mostly were confined to V1 and nearby portions of the lingual and fusiform gyri. These results confirm the presence of adaptations in visual cortex of blind people but argue against the notion that this activity during Braille reading represents somatosensory (haptic) processing. Rather, we suggest that these responses can be most parsimoniously explained in terms of linguistic operations. It remains possible that these responses represent adaptations which initially are for processing either sound or touch, but which are later generalized to the other modality during acquisition of Braille reading skills.
INTRODUCTION
Several groups have investigated the physiological correlates of Braille reading in blind people using functional magnetic resonance imaging (fMRI) (Burton et al. 2002; Melzer et al. 2001), positron emission tomography (PET) (Büchel 1998a; Sadato et al. 1996, 1998) and transcranial magnetic stimulation (TMS) (Cohen et al. 1999; Hamilton and Pascual-Leone 1998). These studies established that visual cortex in the blind plays a functional role in Braille reading. This conclusion is reinforced by the clinical observation of acquired Braille alexia without tactile agnosia following bilateral occipital strokes in one blind patient (Hamilton et al. 2000). Controversy persists concerning differences in the engagement of primary visual cortex between persons who become blind early in life versus those who acquire blindness later (e.g., before age 3 vs. after age 12). The differing results with early versus late onset blindness raise questions regarding the period of susceptibility for cross-modal reorganization (Cohen et al. 1999). Our previous fMRI study of verb generation for Braille nouns (Burton et al. 2002) revealed anatomically distinct activation foci corresponding to V1, V2, V3, VP, and LO (DeYoe et al. 1996; Engel et al. 1997; Sereno et al. 1995; Tootell et al. 1996, 1997) in both early blind and late blind subjects. This result, by analogy to vision in sighted subjects, suggests a distributed network of specialized functional areas. However, assignment of specific functionality remains unclear.
One possibility is that blindness leads to visual cortex adaptation for the analysis of tactile information (Büchel 1998a; Sadato et al. 1996, 1998). Thus the same functionality used for the analysis of print orthography in sighted persons is applied to Braille decoding in the blind. Support for this view comes from TMS experiments in which stimulation of sensorimotor cortex 20 ms after stimulus presentation interfered with the detection of the Braille stimuli whereas stimulation of occipital cortex with onset asynchrony of 50–80 ms disrupted discrimination but not detection (Hamilton and Pascual-Leone 1998). A corollary postulate is that cortico-cortical connections between somatosensory and visual areas supports Braille reading (Hamilton and Pascual-Leone 1998).
Another possibility is that core language processes, e.g., semantic, phonological, or syntactic, acquire representation in visual cortex as a result of adaptations to blindness. The objective of the present study was to test this hypothesis by studying blind individuals using a language task involving audition instead of Braille reading. We again selected verb generation but to heard nouns as the behavioral task. This potent, semantic paradigm has been extensively studied in sighted subjects (see reviews in Gabrieli et al. 1998; Seger et al. 1999). The present experiment parallels our previous study of verb generation to Braille read nouns (Burton et al. 2002). The tasks are identical except for input word modality, i.e., auditory versus Braille. Many of the same Braille literate blind subjects participated in both experiments. We also studied sighted subjects using the identical fMRI protocol. Data presentation was organized to facilitate comparisons among subject groups in this and our prior study (Burton et al. 2002).
METHODS
Subjects
All subjects were paid volunteers who provided informed consent following guidelines approved by the Human Studies Committee of Washington University. Eight early blind, six late blind, and eight sighted subjects contributed to the present results (Table 1). Identification numbers for blind subjects (Table 1 and Figs. 1-7) are retained from our prior study (Burton et al. 2002) for those who participated in both experiments. Results were excluded from one early blind, five late blind, and three sighted individuals because of excessive head movement, abnormal brain anatomy by structural imaging, or inadequate performance on post-fMRI scan recall testing (see Fig. 2). Early blind subjects had no sight at birth or by 3 yr of age. The average age at onset of blindness in the late blind group was 19.2 yr (range, 7–36 yr). All blind subjects, but no sighted subjects, were fluent Braille readers. Average Braille reading rates for the early blind and late blind subjects were 106.3 words/min (wpm) and 79.7 wpm, respectively (Table 1).
TABLE 1.
Blind subject characteristics
| Subject | Age | Sex | Preferred Hand | Braille Hand | Words per Minute | Years Blind | Age of Blindness Onset | Light Sense | Years Reading Braille | Cause of Blindness |
|---|---|---|---|---|---|---|---|---|---|---|
| Early Blind | ||||||||||
| Early 1* | 51 | F | Right | Right | 145.4 | 49 | 2 | None | 45 | ROP/RLF† |
| Early 2* | 50 | M | Right | Right | 152 | 50 | 0 | None | 44 | ROP/RLF |
| Early 4* | 34 | F | Left | Right | 76 | 34 | 1 | Some | 26 | Leber’s disease |
| Early 5* | 39 | F | Right | Right | 99.7 | 36 | 3 | None | 34 | Glaucoma |
| Early 8* | 54 | M | Right | Right | n/a | 54 | 0 | None | 25 | Optic nerve hypoplasia |
| Early 9* | 45 | M | Right | Right | n/a | 43 | 2 | None | 39 | ROP/RLF |
| Early 10 | 33 | M | Right | Left | 88.7 | 33 | 0 | Little | 28 | ROP/RLF |
| Early 11 | 24 | M | Right | Left | 76 | 24 | 0 | Some | 18 | Leber’s disease |
| Late Blind | ||||||||||
| Late 1* | 37 | F | Ambidex | Right | 81.8 | 31 | 7 | Some | 30 | Rubella |
| Late 3* | 41 | M | Right | Right | 75 | 29 | 12 | None | 29 | Coats disease |
| Late 4* | 63 | M | Right | Left | 83.9 | 51 | 12 | None | 55 | Glaucoma |
| Late 5* | 67 | F | Right | Right | n/a | 46 | 21 | None | 48 | Retinitis pigmentosa |
| Late 6* | 48 | F | Right | Right | 125 | 21 | 27 | None | 18 | Retinitis pigmentosa |
| Late 8 | 47 | F | Right | Right | 32.6 | 11 | 36 | None | 10 | Stevens-Johnson syndrome |
Subjects who participated in previous study involving Braille reading (Burton et al. 2002).
ROP/RLF, retinopathy of prematurity/retrolental fibroplasia.
FIG. 1.
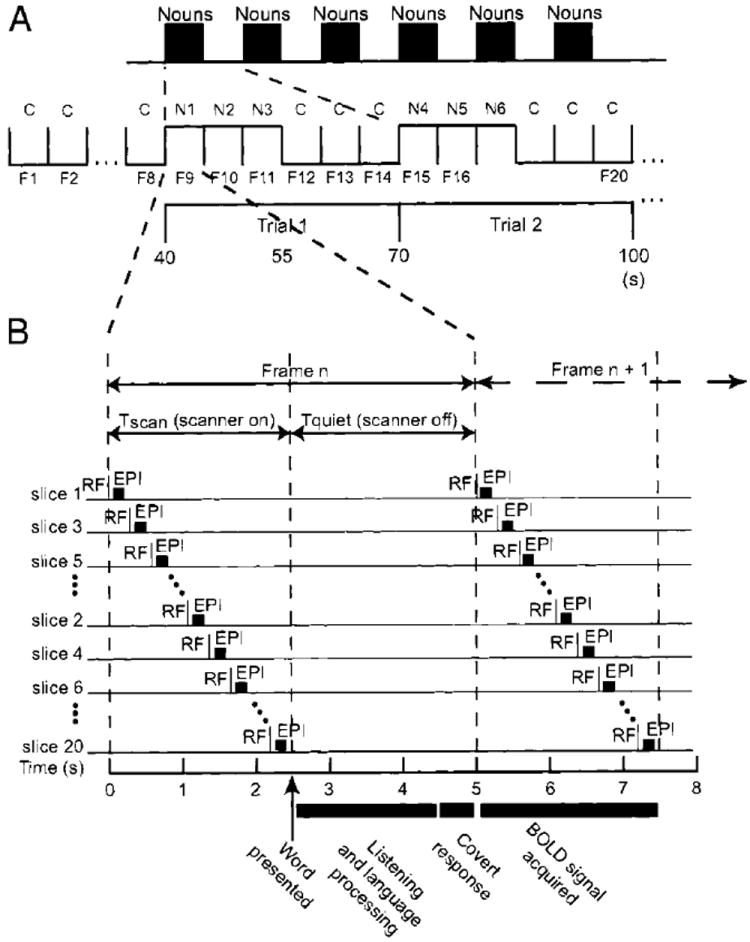
Task paradigm and echo-planar (EPI) timing in relation to frame sequence (F1, F2, F3, … F128). A: each run started with 8 baseline frames (F1–F8) during which subjects heard control sounds. Starting with the 9th frame, we presented 20 sequential mini-blocks of 3 successive nouns alternating with 3 successive control sounds (reverse words). The timing of each word synchronized with 1 repetition time (TR). Each mini-block sequence was defined as 1 ~30-s duration trial (Trial 1, Trial 2, … Trial 20). Every noun was unique throughout all runs (N1, N2, N3, … Nn). The same reverse word was repeated within a run (C, C, C … ), but was different for each run. B: each horizontal line corresponds to 1 of 20 axial, 6-mm slices. EPI occupied only the 1st 2.704 s of each TR (Frame n), leaving a “quiet” time without scanner pulses. During the latter interval, subjects heard the words and covertly generated an appropriate verb if the field contained a noun. Induced BOLD responses were detected by EPI during subsequent frames.
FIG. 7.
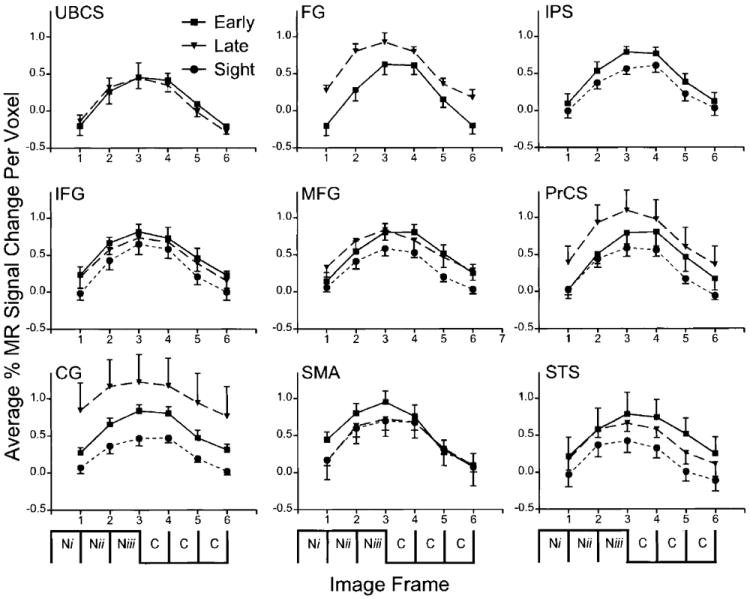
Time course of BOLD responses per voxel in selected regions (see Table 3 to identify abbreviations) for 8 early blind and 6 late blind and, where applicable, 8 sighted subjects. Each data point shows mean ± SE for the selected group. Average percentage change in MR signal was obtained by analyses of signal magnitudes per frame throughout the region volumes that were individually defined in each subject (see METHODS). Incorporated into the abscissa is a schematic representation of the sequence of noun (Ni, Nii, Niii) and control sounds (C, C, C) used for each trial. Note the delayed increase in MR signal to frames 2–4 that follow noun presentations.
FIG. 2.
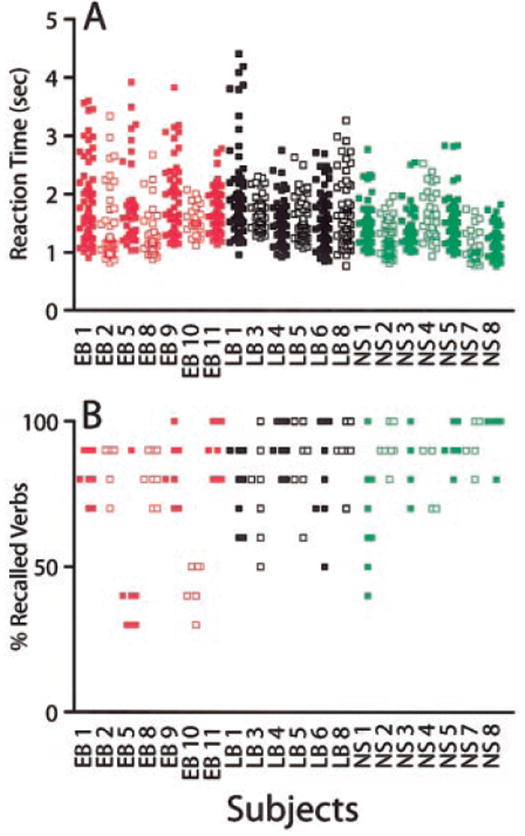
Performance data for individual subjects on recall test. A: reaction times shown for individual words. Total number of scatter dots per subject correspond to time measurements for remembered verbs. Reaction times of equal value for a subject are plotted as slightly displaced points. Red, black, and green symbols are for early blind, late blind, and sighted subjects, respectively. Filled and open symbols are used illustratively to distinguish results from neighboring scatter plots. B: percent recalled verbs shown for each completed functional magnetic resonance imaging (fMRI) run. Differing number of scatter points between subjects reflect variable number of runs (4–8). Labels for blind subjects (e.g., EB 1) correspond to those listed in Table 1.
MRI acquisition
fMRI was acquired on a Siemens 1.5 Tesla Vision scanner using a custom, single-shot asymmetric spin-echo, echo-planar (EPI) sequence and the standard circularly polarized head coil. The EPI was 100% phase over-sampled and used blipped readout (Howseman et al. 1988) and direct 2D-FT reconstruction. The T2* evolution time was 50 ms from a 90° flip angle (Conturo et al. 1996; Ogawa et al. 1990). Whole brain coverage was obtained with 20 contiguous 6-mm axial slices with 3.75 × 3.75-mm pixels (240-mm field of view with 64 × 64 image matrix). The sequence paused after each volume acquisition to allow for auditory stimulus delivery (see Fig. 1). The EPI slice prescriptions were automatically computed by registration of a preliminary, coarse (2-mm cubic voxel) MP-RAGE (Mugler and Brookeman 1990) to an atlas representative target image.
Structural imaging included a T2-weighted spin echo scan (1 × 1 × 6 mm, repetition time (TR) = 3800 ms, echo time (TE) = 22 ms) and a high resolution (1 × 1 × 1.25 mm) T1-weighted sagittal MP-RAGE (TR = 9.7 ms, TE = 4 ms, flip angle = 12°, inversion time (TI) = 300 ms). These images provided the basis for atlas transformation and region analysis as previously described (Burton et al. 2002).
Task
The functional paradigm provided a single contrast comprised of alternating task and control blocks (Fig. 1A). The task was covert generation of a compatible verb for heard nouns (e.g., think “paint” in response to “house”). The nouns were selected from the list used in our prior study of Braille reading (Burton et al. 2002). The concrete: abstract noun ratio was approximately 2:1. During control blocks subjects were presented unintelligible stimuli matched to the nouns in intensity, duration, and spectral content (Binder et al. 2000), which they were instructed to ignore. The stimuli were created by a male voice reading nouns into a Power Macintosh computer running SpeechEdit (www.macinsearch.com/infomac2/applications/speech-edit-104.html). Extraneous noise before and after each word was erased. Sound intensities for all words were equalized. The nouns were randomly assigned to eight different lists, one for each fMRI run. No noun was repeated within or across runs. Otherwise, unused two or three syllable nouns were time reversed to create the control stimuli, which were constant within each run but varied across runs.
Each fMRI run was comprised of 128 frames; each frame was 5 or 5.5 s in duration. We used a “clustered volume acquisition” timing scheme (Bandettini 2001) in which EPI occupied the first 2.704 s of each TR interval (frame), leaving the remaining 2.3 or 2.8 s quiet when the words were heard without scanner noise (Fig. 1B). Slice acquisition of BOLD responses to each word was delayed to the following TR interval (Fig. 1B). Synchronization of stimulus onset with the start of the quiet period was achieved with PsyScope running on a Power Macintosh computer (Cohen et al. 1993). Each run began with eight control frames followed by 20 alternations of task and control blocks, each block containing three frames. For purposes of BOLD response measurement, each six-frame alternation (3 task frames followed by 3 control frames) was treated as a single, 30-, or 33-s single event (Fig. 1A). All subjects completed four to eight runs, thereby providing sufficient data for statistically reliable image analyses within individuals.
Immediately following each run, subjects were asked to overtly recall the verb generated for each of 10 randomly chosen nouns from the preceding list. We noted the number of recalled verbs and digitally recorded the experimenter reading the nouns and the subject’s responses. Subject voice onset latency was measured relative to the end of experimenter’s spoken noun. For each subject, percent verbs recalled and voice onset latencies were averaged over runs. Postrun recall statistics compiled over subjects are presented in Table 2.
TABLE 2.
Assessed recall of covertly generated verbs
| Early (n = 7)*
|
Late (n = 6)
|
Sighted (n = 7)*
|
||||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Reaction time (s) | 1.7† | 0.03 | 1.63 | 0.03 | 1.25 | 0.02 |
| Percent recalled | 63.4‡ | 4.8 | 86.7 | 11.5 | 93.8 | 7.4 |
| Age | 40.1 | 3.2 | 50.5 | 4.9 | 40.3 | 4.4 |
Sample size available for assessment testing does not equal numbers used for image analyses because post hoc testing of performance was not available for all early blind and sighted subjects.
t-test of reaction times for early vs. sight (t = 12.78, P < 0.0001, df = 737); late vs. sight (t = 11.8, P < 0.001, df = 779).
t-test of percent recalled for early vs. late blind subjects (t = 4, P = 0.0001, df = 93); early blind vs. sighted subjects (t = 4.5, P < 0.0001, df = 90). SE, standard error.
Image analysis
Functional data were compensated for systematic slice-dependent time shifts and systematic odd/even slice intensity differences due to interpolated acquisition. These images then were realigned within and across runs to correct for head movement. Atlas transformation of the EPI data were achieved as previously described (Ojemann et al. 1997) by computing a sequence of affine transforms (first frame EPI to MP-RAGE to atlas representative target MP-RAGE), which were combined by matrix multiplication. The atlas representative target was made to conform to the Talairach atlas (Talairach and Tournoux 1988) using the method of Lancaster and colleagues (Lancaster et al. 1995). Before statistical analysis the data were re-sampled in atlas space using 2-mm cubic voxels and smoothed with a 2-voxel Gaussian kernel.
Statistical analyses based on individual subject data
Within subject analyses were based on event-related methods that estimated the time course of the BOLD responses per trial and at each voxel using the general linear model (Friston et al. 1995; Zarahn et al. 1997). For each subject, scans across all runs were concatenated. The time course was estimated over a 30- or 33-s (6 frames) interval without assuming a particular hemodynamic response. To generate statistical maps, response magnitudes per voxel were computed by cross-correlation of the estimated time courses against an assumed hemodynamic response. The latter was obtained by convolving a delayed gamma function (Boynton et al. 1996; Dale and Buckner 1997) with a rectangular function specified by the trial duration (Ollinger et al. 2001; Shulman et al. 1999). Residuals provided an estimate of variance at each voxel. t-Statistics were computed as the ratio of these magnitudes to ±SE. The t-statistics were converted to normally distributed z-scores with the same significance probabilities (Gaussianized). These z-score maps for each subject were corrected for multiple comparisons using prior Monte Carlo simulations on random noise patterns (based on methods described in Forman et al. 1995) and a whole brain P value of 0.05. We used a threshold of z = 4.5 over ≥3 contiguous voxels.
Region analyses involved manual definition of activated regions, which started with averaged z-score maps per group followed by refined definition of the same regions within each subject. Average z-score maps were obtained by summing the corrected z-score images across subjects for each group in atlas space (2 × 2 × 2-mm voxels) and dividing by the squared root of the sample size (Bosch 2000). Boundaries of three-dimensional (3-D) regions of interest were determined on these average composite images using z-score intensities and anatomical borders employing the interactive image display software ANALYZE (Biodynamics Research Unit, Mayo Clinic, Rochester, MN). For example, regions showing significant BOLD responses in the left and right upper and lower banks of the calcarine sulcus were first outlined by setting anatomical boundaries per image slice for the calcarine and collateral sulci, fusiform and cuneus gyri, and brain midline. Next a region was delimited within the anatomical borders using an automatic intensity selection option set to find z-scores with a minimum value with a P = 0.01 and the maximum z-value for the entire volume. The procedure for each region was iterative across multiple sections and involved adjusting each region’s boundaries to obtain a consistent definition in three orthogonal planes. The regions identified from the analysis of the average z-score images provided only the initial demarcation. Next, the defined regions for group averages were imported into displays of the z-score maps (in ANALYZE) for each subject within a specified group. Boundaries for each specific region then were adjusted on the basis of individual cortical anatomy and range of z-scores for that subject. The minimum in the analysis of subject data were also set to a z-score with P = 0.01. For each subject we computed coordinates and z-score peak magnitude for every region found in that subject. For all regions in each subject we computed z-score peak magnitude and the coordinates for center of mass and z-score peak. Additional calculations determined spatial extent, average percent change, and response time-course for each region in every subject.
Statistical analyses based on group data
Group level, voxel-based analysis of variance (ANOVA) (Corbetta et al. 2000; Shulman et al. 1999) and t-test statistical analyses provided additional assessment of the BOLD responses. Subject was a random variable in the ANOVA and t-test analyses. The ANOVA extracted the percentage change in MR signal from baseline using estimates from the general linear models obtained in each subject. The F ratios from the ANOVA were converted to z-scores and corrected for multiple comparisons using a Monte Carlo distribution from random noise as noted above for the within subject z-score calculations for the general linear model. The ANOVA analysis was executed with a three level subject group factor (early blind, late blind, and sighted) and a six level time factor. The z-score map from the subject group by time interaction term of the ANOVA showed the distribution of activity where variations in BOLD responses differed significantly between the groups. The statistical maps were set to a threshold of P = 0.05 for regions consisting of ≥45 contiguous, face connected voxels. The results were further subjected to t-tests to determine the distribution of activity where particular subject groups had the greatest magnitude of responses. The resulting t-statistic images were corrected for multiple comparisons, using Monte Carlo simulations (see above), and displayed as z-score statistical maps.
RESULTS
On average, subjects recalled more than 60% of covertly generated verbs from a preceding run. Early blind recalled significantly fewer verbs (Table 2). Figure 2B shows that this difference mainly represented fewer recalled verbs in two subjects (EB5 and EB10). The fMRI results from these two subjects were indistinguishable from other early blind subjects. Average reaction times for recalled verbs were significantly shorter for sighted subjects (Table 2). This difference primarily reflected greater consistency in the performance of nearly all sighted subjects compared with several blind subjects (Fig. 2A). The predominant reaction times even in the latter (EB1, EB2, EB5, EB9, and LB1) were similar to those observed in the sighted group. Despite these differences, all subjects reported having no difficulty doing the task.
The image analyses proceeded on two levels. First, we determined the distribution of activated regions in the three subject groups by examining results in individual subjects. Second, we contrasted active foci between groups using voxel-wise, group level ANOVA and t-test analyses.
Table 31 contains the results derived from individual subject analyses. It lists the anatomical names and atlas coordinates of peak z-scores for activated foci present in three or more individuals in each group. No entries appear in Table 3 where only one or two subjects showed significant activity in a region. These were conservatively considered outliers. These results were based on using a constant threshold of a z-score ≥ 4.5 (P ≤ 0.05) over ≥3 contiguous, face connected voxels for within subject analyses.2 The number of regions was 19 in early blind, 16 in late blind, and 9 in sighted subjects. Except for two regions (right inferior frontal in sighted subjects and right intraparietal sulcus in early blind individuals), similar active sites appeared in two or all three groups. The coordinates of peak z-scores and centers of mass overlapped nearly completely for most regions observed in all blind individuals. Seven of the nine regions noted in sighted also were coextensive with corresponding sites in the blind subjects. In many regions the peak z-score was highly significant (P < 0.0001) even after correction for multiple comparisons (Table 3). More than 90% of response foci included >0.8 cm3 volumes (Table 3) with z-score probabilities <0.05.
TABLE 3.
Activated regions based on mean peak z-scores
| Regions | BA* | Early Blind
|
Late Blind
|
Sighted
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x, y, z† | SE ± x, y, z | Vol (cm3) | N‡ | Peak z-Score | x, y, z† | SE ± x, y, z | Vol (cm3) | N‡ | Peak z-score | x, y, z† | SE ± x, y, z | Vol (cm3) | N‡ | Peak z-Score | ||
| 1 Lower calcarine (LBCS) | 17 | −7,−81,−3 | 1.5,3.5,3 | 2.4,±.4 | 8 | 14.1,±2 | −9,−86,−3 | 2,5.3,3 | 1.7,±.6 | 5 | 13.9,±2.3 | 0 | ||||
| 2 Lower calcarine (LBCS) | 17 | 12,−87,0 | 4.3,2.9,0.8 | 1.5,±.5 | 4 | 14.6,±2.7 | 11,−83,−2 | 2.1,3.1,1.7 | 1.3,±.4 | 5 | 12.5,±1.6 | 0 | ||||
| 3 Upper calcarine (UBCS) | 17 | −6,−85,4 | 1.4,2,1.3 | 1.3,±.4 | 8 | 13.2,±2.1 | −8,−85,1 | 2.6,4.6,3.2 | 1.7,±.4 | 5 | 14.1,±2.6 | 0 | ||||
| 4 Upper calcarine (UBCS) | 17 | 10,−84,6 | 3.6,4.4,2 | .9,±.2 | 4 | 14.6,±2.9 | 11,−85,−1 | 2.1,3.1,1.6 | 1.6,±.6 | 5 | 13.2,±2 | 0 | ||||
| 5 Cuneus (Cun) | 18 | −2,−87,24 | 2.2,1.6,4.9 | .6,±.1 | 5 | 13.6,±2.2 | −5,−95,23 | 2,2,5 | .4,±.2 | 3 | 10.8,±2.2 | 0 | ||||
| 7 Lat occipital G (LOG) | 18 | −30,−85,2 | 3.7,2.7,3 | 3.7,±.6 | 8 | 15.3,±1.6 | −17,−94,−4 | 5.7,3.1,2.9 | .8,±.3 | 4 | 11.8,±2.2 | 0 | ||||
| 9 Fusiform gyrus (FG) | 19 | −20,−72,−15 | 3.4,3.1,1.3 | 5.3,±.8 | 8 | 16.4,±1.3 | −17,−81,−12 | 6.7,6.8,2.5 | 1.9,±.5 | 4 | 11.7,±1.5 | 0 | ||||
| 10 Fusiform gyrus | 19 | 18,−75,−9 | 2.4,7.2,0.7 | 2.2,±.9 | 3 | 14.3,±2.9 | 20,−85,−14 | 4.7,2.9,2.2 | 2.2,±.5 | 3 | 12.7,±1 | 0 | ||||
| 11 Intraparietal S (IPS) | 19 | −25,−82,19 | 1.6,2.3,3.3 | 5.1,±1.3 | 8 | 15.8,±1.5 | 2 | −34,−61,43 | 3.8,4.1,2.8 | 4.1,±1.2 | 7 | 12.2,±0.7 | ||||
| 12 Intraparietal S | 19 | 28,−76,21 | 2.9,5.7,5.5 | 1.8,±1.3 | 3 | 14.8,±3.6 | 1 | 1 | ||||||||
| 13 Inf temporal G (ITG) | 19,37 | −37,−69,−15 | 2.2,1.9,1.4 | 3.9,±1 | 8 | 14.8,±1.8 | −44,−65,−9 | 3.7,6.9,2.7 | 1,±.8 | 3 | 9.2,±1.6 | 0 | ||||
| 15 Mid temporal G (MTG) | 21,37 | −44,−68,−2 | 3.2,3.5,2.4 | 2.4,±.5 | 7 | 15.2,±1.6 | −41,−70,−3 | 7.7,6.8,7 | .5,±.2 | 4 | 9.1,±1.5 | −55,−60,−1 | 0,0.7,2.9 | 1.2,±.2 | 3 | 10.3,±0.6 |
| 24 Mid temporal G (MTG) | 21 | 43,−61,2 | 5.7,9,6 | 1.9,±.2 | 4 | 16.7,±1.2 | 2 | 0 | ||||||||
| 25 Post/sup temporal | 22,37 | −53,−46,3 | 1.7,1.3,2.6 | 1.4,±.4 | 8 | 11.4,±1.3 | −51,−51,5 | 2.9,2.2,4.2 | .9,±.3 | 6 | 10.3,±2.1 | −56,−47,1 | 2.3,2.8,2.6 | .8,±.1 | 6 | 9.9,±0.7 |
| 17 Inf frontal G (IFG) | 45,47 | −41,17,5 | 2.8,3.1,1.4 | 1.6,±.8 | 7 | 10.7,±2 | −47,15,4 | 2.3,4.8,3.9 | 1.4,±.7 | 5 | 10.0,±2.4 | −47,20,1 | 2.4,5,1.5 | 3,±1 | 8 | 11.4,±0.8 |
| 26 Inf frontal G (IFG) | 45,47 | 0 | 0 | 49,19,3 | 1.4,6.3,3.1 | 2.4,±1.1 | 4 | 10.6,±2.1 | ||||||||
| 18 Inf/mid frontal G (I/MFG) | 9,46 | −39,3,31 | 1.9,2.2,2.5 | 3.7,±.6 | 8 | 13.4,±1.5 | −41,4,32 | 2.1,4.1,2.3 | 2,±1.1 | 5 | 10.4,±2.4 | −45,17,25 | 1.6,4.2,2.4 | 4.9,±.8 | 8 | 11.7,±0.9 |
| 19 Pre central S | 6 | −40,−5,46 | 3,2.4,3.7 | 1.2,±.3 | 8 | 11.7,±1.3 | −39,−6,49 | 3,2.6,1.8 | 1.7,±1 | 6 | 10.2,±2.3 | −43,1,43 | 1.5,2.1,3.3 | 3.2,±1 | 8 | 12.3,±1.2 |
| 21 Cingulate G (CG) | 24,32 | −6,7,49 | 0.6,2,1.4 | .8,±.2 | 7 | 11.1,±1.3 | −5,8,46 | 1.7,2.7,1.2 | .6,±.3 | 4 | 10.3,±2.1 | −6,17,44 | 0.8,4.5,1.9 | 1.5,±.5 | 6 | 9.7,±0.7 |
| 23 Med sup frontal G | 6 | −5,−1,59 | 1,2.3,3.5 | 1,±.4 | 6 | 10.8,±1.1 | −3,2,51 | 1.5,2.8,2.4 | .8,±.5 | 5 | 10.,±2.8 | −4,7,52 | 0.8,2.4,1.8 | 1.7,±.3 | 8 | 12.5,±1.1 |
BA designates Brodmann Area labeled in Talairach atlas (Talairach and Tournoux 1988) for nearest section with corresponding coordinates.
x, y, z are the atlas coordinates for each region’s peak z-score.
The number of subjects within a group that showed significant z-scores in listed region. SE, standard error.
Activity along the banks of the calcarine sulcus: primary visual cortex
The average z-score maps, which were based on individual subject analyses, showed bilateral activations surrounding the posterior calcarine sulcus in both groups of blind subjects (Fig. 3, A and B, Y = −77 and −77, respectively). In all early blind individuals, the activation extended onto the adjoining lingual gyrus and in five of eight onto the cuneus gyrus as well. The spatial extent of responses in the primary visual regions on both sides of the calcarine sulcus (Table 3, regions 1–5) was greater in the left hemisphere in most early blind subjects. More symmetrical activations occurred in some early blind (Fig. 3D, Y = −89). In contradistinction, the spatial extent of V1 responses in the average z-score map from late blind subjects were more medially confined to the banks of the calcarine sulcus (Fig. 3B, Y = −77; Fig. 4), although left lateralization was observed in some late blind subjects at the occipital pole (Fig. 3E, Y = −93; Fig. 4, subjects Late 3 and 5). The spatial extent of V1 responses for early and late blind subjects were comparable in the upper and lower banks of the calcarine sulcus (Table 3, regions 1– 4).
FIG. 3.

Location of regions with significant BOLD responses during covert verb generation to heard words. A: average z-score maps from 8 early blind subjects are shown overlaid on average structural images from the same subjects Axial (Z= 2) and 2 coronal slices (Y= −77 and −51) are aligned and interpolated to Talairach atlas coordinates (Talairach and Tournoux 1988) using 2-mm voxels. Table 3 lists anatomical identifiers for region number labels. The white horizontal lines drawn on the axial slice show the y axis position for the coronal sections; the horizontal line on each coronal slice marks the corresponding z axis position of the axial slice. Applicable z-score scale is shown below axial slice. B: average z-score maps from 6 late blind subjects. See A for additional details. Applicable z-score scale is shown below axial slice for sighted subjects. C: average z-score maps from 8 sighted subjects. See A for additional details. Applicable z-score scale is shown below axial slice. D: within subject statistical map of significant active foci observed in the vicinity of the calcarine sulcus (V1) and adjacent parts of visual cortex of 1 early blind subject (Early 1, see Table 1). Images show Gaussinized t-statistics (z-scores) calculated with the general linear model (see METHODS). Functional data were reconstructed to high resolution structural images obtained in the same subject using the same atlas alignment and interpolation described in A. Vertical white line marks the sagittal position of slice X = −7 and horizontal white line marks the coronal position of slice Y = −89. E: within-subject statistical map of significant active regions observed in the vicinity of the calcarine sulcus (V1) and adjacent parts of visual cortex of 1 late blind subject (Late 3, see Table 1). See D for additional details. Scale of z-score values applies to D and E.
FIG. 4.
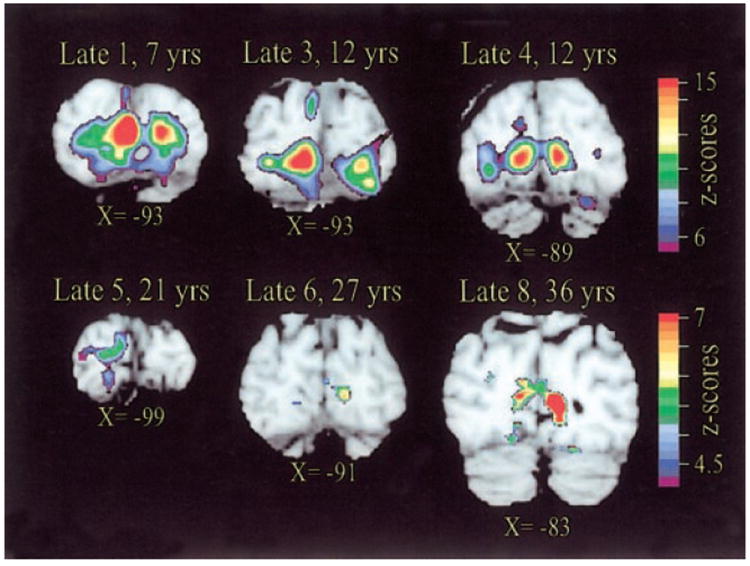
Within-subject statistical maps of significant active regions overlying V1 in 6 late blind individuals. Subject identifiers cross reference Table 1, which lists Braille reading fluency and cause of blindness. The age of blindness onset ranged from 7 (top-left image) to 36 (bottom-right image) years. Scaling (shown to the right) optimized for data from each subject. See Fig. 3D for additional details.
Statistical maps from individual late blind subjects showed that the spatial extent of V1 responses tended to be more extensive in those with earlier (<12 yr) age at onset of blindness (Fig. 4). For example, late blind 1, who lost sight at 7 yr, exhibited a larger spatial extent of activity over V1 (Fig. 4). However, it should be emphasized that V1 responses occurred in subjects who lost sight at a relatively advanced age. For example, late blind 8 (sight lost at age 36 yr) exhibited clear, bilateral responses along the banks of the calcarine sulcus (Fig. 4). The presence of activity in V1 did not correlate with Braille fluency. For example, late blind 8, with fairly extensive responses, was a very slow reader; late blind 6, with a small focus in V1, read faster than many of the early blind subjects (Table 1).
The individual subject analyses found that only two sighted subjects displayed responses in V1 that were of sufficient magnitude to pass threshold. This activity was in the right hemisphere. Thus the average z-score map for the sighted subjects showed a small focus of activity in the right visual cortex (Fig. 3C, Y = −77).
Activity in higher visual areas
Individual subject analyses showed that the three groups had marked differences in the distribution of responses in the fusiform gyrus. All early blind individuals had significant activity on the left; three of eight also did so on the right. As shown in the average z-score map, early blind subjects typically exhibited activations in the fusiform gyrus that extended across the entire inferior surface of the occipital cortex bilaterally (Fig. 3A, Y = −77) and reached rostrally >1 cm (Fig. 3A, Y = −51). In late blind subjects, the spatial extent of responses in the fusiform gyrus was smaller, more clearly separated from foci along the banks of the calcarine sulcus, and restricted to the more posterior parts of the infero-temporal surface (Fig. 3B, Y = −77 and −51). No sighted subjects showed BOLD responses in or adjoining the fusiform gyrus.
The individual subject analyses found that all early blind subjects showed left dominant responses in the lateral occipital gyrus (LOG; Fig. 3A, Z = 2, region 7; Fig. 3D, Y = −89). Only four of six late blind subjects had minimal activity in this region (Fig. 3E, Y = −93). The early blind versus late blind difference in volume was about a factor of 6 (Table 3, region 7, 3.7 vs. 0.6 cm3). No sighted subject showed responses in LOG.
The left posterior, inferior temporal gyrus and sulcus (Table 3, region 13, Fig. 3A, Y = −77) was activated in eight of eight early blind, three of six late blind, and zero of eight sighted subjects. The spatial extent of activity in early blind was four times more extensive than in the late blind (Table 3, region 13). In early blind, the region occupied most of the inferior temporal gyrus, adjoined active sites located medially in the fusiform gyrus, and extended anterior and lateral to another region in the middle temporal gyrus (Fig. 3A, region 15, Y = −77).
The left middle temporal gyrus (Table 3, region 15) was activated in seven of eight early blind and four of six late blind (early blind: Fig. 3A, Z = 2 and Y = −51; Fig. 6A, X = −41 and −45; late blind: Fig. 3B, Z = 2; Fig. 6B, X = −41 and −45). The spatial extent of this response was approximately four times greater in early blind compared with late blind (Table 3, region 15). This region probably corresponds to V5/MT. In four of eight early blind subjects, the right middle temporal gyrus response was also activated (Fig. 3A, Y = −51, region 24; Table 3). Three of eight sighted subjects showed a small response in the left middle temporal gyrus (Fig. 3C).
FIG. 6.
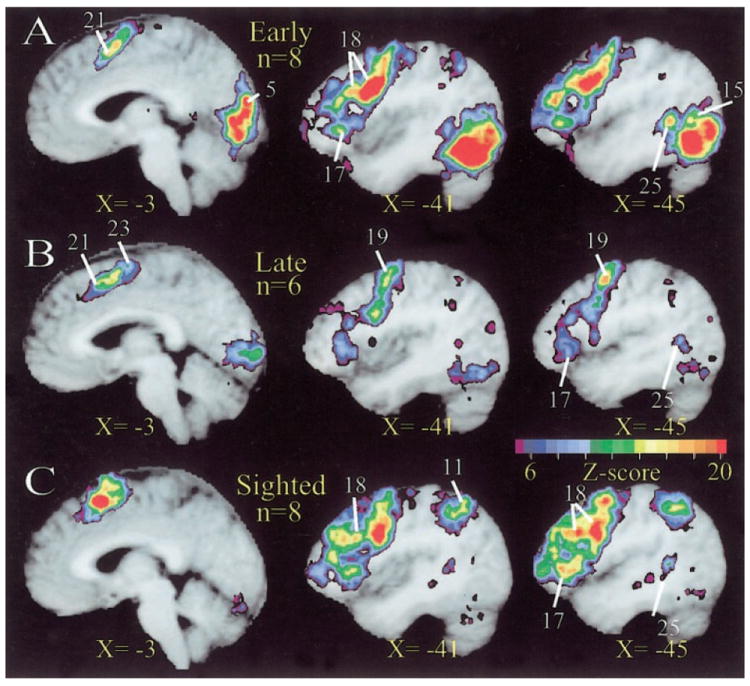
Regions in left frontal cortex with significant BOLD responses during covert verb generation to heard words. A: results are shown on 3 selected sagittal slices from 8 early blind subjects. These average z-score images illustrate regions in left frontal cortex. See Fig. 1A for additional details regarding alignment and interpolation of functional and anatomical images to atlas space. B: results are shown from 6 late blind subjects. See A for additional details. C: results are shown from 8 sighted subjects. See A for additional details. Scale bar for z-scores applies to all images.
Confinement of significant BOLD modulation differences to the left visual cortex was demonstrated in the statistical maps derived from the subject group by time interaction term of the voxel-wise, group level ANOVA analysis (Fig. 5A). The affected regions surrounded V1 along the calcarine sulcus, and higher visual areas in fusiform, posterior inferior temporal, lateral occipital, and posterior middle temporal gyral regions. This result indicated group differences in evoked BOLD waveforms without recourse to assumptions about hemodynamic response functions, but did not show which groups had the larger responses that contributed to the significant variance detected by the ANOVA.
FIG. 5.
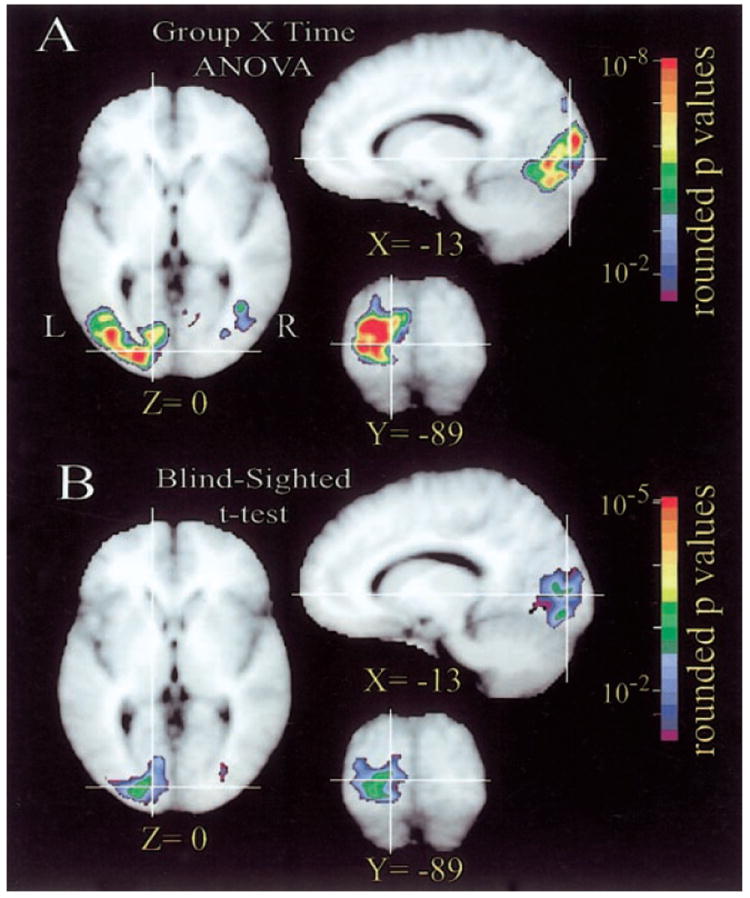
Group level analyses of BOLD responses in parts of visual cortex. A: ANOVA F ratio statistical map for the interaction term of group (8 early blind, 6 late blind, and 8 sighted subjects) by time (6 frames per task alternation). B: t-test statistical map of between group comparison (14 blind vs. 8 sighted subjects). Vertical and horizontal white lines show location of corresponding slice orientations.
The group level statistical maps based on post hoc, voxel-wise t-test analyses showed that the response magnitudes in the same left visual cortex regions were significantly greater in the blind (early plus late) compared with sighted subjects (Fig. 5B). Three additional voxel-wise t-test analyses compared each pair of subject groups. For these tests the threshold was set at 0.017 (=0.05/3) to correct for multiple comparisons. The statistical map based on comparing early blind versus sighted subjects similarly showed greater responses in the same left visual cortex regions illustrated in Fig. 5B. The map obtained from comparing late blind versus sighted subjects also showed greater responses over the left primary and higher visual areas. These results indicated that each group of blind subjects had greater magnitude BOLD responses than sighted subjects in portions of the left visual cortex that included V1. The map obtained from comparing early versus late blind subjects showed a ring of greater activity surrounding the banks of the calcarine sulcus, but no differences in the calcarine region. This result showed that the two groups of blind subjects had relatively equal magnitude responses in V1 but that early blind subjects had greater response magnitudes in adjacent higher visual areas.
Other activated regions
The average z-score maps based on individual subject analyses showed significant BOLD responses in several nonvisual cortex regions in all groups (Figs. 3 and 6). These included foci in the parietal, temporal, and frontal regions that respectively indicated effects on attention, auditory, and language areas (Table 3). None of these regions showed significant group × time effects in the voxel-wise ANOVA (Fig. 5A), which indicated that the response modulations did not differ between the groups in nonvisual regions. The following describes some features of the activity in nonvisual regions that were noted in the average z-score maps.
Regions in parietal-occipital cortex
All early blind subjects displayed left intraparietal sulcus activation (Fig. 3A, Y = −77, region 11) in what appears to be a portion of the extrastriate visual area 19. A minority (3/8) of individual early blind subjects also had a smaller focus in the right intraparietal sulcus (Table 3, region 12). Comparable responses were not observed in the late blind group. Most (7/8) of the sighted group activated a left parietal site (Fig. 3C, Y = −45, also labeled region 11) 12 mm lateral, 16 mm anterior, and 14 mm superior to the early blind response. The left parietal focus in sighted subjects probably is in BA 7. However, the superior-anterior extension of region 11 in early blind incorporated the coordinates of BA 7. It therefore appears likely that the more extended region 11 in early blind included portions of BA 7 and 19.
Regions in auditory cortex
All blind subjects showed significant BOLD responses in the left posterior superior temporal gyrus (Fig. 6, A and B, X = −45, region 25) in what is probably BA 22. This focus extended from X = −43 to the lateral edge of the brain. This response was present also in six of eight sighted subjects, although smaller in extent and amplitude (Fig. 6A, Y = −45, region 25; Table 3). No groups showed evidence of BOLD modulation in the planum temporale or more anterior portions of the superior temporal gyrus, i.e., first and second tier auditory cortex.
Regions in frontal cortex
Language associated areas in left prefrontal cortex (Table 3, regions 17, 26, 18, and 19) were, on the whole, similarly activated in all three subject groups (Fig. 6, A–C, X = −41 and −45). The involved regions included the inferior frontal gyrus/frontal operculum (BA 45/47), the dorsolateral convexity (BA 9/46), and the precentral gyrus with variable anterior extension (BA 6/8).
The spatial extent of the BA 45/47 focus was greatest in the sighted subjects (Table 3, region 17) in whom this response extended toward the frontal pole (Fig. 6C, X = −41, −45). In four of eight sighted subjects, but no blind subjects, the homologous region on the right also was activated (Fig. 3C, Z = 2; Table 3, region 26). The left BA 9/46 response extended further anterior in the sighted as compared with both blind groups (Fig. 6C, X = −41 and −45).
A clear focus of activation centered on the pars opercularis portion of the left inferior frontal gyrus (BA 44) did not emerge in this experiment. In all groups, activation of this part of the brain was not separable from the BA 45/47 focus (Fig. 6, A–C, X = −45).
Mesial surface activations included the frontal gyrus, (mSFG, BA 6) and the anterior cingulate gyrus (BA 24/32) in all groups (Fig. 6, X = −3, regions 21, 23). Figure 3, D and E (X = −7 and −11, respectively), illustrates this response in selected individuals.
Time course of BOLD responses
Where present, mean BOLD temporal response profiles were similar across all subject groups and regions (Fig. 7). Signal increased during task frames and decreased during control frames. Peak modulation appeared one to two frames after the task switch, i.e., during frames 2–3 and 5– 6. Small standard error bars about the means demonstrate response consistency for all subjects.
DISCUSSION
These results confirm prior reports of visual cortex activation in blind persons performing nonvisual tasks (Büchel et al. 1998a; Burton et al. 2002; De Volder et al. 1997; Melzer et al. 2001; Sadato et al. 1996, 1998; Wanet-Defalque et al. 1988). Auditory tasks were used in several prior studies of early blind (Arno et al. 2001; Kujala et al. 1995a,b, 1997; Leclerc et al. 2000; Lessard et al. 1998; Liotti et al. 1998; Röder et al. 1996, 2000, 2001; Weeks et al. 2000) and late blind (Kujala et al. 2000) individuals. These tasks included the following: 1) detecting and counting tones with a deviant frequency versus ignoring the tones during Braille reading (Kujala et al. 1995b), 2) detecting a deviant tone in one ear while ignoring distracting sounds in the other (Liotti et al. 1998), and 3) sustaining attention to binaural sound localization in the azimuth domain (Leclerc et al. 2000). The phenomenon of visual cortex activation by auditory tasks in blind people has a counterpart in hearing impaired individuals showing responses in auditory areas during visual processing of sign language (Petitto et al. 2000). These examples of cross-modal plasticity presumably reflect compensatory adaptations to loss of sight or hearing (Röder et al. 2001; Zatorre 2001). Before addressing the issues concerned with cross-modal adaptations, we consider our prior study of verb generation in response to Braille nouns (Burton et al. 2002). The following discusses only those regions showing differences in responses in the group level analyses.
Regions activated by verb generation in response to Braille read versus heard nouns
All presently activated regions in visual cortex during verb generation to heard nouns (numbered 1–5, 7, 9–13, and 15 in Table 3) were also responsive in the previous experiment involving verb generation to Braille read nouns (see similarly numbered regions in Table 2 of Burton et al. 2002). Both experiments demonstrate prominent activation of visual areas generally believed to support various specialized visual features in sighted people (DeYoe et al. 1996; Dumoulin et al. 2000; Hadjikhani et al. 1998; Malach et al. 1995; Sereno et al. 1995; Tootell et al. 1995–1998; Watson et al. 1993). Also, in both experiments, late blind showed smaller responsive volumes and fewer active regions. We previously suggested that visual cortex activation during Braille reading reflects normal functionality, in particular, orthographic feature analysis (Tagamets et al. 2000), which, consequent to adaptation, becomes connected to somatic sensory/motor (haptic) input. A similar conclusion was reached by Meltzer and colleagues (Melzer et al. 2001). However, the present results require that a complete account of visual cortex functionality in blind persons must include more than reconfigured haptic processing. The experimental design feature common to both of our studies was a demanding semantic task. Listening to words did not produce occipital cortex activity in a previous PET study of blind subjects (Büchel et al. 1998a). Thus it may be that visual cortex activation in blind persons reflects higher level lexical processes. The range of effective language tasks remains to be determined.
In the previous Braille reading experiment, the spatial extent of activations in left V1 were significantly greater in early blind subjects (Burton et al. 2002). Responses were attributed to V1 when significant activity was located over the upper and lower banks of the calcarine sulcus. In the present study, both groups of blind subjects showed nearly comparable spatial extent of responses in V1 bilaterally. Like the findings shown here in Table 3, the earlier results were based on within subject assessments after anatomical definition of region borders in individual subjects. These differences in the spatial extent of activity in V1 between the two studies may be related to the source of sensory input. In the prior study, the left V1 was contralateral to the Braille reading hand for most subjects. Here, all subjects received binaural stimulation, which possibly was responsible for the relatively equal bilateral spatial extent of activity in V1.
A different distribution of activity occurred in higher visual areas. In both the Braille reading and auditory noun experiments, activation of higher visual areas in fusiform, lateral occipital, and inferior temporal cortex was strongly left lateralized in both groups of blind subjects. As in the prior study, however, the left fusiform gyrus in early blind subjects again showed a greater volume of activation (Table 3). Early blind subjects additionally showed a greater spatial extent of activity in occipital-temporal and occipital-parietal areas. Thus the side with predominant activity in higher visual areas does not appear to reflect the Braille reading hand because this distribution of responses was the same with a binaural source of sensory information.
Left dominance of all parts of visual cortex was also apparent in all voxel level, whole brain ANOVA and t-test analyses (Figs. 3 and 5). Group level voxel-wise re-analyses of the results from our prior study (Burton et al. 2002) similarly found significantly greater MR signals in the left visual cortex. These group level assessments in both studies indicated that the magnitude of activity in V1 and all higher visual areas was significantly greater in early blind subjects.
Based on the incidence of responses using within subject analyses, most blind and two sighted subjects showed responses in right visual cortex. However, these results required estimates of hemodynamic responses and correlation with assumed response functions. There were no significant differences in response magnitudes on the right between the groups in the voxel-wise t-test analyses. t-test voxel-wise analysis, however, indicate significant activations in the left visual cortex of blind people. Thus blindness results in the appearance of some novel process or mechanism in the left visual cortex. These changes reach across multiple visual areas especially in early blind people and become more restricted with older ages of blindness onset.
The lateralization finding presumably relates to language dominance of the left hemisphere for most language processes (Geschwind and Galaburda 1985; Petersen and Fiez 1993; Petersen et al. 1990; Petitto et al. 2000). Accordingly, the early versus late blind distribution differences argue that age at onset of blindness affects the degree to which extrastriate cortex can acquire representation of language. Alternative explanations previously suggested for activity in these extrastriate regions are processes associated with object recognition, mental imagery, or spatial localization.
Several studies have identified areas in extrastriate cortex associated with object recognition both in sighted (Amedi et al. 2001; Grill-Spector et al. 2001; Malach et al. 1995) and blind individuals (Büchel et al. 1998b). The affected regions include BA areas 19/37 in anterior fusiform gyrus, posterior inferior temporal gyrus, and the neighboring lateral occipital gyrus, all of which were activated in the present study. These regions are thought to be multimodal as they responded to tactile as well as visual objects (Amedi et al. 2001; Büchel et al. 1998b). A possible interpretation of the present results would be the suggestion that auditory noun recognition-identification might be construed as auditory object recognition and as such acquired shared representation with objects (tactile or visual) at least in early blind people. Limiting the general applicability of an object recognition hypothesis for these responses is that no sighted subject showed activation in these areas and, in late blind subjects, the responses were reduced.
It has been suggested that these extrastriate responses reflect normal mental imagery (De Volder et al. 2001) rather than compensatory adaptations to blindness. The regions described in this study especially included posterior fusiform gyrus (BA 19/37) on the left side, and the posterior inferotemporal gyrus (BA 37) (De Volder et al. 2001). Normal mental imagery does not explain our extrastriate responses because it would have to be supposed that such imagery occurred only in the early blind subjects. No sighted and few late blind subjects in the current study showed extrastriate functional anatomy corresponding to that observed in the early blind subjects. It seems illogical that only early blind subjects “mentally visualized” when doing verb generation as these people have the least knowledge of objects. It also seems implausible that activations in some blind people arise from mental imagery when the same task does not lead to such responses in all subjects when listening to words.
All groups in the current study showed left posterior middle temporal gyrus responses (region 15, Table 3) at a locus previously identified as MT/V5 (Dumoulin et al. 2000; Tootell et al. 1995). In our prior study (Burton et al. 2002) this focus was activated only in the early blind group. We suggested that MT specialization for the analysis of coherent visual motion had become applied to the haptics of reading Braille as a result of adaptations to blindness. The present results do not support this hypothesis as auditory nouns do not embody motion as commonly understood. The presence of left MT responses in a minority (3/8) of sighted subjects suggests that the adequate stimulus may be intrinsic to a demanding semantic task. If so, then the data suggest that early blindness may enhance MT responsiveness bilaterally. Only early blind subjects (4/8) showed MT responses on the right (region 24, Table 3). Possibly, these activations reflect responses to the auditory nouns as temporal events (Zacks et al. 2001). MT should be distinguished from another focus more than 3 cm anterior in the middle temporal gyrus (Bokde et al. 2001; Rumsey et al. 1997) thought to be involved in word recognition, but not activated in the present study.
The principal finding in parietal-occipital cortex was left lateralized activation of the posterior intraparietal sulcal cortex (IPS) in early blind subjects (possibly BA 19). All but one sighted subject exhibited activation at a nearby focus (region 11, Table 3) probably in left BA 7. However, the voxel-wise t-test analyses showed no significantly greater response magnitudes in this region, which suggests that all subjects had some activity in the BA 19 part of IPS. We previously suggested that IPS activation indicated attention directed toward the Braille reading hand (Burton et al. 2002). However, this hypothesis does not explain IPS activation in the absence of a somato-motor task. The IPS region is generally believed to mediate eye movements (Petit and Haxby 1999) and spatial attention (Corbetta 1998; Corbetta et al. 1998a,b, 2000; Shulman et al. 1999). The current IPS activations can be formulated in terms of attention by supposing that the task required intentional focusing on the auditory/phonological features of the noun stimuli (delivered through headphones) in the noisy environment of the scanner. Virtually all subjects in all groups admitted to occasional uncertainty regarding the identity of the delivered noun. This interpretation of IPS functionality is admittedly speculative.
A critical age for developing cross-modal adaptations
These data and our preceding study pertain to the age of blindness affecting cortical reorganization. The age at which our late blind subjects lost sight varied between 7 and 36 yr. Late blind who lost sight before age 12 yr more closely resembled the early blind than did those who lost sight later in life (Fig. 4), but physiological evidence of cortical reorganization was observed in all blind subjects. Thus in contradistinction to some previous reports (Cohen et al. 1999), we conclude that cortical plasticity persists after age 20. This is consistent with an extensive literature documenting instances where sensory deprivation altered cortical functions in adult animals and humans (e.g., Buonomano and Merzenich 1998; Ramachandran and Hirstein 1998). Recent studies with adult deaf people (average age > 30 yr) also demonstrated cross-modal responses to cochlear implants (Giraud et al. 2001a,b). These findings demonstrate adaptive changes to sensory deprivation possibly throughout life rather than a limited period of susceptibility.
An associative coupling mechanism for cross-modal plasticity in sensory deprived individuals
Ideas developed from recent studies with individuals who received cochlear implants may be applicable to the adaptations noted in blind people. Giraud and colleagues (Giraud et al. 2001b) showed that users of cochlear implants developed concomitant activation of auditory and visual cortex during perception of speech and other meaningful sounds. The degree of V1/V2 activity correlated strongly with lipreading skills and progressed with the ability to interpret cochlear implant signals. Visual cortex activity persisted when these subjects processed cochlear implant sounds in the absence of visual information. The authors proposed that visual cortex was activated by lipreading as these people learned to use the cochlear implants for speech. They hypothesized that learned associative coupling of visual and auditory cortex activity is critical to the development of speech discrimination using cochlear implants. Thus the subjects had “audio-visual coupling as the probable substrate for long-term functional improvement in speech discrimination” (Giraud et al. 2001b).
A parallel may be drawn to the process of learning Braille. Braille literacy requires associative coupling between phonics and the tactile experience of Braille. All of our subjects learned Braille after they became blind, and they had extensive experience associating the feel of the Braille fields and auditory instruction as to the lexical meaning of the tactile sensations. Hence, we argue that Braille literacy is not primarily haptic but rather lexical and that the lexical processor resides at least in part in left occipital cortex (Burton et al. 2002). The associative coupling hypothesis predicts that prolonged Braille experience underlies the lexical processor responding to auditory words even in the absence of Braille. The analogy to cochlear implants is imperfect because Braille is not a true prosthetic device. Moreover, “lexical” is a broad and incompletely defined descriptor. Nevertheless, the converse of the associative coupling hypothesis predicts that blind people who do not know Braille might show little or no left visual cortex activation when generating verbs to auditory nouns. Confirmation of the hypothesis would suggest that acquisition of Braille literacy leads to expansion of the brain regions engaged by language during listening as well as reading. This outcome would have important implications for education in the blind, and by extension, rehabilitation in general.
Acknowledgments
P. Schonlau, the Braille Reading Teacher at the Missouri School for the Blind, recruited blind subjects for this project. We are indebted to N. Thompson and J. Kakkanathu for assistance with preparing the subjects and help in collecting and processing image data. We also thank Drs. T. Conturo and E. Akbudak for providing scanner pulse sequences and Drs. J. Ollinger and M. McAvoy for analysis routines used to create the statistical parameter maps.
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-37237 to H. Burton and the McDonnell Center for Higher Brain Function.
Footnotes
Cortical region names and label numbers match those used previously (see Table 2 in Burton et al. 2002). These same label numbers mark selected regions in Figs. 3, 5, and 6.
Use of less stringent threshold criteria might have affected the number of subjects with identified responses in some regions. The selected criteria represent a compromise intended to protect against falsely concluding that a particular region showed no activity in one or another group.
References
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nature Neurosci. 2001;4:324–330. doi: 10.1038/85201. [DOI] [PubMed] [Google Scholar]
- Arno P, De Volder AG, Vanlierde A, Wanet-Defalque MC, Streel E, Robert A, Sanabria-Bohorquez S, Veraart C. Occipital activation by pattern recognition in the early blind using auditory substitution for vision. Neuroimage. 2001;13:632–645. doi: 10.1006/nimg.2000.0731. [DOI] [PubMed] [Google Scholar]
- Bandettini P. Selection of the optimal pulse sequence for functional MRI. In: Jezzard P, Matthews P, Smith S, editors. Functional Magnetic Resonance Imaging: An Introduction to Methods. Oxford, UK: Oxford Press; 2001. pp. 124–143. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Bosch V. Statistical analysis of multi-subject fMRI data: assessment of focal activations. J Magn Reson Imaging. 2000;11:61–64. doi: 10.1002/(sici)1522-2586(200001)11:1<61::aid-jmri9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Price C, Frackowiak RS, Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998a;121:409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- Büchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998b;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol. 2002;87:589–611. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Macwhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instruments Comput. 1993;25:257–271. [Google Scholar]
- Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M. Period of susceptibility for cross-modal plasticity in the blind. Ann Neurol. 1999;45:451–460. doi: 10.1002/1531-8249(199904)45:4<451::aid-ana6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Conturo TE, McKinstry RM, Akbudak E, Snyder AZ, Yang T, Raichle ME. Sensitivity optimization and experimental design in fMRI. Soc Neurosci Abstr. 1996;22:7. [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, VanEssen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- De Volder AG, Bol A, Blin J, Robert A, Arno P, Grandin C, Michel C, Veraart C. Brain energy metabolism in early blind subjects: neural activity in the visual cortex. Brain Res. 1997;750:235–244. doi: 10.1016/s0006-8993(96)01352-2. [DOI] [PubMed] [Google Scholar]
- De Volder AG, Toyama H, Kimura Y, Kiyosawa M, Nakano H, Vanlierde A, Wanet-Defalque MC, Mishina M, Oda K, Ishiwata K, Senda M. Auditory triggered mental imagery of shape involves visual association areas in early blind humans. Neuroimage. 2001;14:129–139. doi: 10.1006/nimg.2001.0782. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization: biological mechanisms, associations, and pathology. III. A hypothesis and a program for research. Arch Neurol. 1985;42:634–654. doi: 10.1001/archneur.1985.04060070024012. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Price CJ, Graham JM, Frackowiak RS. Functional plasticity of language-related brain areas after cochlear implantation. Brain. 2001a;124:1307–1316. doi: 10.1093/brain/124.7.1307. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Price CJ, Graham JM, Truy E, Frackowiak RS. Cross-modal plasticity underpins language recovery after cochlear implantation. Neuron. 2001b;30:657–663. doi: 10.1016/s0896-6273(01)00318-x. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Liu AK, Dale AM, Cavanagh P, Tootell RB. Retinotopy and color sensitivity in human visual cortical area V8. Nature Neurosci. 1998;1:235–241. doi: 10.1038/681. [DOI] [PubMed] [Google Scholar]
- Hamilton R, Keenan JP, Catala M, Pascual-Leone A. Alexia for Braille following bilateral occipital stroke in an early blind woman. Neuroreport. 2000;11:237–240. doi: 10.1097/00001756-200002070-00003. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Pascual-Leone A. Cortical plasticity associated with Braille learning. Trends Cogn Sci. 1998;2:168–174. doi: 10.1016/s1364-6613(98)01172-3. [DOI] [PubMed] [Google Scholar]
- Howseman AW, Stehling MK, Chapman B, Coxon R, Turner R, Ordidge RJ, Cawley MG, Glover P, Mansfield P, Coupland RE. Improvements in snap-shot nuclear magnetic resonance imaging. Brit J Radiol. 1988;61:822–828. doi: 10.1259/0007-1285-61-729-822. [DOI] [PubMed] [Google Scholar]
- Kujala T, Alho K, Huotilainen M, Ilmoniemi Rj, Lehtokoski A, Leinonen A, Rinne T, Salonen O, Sinkkonen J, Standertskjold-Nordenstam CG, Naatanen R. Electrophysiological evidence for cross-modal plasticity in humans with early- and late-onset blindness. Psychophysiology. 1997;34:213–216. doi: 10.1111/j.1469-8986.1997.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Kujala T, Alho K, Kekoni J, Hamalainen H, Reinikainen K, Salonen O, Standertskjold-Nordenstam CG, Naatanen R. Auditory and somatosensory event-related brain potentials in early blind humans. Exp Brain Res. 1995a;104:519–526. doi: 10.1007/BF00231986. [DOI] [PubMed] [Google Scholar]
- Kujala T, Alho K, Naatanen R. Cross-modal reorganization of human cortical functions. Trends Neurosci. 2000;23:115–120. doi: 10.1016/s0166-2236(99)01504-0. [DOI] [PubMed] [Google Scholar]
- Kujala T, Huotilainen M, Sinkkonen J, Ahonen A, Alho K, Hamalainen M, Ilmoniemi R, Kajola M, Knuutila J, Lavikainen J, Salonen O, Simola J, Standerskjold-Nordenstam C-G, Tiitinen H, Tissari S, Naatanen R. Visual cortex activation in blind humans during sound discrimination. Neurosci Lett. 1995b;183:143–146. doi: 10.1016/0304-3940(94)11135-6. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomo-graphic images of the human brain. Hum Brain Mapp. 1995;3:209–223. [Google Scholar]
- Leclerc C, Saint-Amour D, Lavoie ME, Lassonde M, Lepore F. Brain functional reorganization in early blind humans revealed by auditory event-related potentials. Neuroreport. 2000;11:545–550. doi: 10.1097/00001756-200002280-00024. [DOI] [PubMed] [Google Scholar]
- Lessard N, Paré M, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395:278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ryder K, Woldorff MG. Auditory attention in the congenitally blind: where, when and what gets reorganized? Neuroreport. 1998;9:1007–1012. doi: 10.1097/00001756-199804200-00010. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas Jb, Benson RR, Kwong Kk, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer P, Morgan VL, Pickens DR, Price RR, Wall RS, Ebner FF. Cortical activation during Braille reading is influenced by early visual experience in subjects with severe visual disability: a correlational fMRI study. Hum Brain Mapp. 2001;14:186–195. doi: 10.1002/hbm.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler JPD, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood level oxygenation. Proc Nat Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI I. The method. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Petersen S, Fiez J. The processing of single words studied with positron emission tomography. Annu Rev Neurosci. 1993;16:509–530. doi: 10.1146/annurev.ne.16.030193.002453. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Petit L, Haxby JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999;82:463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Zatorre RJ, Gauna K, Nikelski EJ, Dostie D, Evans AC. Speech-like cerebral activity in profoundly deaf people processing signed languages: implications for the neural basis of human language. Proc Natl Acad Sci USA. 2000;97:13961–13966. doi: 10.1073/pnas.97.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Hennighausen E, Näcker F. Event-related potentials during auditory and somatosensory discrimination in sighted and blind human subjects. Cogn Brain Res. 1996;4:77–93. [PubMed] [Google Scholar]
- Röder B, Rösler F, Neville HJ. Event-related potentials during auditory language processing in congenitally blind and sighted people. Neuropsychologia. 2000;38:1482–1502. doi: 10.1016/s0028-3932(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Neville HJ. Auditory memory in congenitally blind adults: a behavioral- electrophysiological investigation. Cognit Brain Res. 2001;11:289–303. doi: 10.1016/s0926-6410(01)00002-7. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Deiber MP, Ibanez V, Hallett M. Neural networks for Braille reading by the blind. Brain. 1998;121:1213–1229. doi: 10.1093/brain/121.7.1213. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Seger CA, Rabin LA, Desmond JE, Gabrieli JD. Verb generation priming involves conceptual implicit memory. Brain Cogn. 1999;41:150–177. doi: 10.1006/brcg.1999.1116. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagamets MA, Novick JM, Chalmers ML, Friedman RB. A parametric approach to orthographic processing in the brain: an fMRI study. J Cogn Neurosci. 2000;12:281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. p. 122. [Google Scholar]
- Tootell RB, Dale AM, Sereno MI, Malach R. New images from human visual cortex. Trends Neurosci. 1996;19:481–489. doi: 10.1016/S0166-2236(96)10053-9. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci USA. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet-Defalque MC, Veraart C, De Volder A, Metz R, Michel C, Dooms G, Goffinet A. High metabolic activity in the visual cortex of early blind human subjects. Brain Res. 1988;446:369–373. doi: 10.1016/0006-8993(88)90896-7. [DOI] [PubMed] [Google Scholar]
- Watson JDG, Myers R, Frackowiak RSJ, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, Hallett M, Rauschecker JP. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Braver TS, Sheridan MA, Donaldson DI, Snyder AZ, Ollinger JM, Buckner RL, Raichle ME. Human brain activity time-locked to perceptual event boundaries. Nature Neurosci. 2001;4:651–655. doi: 10.1038/88486. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Do you see what I’m saying? Interactions between auditory and visual cortices in cochlear implant users. Neuron. 2001;31:13–14. doi: 10.1016/s0896-6273(01)00347-6. [DOI] [PubMed] [Google Scholar]


