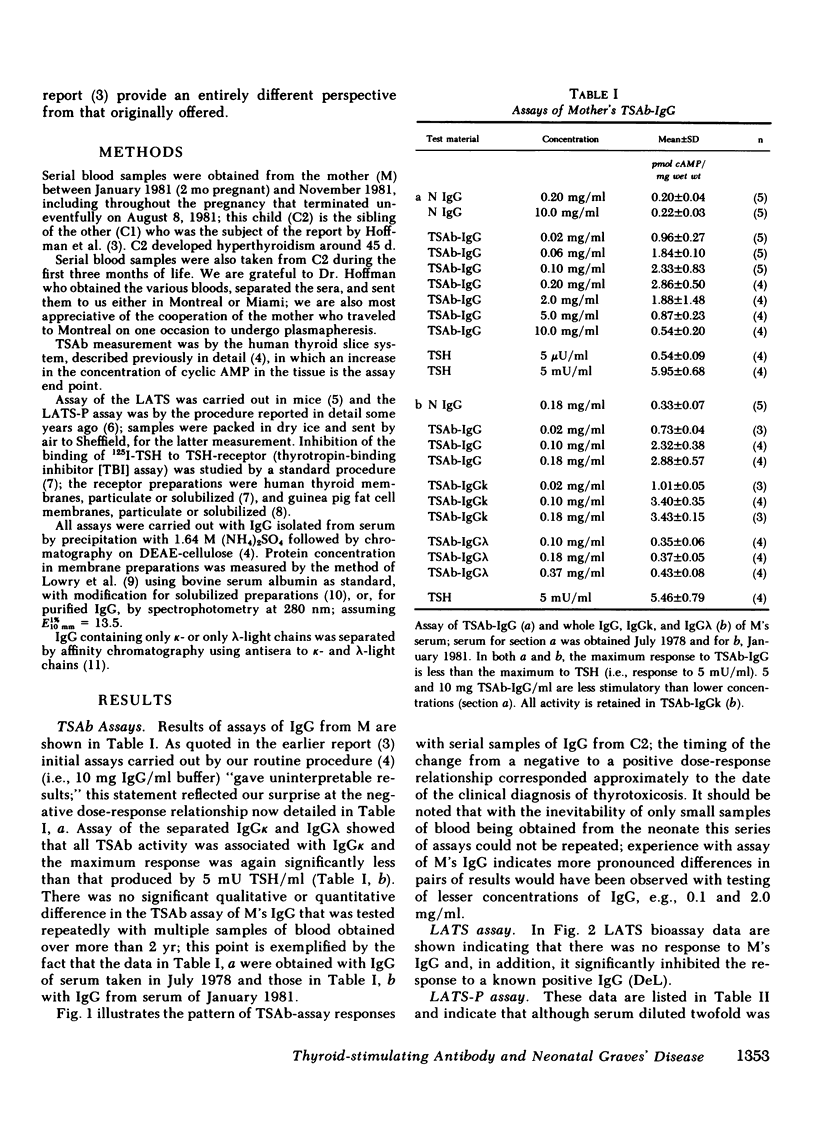
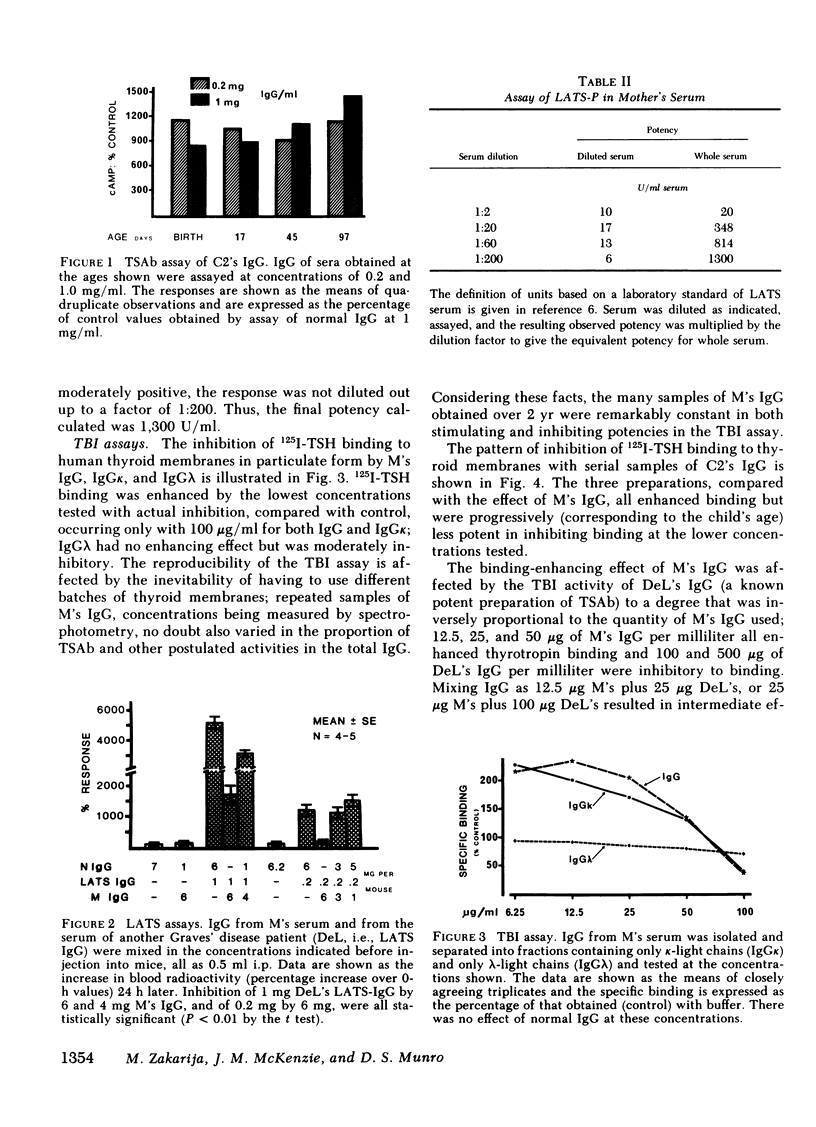
Abstract
Studies were carried out with the serum IgG from a mother and her two children who developed neonatal Graves' disease several weeks after birth. The maternal IgG: (a) stimulated the human thyroid in vitro, but maximal stimulation was found only with dilution of the IgG; (b) was very potent in the long-acting thyroid stimulator (LATS)-protector assay, but only when an inhibitor of the system was diluted out; (c) inhibited a standard preparation of LATS in the mouse bioassay; (d) was biphasic in the thyrotropin-binding inhibition (TBI) assay, i.e., enhanced binding at low concentrations of IgG and inhibited binding at high levels. Enhancement in the TBI assay was found only with particulate preparations of human thyroid membranes as receptor and not when that material was solubilized, nor with guinea pig fat cell membranes as receptor. Serial blood samples from the second child were obtained at birth and until 3 mo of age. In the thyroid slice (cyclic AMP) assay system there was a negative dose-response relationship in testing IgG until age 45 d when it became positive, coinciding with the clinical recognition that hyperthyroidism had developed. The data are compatible with a concept that this mother's IgG contained thyroid-stimulating antibody (TSAb) and another moiety that inhibited TSAb through an action on the thyroid cell membrane, thus delaying the onset of hyperthyroidism in the neonate until the inhibiting IgG was metabolically cleared to an ineffective concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Hoffman W. H., Sahasrananan P., Ferandos S. S., Burek C. L., Rose N. R. Transient thyrotoxicosis in an infant delivered to a long-acting thyroid stimulator (LATS)- and LATS protector-negative, thyroid-stimulating antibody-positive woman with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1982 Feb;54(2):354–356. doi: 10.1210/jcem-54-2-354. [DOI] [PubMed] [Google Scholar]
- Holmes S. D., Dirmikis S. M., Martin T. J., Munro D. S. Evidence that both long-acting thyroid stimulator and long-acting thyroid stimulator-protector stimulate the human thyroid gland. J Endocrinol. 1979 Feb;80(2):215–221. doi: 10.1677/joe.0.0800215. [DOI] [PubMed] [Google Scholar]
- Kishihara M., Nakao Y., Baba Y., Matsukura S., Kuma K., Fujita T. Interaction between thyroid-stimulating immunoglobulins and thyrotropin receptors in fat cell membranes. J Clin Endocrinol Metab. 1979 Nov;49(5):706–711. doi: 10.1210/jcem-49-5-706. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKenzie J. M., Zakarija M. A reconsideration of a thyroid-stimulating immunoglobulin as the cause of hyperthyroidism in Graves' disease. J Clin Endocrinol Metab. 1976 Apr;42(4):778–781. doi: 10.1210/jcem-42-4-778. [DOI] [PubMed] [Google Scholar]
- McKenzie J. M., Zakarija M. Pathogenesis of neonatal Graves' disease. J Endocrinol Invest. 1978 Apr;1(2):183–189. doi: 10.1007/BF03350370. [DOI] [PubMed] [Google Scholar]
- Mullin B. R., Aloj S. M., Fishman P. H., Lee G., Kohn L. D., Brady R. O. Cholera toxin interactions with thyrotropin receptors on thyroid plasma membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1679–1683. doi: 10.1073/pnas.73.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro D. S., Dirmikis S. M., Humphries H., Smith T., Broadhead G. D. The role of thyroid stimulating immunoglobulins of Graves's disease in neonatal thyrotoxicosis. Br J Obstet Gynaecol. 1978 Nov;85(11):837–843. doi: 10.1111/j.1471-0528.1978.tb15839.x. [DOI] [PubMed] [Google Scholar]
- Zakarija M. Immunochemical characterization of the thyroid-stimulating antibody (TSAb) of Graves' disease: evidence for restricted heterogeneity. J Clin Lab Immunol. 1983 Feb;10(2):77–85. [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M., Banovac K. Clinical significance of assay of thyroid-stimulating antibody in Graves' disease. Ann Intern Med. 1980 Jul;93(1):28–32. doi: 10.7326/0003-4819-93-1-28. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Influences of cholera toxin on thyroid stimulation by thyrotropin and thyroid-stimulating antibody. Endocrinology. 1980 Dec;107(6):2051–2054. doi: 10.1210/endo-107-6-2051. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Zoological specificity of human thyroid-stimulating antibody. J Clin Endocrinol Metab. 1978 Aug;47(2):249–254. doi: 10.1210/jcem-47-2-249. [DOI] [PubMed] [Google Scholar]
- Zakarija M., Witte A., McKenzie J. M. Influence of cholera toxin on in vitro refractoriness to thyrotropin of thyroids from rats fed propylthiouracil. Endocrinology. 1980 Dec;107(6):2045–2050. doi: 10.1210/endo-107-6-2045. [DOI] [PubMed] [Google Scholar]


