Abstract
Intestinal microfold (M) cells are an enigmatic lineage of intestinal epithelial cells that initiate mucosal immune responses by uptake and transcytosis of luminal antigens. The mechanisms of M-cell differentiation are poorly understood as the rarity of these cells has hampered analysis. Exogenous RANKL administration can synchronously activate M-cell differentiation in mice. Here we show the Ets transcription factor Spi-B was induced early during M-cell differentiation. Absence of Spi-B silenced the expression of multiple M-cell markers and prevented the differentiation of M cells in mice. Oral antigen-specific T cell activation was significantly impaired in the intestine of Spib−/− mice. Our study demonstrates that intestinal M-cell lineage commitment requires Spi-B as a candidate master regulator.
Introduction
The mucosal surface of the mammalian gut is continuously exposed to a variety of foreign proteins and microorganisms, some of which are potentially harmful for the host. To protect from these dangers, the intestinal mucosa has evolved specialized organized lymphoid tissues. Gut-associated lymphoid tissue (GALT) including Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs) is the inductive site for intestinal immunity. Different from other peripheral lymphoid tissues, GALT lacks afferent lymphatics, and directly samples mucosal antigens across the epithelial barrier to initiate immune responses. This task is accomplished by specialized epithelial cells within the follicle-associated epithelium (FAE) covering the lymphoid follicles of GALT known as microfold (M) cells. M cells possess a high capacity for phagocytosis and transcytosis; these functions allow the rapid transport of antigens to underlying lymphoid tissues, especially antigen-presenting cells. Antigens are then presented to T cells that support B cell activation, resulting ultimately in the generation of IgA-producing plasma cells. Thus, M-cell–mediated antigen transport is an important step in the initiation of mucosal immune responses1–3.
M cells display characteristic morphological features that set them apart from the other intestinal epithelial cell subsets. They have shorter and irregular microvilli on their apical surface, and a pocket-like basolateral invagination of the plasma membrane which houses lymphocytes and antigen-presenting cells3. These morphological features enable recognition of M cells by transmission or scanning electron microscopy. Although the functional and morphological features of M cells were initially described nearly 40 years ago4,5, many basic questions concerning M-cell differentiation and function remain unsolved. Gene expression profiling of M cells has been used as one approach in recent years to learn more about M cells, and several M-cell-specific molecules were identified, including glycoprotein-2 (GP2), Marcksl1, M-Sec, Sgne-1, Annexin V and CCL9 (refs 6–11). Among them, GP2 mediates the uptake of FimH-expressing bacteria to initiate subsequent bacteria-specific immune responses6.
M cells are a small subset of intestinal epithelial cells derived from the intestinal epithelial stem cells12,13. Because of the restricted localization of M cells in the FAE, it has been postulated that the immune cells in GALT are involved in M-cell differentiation. Among them, the contribution of B cells in M-cell differentiation has been demonstrated in vivo and in vitro14–16. Besides the contribution of hematopoietic cells, mesenchymal cells in the subepithelial dome (SED) of PPs play an essential role in M-cell differentiation by producing receptor activator of nuclear factor-κB ligand (RANKL), which is one of tumor necrosis factor (TNF) superfamily cytokines17. Indeed, RANKL-deficient mice show a severe reduction of M cells, and exogenously administered recombinant RANKL restores the number of M cells in these mice. Furthermore, RANKL treatment can induce ectopic differentiation of the villous epithelium into M cells in wild-type mice18.
Multiple transcription factors are involved in the cell fate decisions that occur as intestinal epithelial stem cells in the crypt differentiate into one of the recognized types of terminally differentiated intestinal epithelial cells. Math1 (also known as Atoh1), which is repressed by Notch effector Hes1, is essential for the commitment of epithelial progenitor cells into the secretory lineage, including goblet, Paneth, enteroendocrine, and tuft cells19,20. Downstream of Math1, the specification of the individual secretory cell lineages requires at least one additional transcription factor; KLF4 is required for the maturation of goblet cells21, Sox9 for Paneth cells22,23, and neurogenin 3 for enteroendocrine cells24. By analogy, M-cell differentiation is hypothesized to require the regulation by one or more distinct transcription factors, although their identity has yet to be determined.
In this study, we took advantage of the exogenous administration of recombinant RANKL as a means of synchronously inducing M-cell differentiation throughout the small intestinal epithelium18 to identify genes induced during the process of M-cell differentiation. We found that the transcript of the Ets family transcription factor Spi-B is highly upregulated at an early stage of RANKL-induced M-cell differentiation, and is specifically expressed by naturally occurring M cells in the FAE of PPs. Previously, Spi-B has only been shown to be expressed in hematopoietic cells, such as B and T cells25 and plasmacytoid dendritic cells (pDCs)26; therefore, this study is the first evidence that Spi-B is also expressed in non-hematopoietic cells. We further demonstrate that Spi-B is a regulatory factor for the differentiation of intestinal M cells.
Results
Sequential induction of M-cell markers by RANKL
Systemic treatment of mice with RANKL was recently identified as a means of inducing ectopic M-cell differentiation in the villous epithelium (VE) of the small intestine18. Besides the RANKL-induced M cells, a few intrinsic M cells have also been identified in the VE, which are called villous M-like cells7,27. Unlike the authentic M cells in the PP FAE, villous M-like cells do not express M-cell markers GP2 and Marcksl1 (ref. 7).
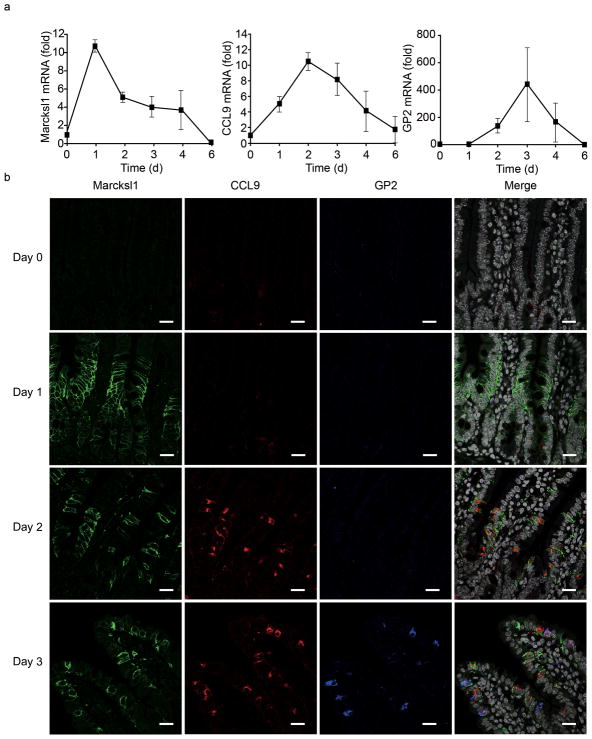
To characterize the RANKL-induced M cells in detail, we first examined the kinetics of expression of M-cell markers such as GP2, Marcksl1, CCL9, M-Sec, Sgne-1 and Annexin V on these cells. We have previously demonstrated that CCL9 is highly expressed in the FAE10, and here we confirmed the preferential expression of CCL9 in M cells by dual staining with GP2 (Supplementary Fig. 1). All of these transcripts were upregulated in the VE of distal ileum upon RANKL treatment (Fig. 1a and Supplementary Fig. 2), suggesting that RANKL-induced ectopic M cells in the villi more closely resemble FAE M cells than the rare naturally occurring villous M-like cells. Of note, the kinetics of expression of these M-cell markers after RANKL treatment were distinct; the expression of Marcksl1, CCL9 and GP2 peaked after 1, 2 and 3 days respectively, both at the mRNA (Fig. 1a) and the protein levels (Fig. 1b). Furthermore, the localization of these M-cell markers moved from the crypt zones towards the tips of the villi from days 1 to 3 after the RANKL treatment, with Marcksl1 limited to the crypt-villus junction at day 1 and GP2 expression restricted to the upper part of villi on day 3 (Fig. 1b). Considering that the position of cells along the intestinal crypt-villus axis reflects their degree of maturation, these observations indicate that Marcksl1 expression begins at an early stage of M-cell differentiation, whereas expression of CCL9 and GP2 requires further maturation of the RANKL-induced M cells.
Figure 1. Distinct expression patterns of M-cell markers in mouse villous epithelium after RANKL-treatment.
(a) The expression of M-cell markers in mouse intestinal epithelial cells after GST-RANKL treatment was assessed by qPCR. RANKL injections were given twice a day for up to 4 days followed by 2 days without further RANKL treatment. Intestinal epithelial cells were isolated from the ileum at 1, 2, 3, 4 and 6 days after the initial RANKL treatment for qPCR analysis. Data represent fold change compared to the normalized value of expression of each transcript in epithelial cells from untreated mice. All samples were normalized to the expression level of GAPDH (n = 3). (b) Small intestinal tissues from RANKL-treated mice were stained with antibodies for M-cell markers (Supplementary Table 1). All sections were counterstained with DAPI. Scale bar: 20 μm. Data are representative of three independent experiments (error bars, s.d.)
Since the induction of M-cell differentiation on small intestinal villi following exogenous RANKL administration is a non-physiological pathway for intestinal M-cell induction, we investigated whether naturally developing PP M cells acquire these markers in the same order as observed during RANKL-induced differentiation. To this end, we followed the ontogenic expression of these M-cell markers in the developing mouse PP FAE. It has been reported that the PP FAE structure is clearly distinguishable from embryonic day 18.5 (E18.5)28, with morphologically mature M cells being first identified between 5 and 10 days postpartum (P5–10)29. Therefore, the first appearance of M cells should be detectable in this period (E18.5 to P10). We found that Marcksl1 was already expressed on a subset of cells in the FAE by E18.5, whereas CCL9 was first identified on P2 and individual GP2+ cells were not detected until P7 (Supplementary Fig. 3), suggesting that GP2 is only expressed on mature M cells. On the basis of these findings, we propose that the process of M-cell differentiation can be practically divided into a series of distinct stages defined by the expression profile of M-cell markers, and that in vivo RANKL treatment is a powerful experimental tool for tracing the individual steps of M-cell differentiation.
Preferential expression of Spi-B by intestinal M cells
Identification of M-cell lineage-specific transcription factors expressed early in M-cell differentiation is a key to elucidating the molecular mechanisms of M-cell differentiation. Whole-genome expression profiling of mouse VE showed that transcripts encoding Spi-B, an Ets family transcription factor, were highly upregulated shortly after RANKL treatment (Fig. 2a). Real-time PCR analysis confirmed that Spi-B mRNA was highly expressed in PP FAE, but not in VE (Fig. 2b). In situ hybridization (ISH) analysis demonstrated that Spi-B mRNA was localized to a subset of cells in the PP FAE that also bound UEA-I (Fig. 2d). At the protein level, Spi-B was localized to the nuclei of GP2 positive M cells (Fig. 2e), thus establishing the specific expression of Spi-B by M cells within the PP FAE.
Figure 2. Preferential expression of Spi-B transcript in mouse M cells.
(a) The kinetics of Spi-B expression after RANKL-treatment was assessed by qPCR. Data represent fold change compared to the normalized value of expression of each transcript in epithelial cells from untreated mice. (b) Spi-B mRNA expression was measured by qPCR in villous epithelium (VE) and follicle-associated epithelium (FAE). Data represent fold change compared to the normalized value of expression of each transcript in villous epithelial cells. All samples were normalized to the expression level of GAPDH (n = 3). (c) In situ hybridization (ISH) analysis of Spi-B mRNA in the small intestine from RANKL-treated and untreated mice. Scale bar: 50 μm. (d) The top panel shows ISH image of Spi-B mRNA in a PP follicle. Scale bar: 100 μm. Lower three panels show the high magnification images of box in the top panel, after restaining of the section with UEA-I. Dotted lines represent FAE. Arrows indicate the double positive cells with Spi-B and UEA-I. Asterisks indicate goblet cells on adjacent villous epithelium, which are also positive for UEA-I. Scale bar: 20 μm. (e) Top panel shows immunostaining of Spi-B (red) in small intestine from mouse after 18 hours after RANKL-treatment. Lower three panels show dual staining of Spi-B (red) and GP2 (green). Arrows indicate Spi-B+GP2+ cells. All sections were counterstained with DAPI. Scale bar: 20 μm. (f) ISH of Spi-B mRNA in a PP from 18.5 day old wild-type mouse embryo is shown (top panel). Spi-B mRNA was also expressed in other GALT, such as ILFs, colonic patches and cecal patches (lower three panels). Scale bar: 50 μm. Data are representative of three independent experiments (error bars, s.d.).
ISH also demonstrated the distribution of Spi-B mRNA after RANKL treatment. Spi-B mRNA was already observed in the crypt as early as 6 h after treatment. At 1 day Spi-B+ cells were concentrated in the transit amplifying cell compartment in the mid-crypt and migrated further up the crypt-villus axis at later time points (Fig. 2c). Spi-B protein was detected in the nuclei of crypt cells at 18 h after treatment (Fig. 2e). In addition, Spi-B mRNA was observed in a subset of cells in the PP FAE of E18.5 mouse embryos (Fig. 2f). The parallel upregulation of Spi-B transcript during both natural PP M-cell development in ontogeny and following RANKL-induced M-cell differentiation in the VE suggests a pivotal role of Spi-B in the induction of intestinal M-cell differentiation. We also examined Spi-B expression in various GALT besides PPs, such as ILFs, colonic patches and cecal patches, and showed that M cells in these tissues also expressed Spi-B mRNA (Fig. 2f). To assess the possibility that human M cells also express Spi-B, we examined the expression of Spi-B in human PPs by ISH, and found that the human M cells in PPs also preferentially expressed Spi-B mRNA (Supplementary Fig. 4).
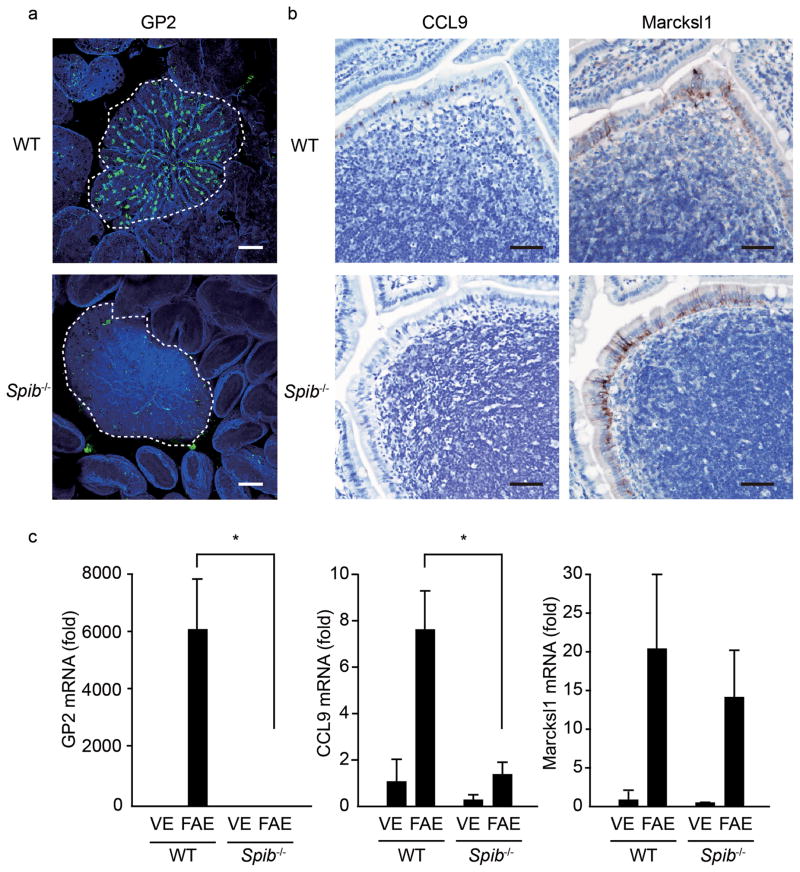
To determine if Spi-B is required for normal M-cell differentiation, we examined Spib−/− mice. Whole mount staining and immunohistochemistry of PPs demonstrated that GP2+ or CCL9+ M cells were completely absent in the PP FAE of Spib−/− mice (Figs. 3a,b). Real-time PCR analysis also revealed a virtually complete loss of GP2, CCL9, M-Sec and Sgne-1 transcripts in these mice (Fig. 3c and Supplementary Fig. 5). The loss in the expression of these M-cell markers in Spib−/− mice was also observed in RANKL-induced M-cell differentiation (Fig. 4 and Supplementary Fig. 6). On the other hand, Marcksl1 and Annexin V mRNA expression both in natural PP M cells and RANKL-induced M cells from Spib−/− mice was maintained at an equivalent level to that from wild-type mice (Fig. 3c, 4b and Supplementary Fig. 5, 6). Based on these observations, the expression of GP2, CCL9, M-Sec and Sgne-1 in M cells is dependent on Spi-B, whereas Marcksl1 and Annexin V expression is independent of Spi-B. Of note, there were cells expressing Marcksl1 (Fig. 3b) and Annexin V (data not shown) near the apex of PP FAE from Spib−/− mice, indicating that Spi-B is required for the expression of late markers of M-cell differentiation but dispensable for the survival of the Marcksl1+Annexin V+ cells, allowing them to migrate towards the top of FAE before cell death.
Figure 3. The expression of M cell-markers in Spib−/− mice.
(a) Whole mount GP2 staining of PPs from Spib−/− and wild-type mice was performed. GP2 was visualized with Alexa 488 anti-rat IgG (green). F-actin was stained with Alexa 647 Phalloidin (blue). Dotted lines depict the position of FAE. Scale bar: 80 μm. (b) Expression of CCL9 and Marcksl1 in mouse FAE was analyzed by immunohistochemistry. Scale bar: 50 μm. (c) Comparison of M-cell marker expression in FAE and VE between wild-type and Spib−/− mice by qPCR analysis. Data represent fold change compared to the normalized value of expression of each transcript in villous epithelial cells from wild-type mice. All samples were normalized to the expression level of GAPDH. Data are representative of three independent experiments (error bars, s.d.). *, P<0.01.
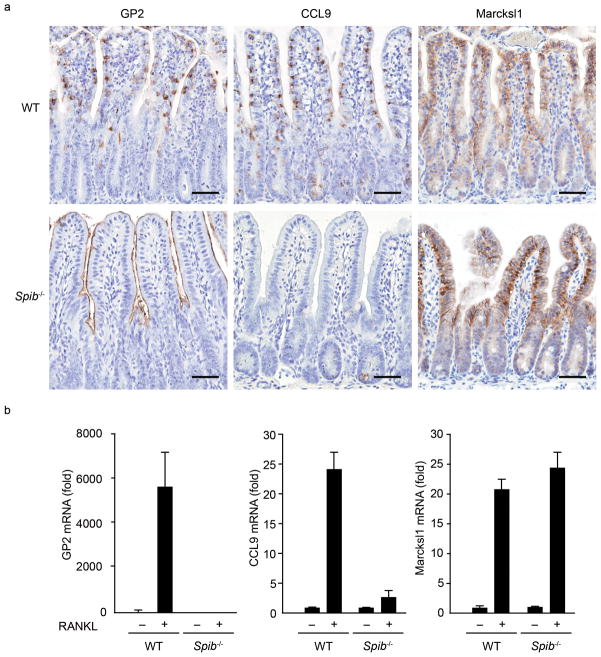
Figure 4. RANKL-induced M-cell differentiation in Spib−/− mice.
(a) Small intestines were dissected from wild-type and Spib−/− mice after 3 days of RANKL-treatment, and subjected to immunohistochemistry. The upper three panels show the immunostaining of M-cell markers GP2, CCL9 and Marcksl1 in wild-type mice, and the lower three panels show those in Spib−/− mice. Scale bar: 50 μm. (b) Quantitative analysis of M-cell marker expression by qPCR. Data represent fold change compared to the normalized value of expression of each transcript in villous epithelial cells from untreated wild-type mice. All samples were normalized to the expression level of GAPDH. Data are representative of two independent experiments (error bars, s.d.).
Spi-B is critical for M-cell differentiation
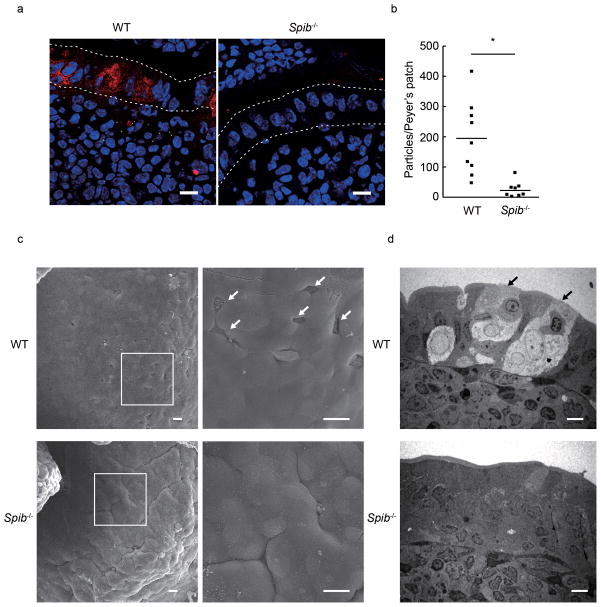
In the PP FAE of Spib−/− mice, cells expressing only Marcksl1 and Annexin V were present but lacked other M-cell markers that are normally acquired at later stages of differentiation. Since GP2 is a functional antigen-uptake receptor on M cells6 and is acquired at a late stage of M-cell differentiation, this molecule is considered a marker for the terminally differentiated, fully functional M cells. We therefore hypothesized that the residual Marcksl1+Annexin V+ cells in the PP FAE of Spib−/− mice are not functionally mature M cells. To explore the possibility, we examined transcytotic activity, a critical function provided by M cells enabling the rapid delivery of particulate luminal antigens across the epithelial barrier. We employed oral administration of fluorescent nanoparticles, an established assay for M-cell transcytosis30, in Spib−/− and wild-type mice. In the wild-type mice, particles were abundantly observed in the SED area of PPs, whereas very few particles were taken up into the PPs of Spib−/− mice (Figs. 5a,b).
Figure 5. Disappearance of functional M cells in Spib−/− mice.
(a) Green fluorescent 200 nm diameter nanoparticles were orally administered to the mice. Three hours later, the distal three PPs were isolated, embedded into OCT-compound, and the sections were stained with DAPI (blue). GP2 was visualized with Dylight549-labeled secondary Ab (red). Dotted lines depict the position of FAE. Scale bar: 10 μm. (b) The number of nanoparticles was counted in 20 serial sections per PP, and were represented in lower graph. Statistical analysis was performed by Student’s t-test (n = 9, P = 0.0269). (c) SEM images of PPs from wild-type mice (upper panels) and Spib−/− mice (lower panels) are shown. Left panels show low magnification images, with the boxed areas shown at higher magnification in right panels. Scale bar = 20 μm. (d) TEM images of PPs from wild-type mice (upper) and Spib−/− mice (lower) are shown. Scale bar: 5 μm. Arrows in (c) and (d) indicate typical M cells, with sparse and irregular microvilli and basolateral pocket-like structure. Data are representative of two independent experiments (error bars, s.d.)
We further investigated the loss of M-cell function in PPs of Spib−/− mice by examining translocation of orally administered bacteria. The uptake of Salmonella enterica serovar Typhimurium (S. Typhimurium) in PPs was prominently decreased in Spib−/− mice. This result is consistent with the absence of GP2+ M cells in Spib−/− mice because the uptake of S. Typhimurium is significantly impaired in GP2-deficient mice6. In addition, PPs from Spib−/− mice exhibited significant reduction in the bacterial load of Yersinia enterocolitica (Y. enterocolitica) when they were orally administered (Supplementary Fig. 7), suggesting that GP2-independent bacterial uptake was also impaired in PPs of Spib−/− mice. Thus, the residual Marcksl1+Annexin V+ cells in Spib−/− mice are defective in their ability to take up luminal antigens.
Consistent with the absence of transcytotic capability by the residual Marcksl1+Annexin V+ cells in Spib−/− mice, the ultrastructure of the FAE showed the absence of cells with typical M-cell morphology. PP M cells exhibit unique morphological characteristics including irregular and sparse microvilli, and a pocket-like invagination of the basolateral plasma membrane4,5. Scanning as well as transmission electron microscopy revealed that the FAE of Spib−/− mice completely lacked cells with these morphological features (Fig. 5c,d). In contrast, no obvious defect was observed in the development of goblet, Paneth or enteroendocrine cells in Spib−/− mice, indicating that Spi-B is not involved in the differentiation of these secretory intestinal epithelial cell lineages (Supplementary Fig. 8). Based on these observations, Spi-B has a crucial role in the differentiation of morphologically and functionally mature M cells, and its role in cellular differentiation is specific to M cells among the known types of intestinal epithelial cells.
Intrinsic Spi-B is required for M-cell differentiation
Within the hematopoietic system, Spi-B is known to be involved in B cell function and development31. In addition, it has been reported that B cells contribute to M-cell differentiation14–16. On the basis of these studies, the possibility cannot be excluded that absence of Spi-B in these immune cells, but not in M cells per se, is responsible for the observed defects in M-cell differentiation. To address this issue, we prepared reciprocal bone marrow (BM) chimeras using Spib−/− and wild-type mice and characterized the differentiation of M cells within the PP FAE. Transfer of wild-type BM cells into irradiated Spib−/− mice failed to restore the expression of M-cell markers and the establishment of normal M-cell morphology in the FAE, whereas the transfer of Spib−/− BM cells into wild-type mice did not interfere with normal M-cell differentiation in the PP of wild-type recipient mice (Fig. 6). Taken together, these data indicate that M-cell–intrinsic expression of Spi-B is critical for the expression of M-cell–specific genes, CCL9, GP2, M-Sec and Sgne-1, as well as the formation of M-cells with typical morphological features.
Figure 6. The maturation of M cells in Spib−/− bone marrow chimeric mice.
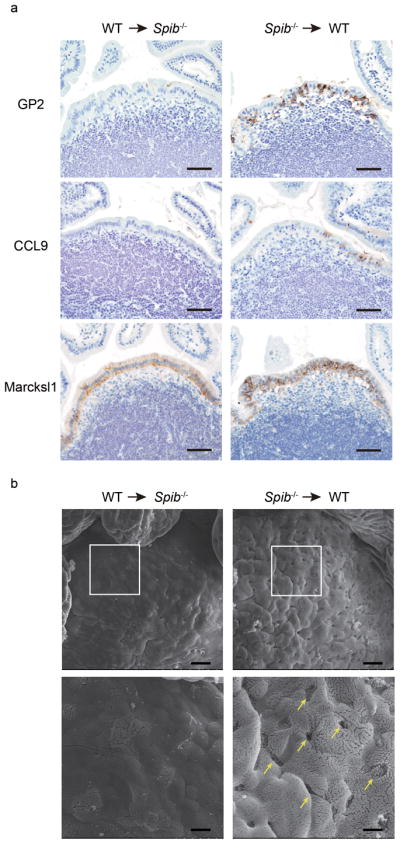
(a) Bone marrow (BM) cells from wild-type (CD45.1) mice were transferred into irradiated Spib−/− (CD45.2) mice (WT → Spib−/−, left column), or vice versa (Spib−/−→ WT, right column). Eight weeks after the transfer, PPs were immunostained with M-cell markers: GP2, CCL9, and Marcksl1, as described in the Experimental Procedures. Scale bar: 50 μm. (b) SEM images of PPs from BM chimeric Spib−/− mice (WT → Spib−/−, left column) and BM chimeric wild-type mice (Spib−/−→ WT, right column) are shown. Lower panels show enlarged images of squares in upper panels. Scale bar = 20 μm (upper), 6 μm (lower). Data are representative of two independent experiments.
Impaired mucosal immune response in Spi-B-deficient mice
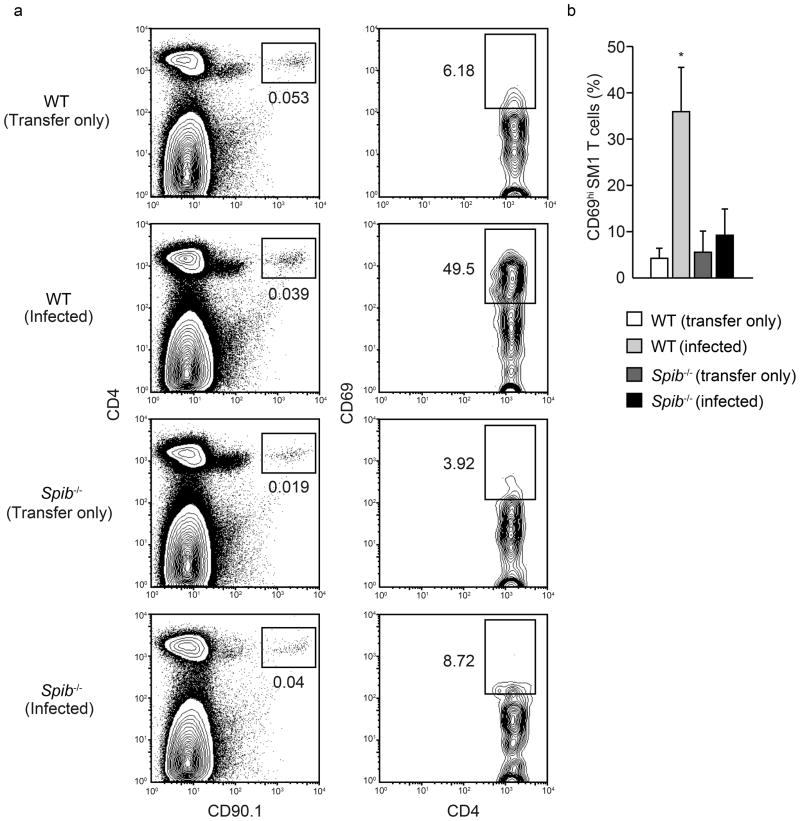
The defect of functionally mature M cells in Spib−/− mice raises the possibility that Spi-B is essential for the induction of adaptive immune responses initiated by M-cell antigen delivery within the mucosal immune system. Since the expression of Spi-B in immune cells25,26 could affect the immune responses in Spi-B–deficient mice, we examined antigen-specific T cell activation in PPs after oral infection with S. Typhimurium in the adoptive transfer model using S. Typhimurium-specific SM1 TCR-transgenic T cells32,33. This is a well-established model to analyze antigen-specific immune response in PPs and we have previously shown the biological significance of M cells for efficient mucosal immune responses after oral challenge with S. Typhimurium6. SM1 T cell-transferred Spib−/− or wild-type mice were orally infected with S. Typhimurium, and PPs were harvested for the analysis of S. Typhimurium-specific T-cell activation. SM1 T cells were detected in the PPs of uninfected mice of both genotypes, indicating that the migration of transferred SM1 T cells into PPs occurred normally in the absence of Spi-B. SM1 T cells in the PPs of uninfected wild-type mice displayed the naïve phenotype with a low surface expression of the CD69 activation marker, which increased markedly 24 h after oral infection with S. Typhimurium. On the other hand, the induction of CD69 on SM1 T cells after infection was significantly impaired in Spib−/− mice (Fig. 7).
Figure 7. Defect in Salmonella-specific T-cell activation in Spib−/− mice.
(a) Wild-type or Spib−/− mice were adoptively transferred with SM1 T cells, and orally inoculated with S. Typhimurium at 24 hours after transfer. Left panels depict dot plots of CD90.1 and CD4 expression on PP cells from uninfected (Transfer only) and infected (Infected) mice at 24 hours after infection. Rectangles in the left panels indicate the gate for CD90.1+ SM1 cells. Figures under the rectangles are the percentage of the gated cells in the total cells. Right panels depict contour plots of CD4 and CD69 expression on the cells in the gate of the left panels. Rectangles in the right panels indicate the CD69+ activated cells among the gated SM1 cells. Figures are the percentage of the gated cells among all SM1 cells. (b) Plot shows CD69 activation data from four independent experiments. Error bar indicates s.d. *, P<0.01.
The defect in T cell activation upon oral antigen challenge is not likely due to the impaired antigen presentation because of absence of Spi-B in hematopoietic cells, since we observed an equivalent systemic T cell activation against S. Typhimurium administered intraperitoneally in SM1 T cell-transferred Spib−/− or wild-type mice (Supplementary Fig. 9). To further exclude effects attributable to the absence of Spi-B in hematopoietic cells, we prepared BM chimeric mice by transferring wild-type bone marrow into wild-type and Spib−/− recipients. After reconstitution, BM chimeric wild-type or Spib−/− mice received CFSE-labeled SM1 T cells and were subsequently orally infected with S. Typhimurium. Three days after infection, we analyzed the proliferation of SM1 T cells in BM chimeric mice. At this time point most SM1 T cells in wild-type marrow into wild-type chimeric recipients had undergone one or more divisions. In contrast, SM1 T cells in wild-type marrow into Spib−/− chimeric recipients showed a muted proliferative response (Supplementary Fig. 10). Taken together, these results confirmed that the M-cell–intrinsic expression of Spi-B is critical for differentiation of M cells required for the host to initiate an efficient antigen-specific mucosal immune response.
Discussion
We here report that Spi-B is a RANKL-induced transcription factor essential for the differentiation of intestinal M cells. Identification of Spi-B as a candidate “master regulator” of M-cell differentiation resolves a long-standing question about the genesis of M cells and reveals a novel and completely unanticipated function for Spi-B. Furthermore, the lack of M cells in Spi-B-deficient mice also provides a unique tool for elucidating physiological and pathological functions of this enigmatic type of epithelial cell.
Spi-B is a member of the Ets family transcription factors34. Spi-B has been reported to play a role in B-cell receptor signaling, antibody responses and germinal center formation35, as well as B-cell development31,36. In addition, Spi-B is required for development of human plasmacytoid dendritic cells (pDCs)37. In this study, we found that Spi-B was highly expressed in both RANKL-induced and PP FAE M cells. This preferential expression of Spi-B in intestinal M cells is the first demonstration that Spi-B is expressed in non-hematopoietic cells. Furthermore, we demonstrated that Spib−/− mice completely lacked M cells in PPs, providing evidence that M-cell differentiation is regulated by a specific transcription factor as previously shown for most other intestinal epithelial cell lineages. In addition, Spi-B expression in M cells was conserved in all mouse GALT tissues, as well as M cells in human PPs, suggesting the essential role of Spi-B in M-cell differentiation.
Multiple Ets transcription factors besides Spi-B are expressed in epithelial cells of the small intestine. However, identifying the function of individual Ets transcription factors in these cells has been challenging, in part due to the potential for compensatory activity by other co-expressed Ets factors in the absence of a single Ets transcription factor38. Although nearly two-thirds of all Ets transcription factors have been genetically inactivated in mice, genetic absence of just two of these factors has been reported to confer an intestinal phenotype. Elf3−/− mice exhibit abnormal morphogenesis of villi and terminal differentiation of enterocytes and goblet cells39. In contrast, homozygous deletion of Spdef impairs the full maturation of both goblet cells and Paneth cells40. The indispensable role of Spi-B in M-cell differentiation indicates that any other Ets transcription factors expressed in M cells are unable to substitute for Spi-B in orchestrating M-cell differentiation.
The relationship between the expression pattern of M-cell markers and the lack of M-cells in Spib−/− mice has greatly improved our understanding of M-cell differentiation. The FAE in Spib−/− mice lacks several M-cell-associated markers including GP2, CCL9, M-Sec and Sgne-1, indicating that these genes are all located downstream of Spi-B expression in M-cell differentiation. GP2 is thought to be a marker of terminally differentiated, functional M cells, because of its role in the uptake of pathogenic and commensal bacteria6. In contrast to the dependence on Spi-B for expression of most M-cell-associated markers and full functional maturation of M cells, we found that Marcksl1 and Annexin V expression were not diminished in Spib−/− mice in either cells from the PP FAE or the VE after RANKL treatment. These Marcksl1+Annexin V+ cells in Spib−/− mice lack not only GP2 expression but also the ability to take up macromolecules, as well as typical M-cell morphologies such as irregular microvilli and a pocket-like basolateral invagination. These observations suggest that Spi-B is essential for the morphological and functional maturation of M cells, and the genes expressed under the transcriptional control of Spi-B are involved in the maturation and/or function of M cells. CCL9 is a chemokine involved in the recruitment of CD11b+ dendritic cells into the SED region in PPs41. Its expression preceded GP2 expression in both RANKL-induced and FAE M-cell differentiation. In addition, we found that Spi-B directly bound the CCL9 promoter region in vitro (data not shown), raising the possibility of a substantial role of CCL9 in M-cell maturation. CD11b+ dendritic cells attracted to the SED by CCL9 may provide signals that contribute to the terminal differentiation of M cells. M-Sec is also dependent on Spi-B expression in RANKL-induced M-cell differentiation. M-Sec has been shown to be involved in tunneling nanotube (TNT) formation11, indicating that the appearance of the TNT structures observed in M cells is also a feature of fully mature M cells.
Marcksl1 was one of the earliest M-cell markers to be expressed in response to RANKL treatment and was predominantly expressed at the crypt-villus junction, but not by fully differentiated villous enterocytes, shortly after treatment. This suggests that the cells in the crypt, most likely undifferentiated transit amplifying cells, can respond to the activation of RANKL signaling through RANK with induction of Marcksl1. Interestingly, the frequency of Marcksl1+Annexin V+ cells within the epithelium decreases at later time points after the onset of RANKL treatment, and the remaining Marcksl1+Annexin V+ cells start co-expressing subsequent M-cell markers, such as CCL9 and GP2. Considering that Spi-B is essential for the expression of CCL9, GP2, M-Sec and Sgne-1 in M cells, we hypothesize that only the cells that achieve successful induction of Spi-B by RANKL fully commit to M-cell differentiation, allowing them to maintain expression of Marcksl1 and Annexin V, progress through M-cell differentiation and express later stage markers characteristic of mature M cells. In contrast, those cells that fail to express Spi-B do not sustain Marcksl1 and Annexin V expression and instead eventually commit to differentiate into other types of mature enterocytes. Lateral inhibition by committed M cells may be one mechanism contributing to the suppression of Marcksl1 and Annexin V expression by the surrounding cells, as observed in secretory lineage cells42. This agrees with the observation that in Spib−/− mice residual Marcksl1+Annexin V+ cells reach the apex of the FAE, as well as the villus tips in the case of RANKL treatment. The Spi-B-independent expression of Marcksl1 and Annexin V in the initial phase of RANKL-induced M-cell differentiation probably indicates that these proteins may be markers for RANKL-responsive intestinal epithelial cells.
In summary, our study demonstrates that Spi-B is an essential transcription factor that regulates M-cell differentiation. The investigation of M-cell differentiation in Spib−/− mice revealed a tight linkage between acquisition of Spi-B-dependent M-cell marker expression and induction of fully functional M cells. In addition, a significant reduction was observed in antigen-specific T-cell activation in Spib−/− mice, confirming the importance of M cells for induction of adaptive immune responses in the intestinal GALT. Spi-B-deficient mice serve as a unique tool for elucidating the physiological and pathological functions of M cells.
Methods
Animals
BALB/c, C57BL/6J (CLEA), Spib−/− (C57BL/6J background; I.S., Ts.K. et al. submitted for publication), CD45.1 congenic (C57BL/6 background), and S. Typhimurium-specific SM1 TCR-transgenic Rag2−/− C57BL/6 expressing CD90.1 (SM1-tg)32,33 mice were used for experiments. All mice were maintained under specific pathogen-free conditions at the RCAI Animal Facility. All animal experiments were approved by the Animal Research Committees of RIKEN Yokohama Research Institute.
RANKL injections
The GST-RANKL fusion protein was prepared as described previously18. The GST-RANKL fusion protein was intraperitoneally administered to mice twice a day for up to 4 days.
Isolation of epithelium
For the preparation of villous epithelium, small pieces of the ileum at 35 cm from pylorus were dissected from mice. For FAE, the epithelial layer was peeled off from lamina propria of the small intestine of mice containing PPs, and the FAE regions were isolated from surrounding villous epithelium. Detailed procedures for isolation of the epithelium were described elsewhere8.
Expression analyses
Total RNA samples of isolated intestinal epithelium were extracted by Trizol reagent (Invitrogen), and reverse transcribed with ReverTra Ace (TOYOBO). The expression of M-cell-marker genes was examined by real-time quantitative PCR with SYBRR Premix Ex TaqTM (TAKARA) and specific primers are summarized in Supplementary Table 2. Target gene expression was normalized to that of Gapdh encoding glyceraldehyde 3-phosphate dehydrogenase.
Microarray analysis
Extracted total RNA samples from isolated intestinal epithelium were used for microarray analysis using GeneChip Mouse Gene 1.0 ST array (Affymetrix). All procedures were performed according to the manufacturer’s instructions. Genes upregulated upon RANKL-treatment were analyzed by GeneSpring software (Agilent Technologies).
Histology
For immunohistochemistry, small intestinal samples including PPs were fixed with Zinc Formalin Fixative (Polysciences) and embedded in paraffin. Deparaffnized sections were incubated with primary antibodies (Abs) overnight at 4 °C. Specific binding of primary Abs were followed with biotinylated secondary Abs and streptavidin-HRP (ABC Elite; Vector Laboratories), and visualized with 3,3-diamino-benzidine (DakoCytomation). For immunofluorescent staining, we used fluorescent-labeled secondary Abs. The primary and secondary Abs used are summarized in Supplementary Table 1. The immunostained sections were examined by DM-IRE2 (Leica) confocal laser microscope. Obtained images were edited by Photoshop CS software (Adobe). For observing the distribution of goblet cells, small intestinal sections were stained with Alcian blue (Wako Pure Chemical Industries).
In situ hybridization (ISH)
For ISH, tissues were fixed with 4% paraformaldehyde (PFA) for 24 h and embedded into paraffin. cDNA fragment for RNA probe of mouse Spi-B, M-Sec, Sgne-1 and Annexin V, and human Spi-B and GP2 were amplified by PCR, and subcloned into pcDNA3 plasmid vector (the primer sequences were summarized in Supplementary Table 3). Digoxigenin-labeled antisense RNA probes were prepared by in vitro transcription with SP6 RNA polymerase (Roche Applied Science), using pcDNA3 including subcloned sequences as templates. All procedures of ISH were performed with a VENTANA DISCOVERY (Roche Diagnostics). For dual staining with UEA-I lectin, ISH sections were incubated with rhodamine-labeled UEA-I (Vector Laboratories).
Human biopsy samples
Biopsy samples of human PPs were collected from healthy small intestinal tissues, and treated for ISH. These studies were approved by the Committee on Human Subjects in RIKEN and Chiba University.
Whole mount staining
For whole mount staining, mouse PPs were fixed with a Cytofix/Cytoperm kit (BD Biosciences). The specimens of PPs were stained with anti-GP2 mAb and Alexa 488-labeled secondary Ab. For counterstaining, F-actin was stained with Alexa 647-conjugated phalloidin (Invitrogen). Stained specimens were examined by confocal laser microscope. The projection images of whole mount staining were edited with ImageJ v1.36b software (http://rsb.info.nih.gov/ij/).
Electron microscopy
PPs were fixed with 4% PFA and 2% glutaraldehyde buffered with 100 mM sodium cacodylate (pH 7.4), and post-fixed with 1% osmium tetroxide in 100 mM cacodylate buffer (pH 7.4). For scanning electron microscopy, samples were dehydrated and substituted with t-butyl alcohol. After freeze drying, samples were coated with platinum and examined by scanning microscope (JSM-6360LV; Jeol). For transmission electron microscopy, the dehydrated samples were embedded in Epon812 resin (Taab). Ultrathin sections were stained with 4% uranyl acetate and then with lead citrate, and examined with a transmission electron microscope (JEM-1011; Jeol, SU1510; Hitachi).
In vivo assessment of M-cell uptake of fluorescent beads
Mice were gavaged with 200-nm diameter green fluorescent polystyrene latex nanoparticles (Fluoresbrite YG; Polysciences) (2 × 1011 particles in 200 μl PBS). After 3 h PPs were isolated from mice, and embedded in OCT compound. Frozen sections were stained with anti-GP2 mAb as previously described6. Quantification of incorporated fluorescent beads was performed as described in a previous report18.
Generation of bone marrow (BM) chimeric mice
BM cells were isolated from femora of wild-type (CD45.1) and Spib−/− (CD45.2) mice and injected intravenously (107 cells per mouse) into Spib−/− and wild-type mice, respectively. Before injection, recipient mice were irradiated with 8 Gy of gamma rays. Eight to ten weeks later, recipient mice were sacrificed. For the evaluation of chimerism, PPs were immunostained with anti-CD45.1 or 45.2 Ab (BioLegend).
Adoptive transfer of SM1 T Cells
Cells from the spleen and mesenteric lymph node were harvested from SM1 transgenic mice. The cell suspensions (3–4 × 106 SM1 T cells/200 μl) were injected intravenously into recipient mice. For analyzing SM1 T cell proliferation in vivo, SM1 T cells were stained with CFSE dye (Invitrogen) before the transfer.
Bacterial infection
Salmonella enterica serovar Typhimurium (S. Typhimurium) carrying a nalidixic acid resistance gene (χ3306) was a gift from H. Matsui43. SM1 T cell-transferred Spib−/− and wild-type mice were orally inoculated with S. Typhimurium χ3306 (5 × 109 c.f.u) 24 h after adoptive transfer. Twenty-four hours later, PPs were harvested for the analysis. In some experiments, additional groups of mice were infected i.p. with S. Typhimurium χ3306 (105 c.f.u) and the spleens harvested 24 h later.
CFU assay after oral infection
Spib−/− and wild-type mice were fed with 5 × 107 c.f.u. of S. Typhimurium χ3306 or 108 c.f.u. of Y. enterocolitica WA (ATCC 27729)44. After 24 h, PPs were dissected and incubated at 20°C in sterile PBS containing 500 μg/ml gentamicin for 30 min with gentle shaking. The pooled PP tissue was weighed and homogenized in sterile PBS. The homogenates were serially diluted in sterile PBS and plated on a Luria–Bertani agar plate containing 25 μg/ml nalidixic acid (for S. Typhimurium) or a Yersinia-selective agar plate (Oxoid) to determine colony-forming units.
Preparation of PP lymphocytes
PP lymphocytes were prepared as described previously8.
Flow cytometry
PP lymphocytes were preincubated with anti-CD16/CD32 mAb (eBioscience) in 2% FBS-PBS for FcγR blocking, and stained with monoclonal Abs for CD antigens (Supplementary Table 1). After staining, cells were analyzed with a FACS Canto II (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
Statistical analysis
Data are shown as averages and standard deviations. We used Student’s t test, Mann-Whitney U-test, and one-way ANOVA followed with Bonferroni post hoc test. P values < 0.05 were considered as statistically significant.
Supplementary Material
Acknowledgments
We would like to thank Z. Guo, Y Obata, Y. Oohara, Y. Fujimura, M. Ohmae, C. Uetake, Y. Usami, and S. Kimura for technical support; T. Kawai (RIKEN Plant Science Center) for electron-microscopic analysis; H. Matsui (Kitasato University) for providing S. Typhimurium. T. Kanaya is a RIKEN Special Postdoctoral Researcher. This study was supported in part by Grants-in-Aid for Young Scientists (A) (22689017 to K.H.) and (B) (23790550 to T. Kanaya), Scientific Research (B) (21390155 to H.O.; 20390146 and 23390124 to Ts.K.) and (C) (18590483 to Ts.K.), Scientific Research in Priority Areas (21022049 to K.H.; 20060033 and 21022048 to Ts.K.), and Scientific Research on Innovative Areas (20113003 to H.O.; 21117003 to Ts.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Precursory Research for Embryonic Science and Technology (PRESTO) (to K.H.) from Japan Science and Technology Agency, Incentive Research Grant Proposals from RIKEN (to T. Kanaya), and also by the Sasakawa Scientific Research Grant from The Japan Science Society (to K.H.), Takeda Science Foundation (to H.O.), The Mitsubishi Foundation (to H.O.), The Uehara Memorial Foundation (to Ts.K.), the National Institutes of Health (DK64730 to I.R.W.), and the Bill & Melinda Gates Foundation (OPP1006977 to I.R.W.). T.D. and I.S. are supported by RIKEN Junior Research Associate grant.
Footnotes
Author Contributions I.R.W., K.H. and H.O. conceived the study; T. Kanaya designed and performed the experiments, analyzed data and wrote the manuscript; K.H. contributed adoptive transfer experiments and data analyses; D.T. and Y.K. helped perform flow cytometric analyses; S.F. and T.J. helped with S. Typhimurium infection experiments; K.N. and A.S. performed expression analyses; K.H., I.S., H.H. and Ts.K. generated Spib−/− mice; K.A.K., N.K. and I.R.W. developed the RANKL treatment protocol; M.S. and K.T. helped with electron microscopy; O.Y. and T. Katsuno provided human PPs samples; S.J.M. provided SM1 transgenic mice; K.H. and I.R.W. edited the manuscript; H.O. directed the research and edited the manuscript.
Accession code. GEO: microarray data, GSE37861.
References
- 1.Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 2.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 3.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 4.Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am J Anat. 1973;136:455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 5.Owen RL, Jones AL. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 6.Hase K, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 7.Terahara K, et al. Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J Immunol. 2008;180:7840–7846. doi: 10.4049/jimmunol.180.12.7840. [DOI] [PubMed] [Google Scholar]
- 8.Hase K, et al. Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and M cells. DNA Res. 2005;12:127–137. doi: 10.1093/dnares/12.2.127. [DOI] [PubMed] [Google Scholar]
- 9.Verbrugghe P, et al. Murine M cells express annexin V specifically. J Pathol. 2006;209:240–249. doi: 10.1002/path.1970. [DOI] [PubMed] [Google Scholar]
- 10.Hase K, et al. The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. J Immunol. 2006;176:43–51. doi: 10.4049/jimmunol.176.1.43. [DOI] [PubMed] [Google Scholar]
- 11.Hase K, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 12.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Gebert A, Fassbender S, Werner K, Weissferdt A. The development of M cells in Peyer’s patches is restricted to specialized dome-associated crypts. Am J Pathol. 1999;154:1573–1582. doi: 10.1016/S0002-9440(10)65410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebisawa M, et al. CCR6hiCD11c(int) B cells promote M-cell differentiation in Peyer’s patch. Int Immunol. 2011;23:261–269. doi: 10.1093/intimm/dxq478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 16.Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RT, et al. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol. 2007;178:5659–5667. doi: 10.4049/jimmunol.178.9.5659. [DOI] [PubMed] [Google Scholar]
- 18.Knoop KA, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen J, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 20.Gerbe F, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz JP, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastide P, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori-Akiyama Y, et al. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Jenny M, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su GH, et al. The Ets protein Spi-B is expressed exclusively in B cells and T cells during development. J Exp Med. 1996;184:203–214. doi: 10.1084/jem.184.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schotte R, et al. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood. 2003;101:1015–1023. doi: 10.1182/blood-2002-02-0438. [DOI] [PubMed] [Google Scholar]
- 27.Jang MH, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rumbo M, Sierro F, Debard N, Kraehenbuhl JP, Finke D. Lymphotoxin beta receptor signaling induces the chemokine CCL20 in intestinal epithelium. Gastroenterology. 2004;127:213–223. doi: 10.1053/j.gastro.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005;206:177–189. doi: 10.1111/j.0105-2896.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 30.Pappo J, Ermak TH. Uptake and translocation of fluorescent latex particles by rabbit Peyer’s patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989;76:144–148. [PMC free article] [PubMed] [Google Scholar]
- 31.Garrett-Sinha LA, et al. PU.1 and Spi-B are required for normal B cell receptor-mediated signal transduction. Immunity. 1999;10:399–408. doi: 10.1016/s1074-7613(00)80040-0. [DOI] [PubMed] [Google Scholar]
- 32.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 33.Salazar-Gonzalez RM, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray D, et al. Characterization of Spi-B, a transcription factor related to the putative oncoprotein Spi-1/PU.1. Mol Cell Biol. 1992;12:4297–4304. doi: 10.1128/mcb.12.10.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su GH, et al. Defective B cell receptor-mediated responses in mice lacking the Ets protein, Spi-B. EMBO J. 1997;16:7118–7129. doi: 10.1093/emboj/16.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeKoter RP, et al. Regulation of follicular B cell differentiation by the related E26 transformation-specific transcription factors PU.1, Spi-B, and Spi-C. J Immunol. 2010;185:7374–7384. doi: 10.4049/jimmunol.1001413. [DOI] [PubMed] [Google Scholar]
- 37.Schotte R, Nagasawa M, Weijer K, Spits H, Blom B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J Exp Med. 2004;200:1503–1509. doi: 10.1084/jem.20041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jedlicka P, Gutierrez-Hartmann A. Ets transcription factors in intestinal morphogenesis, homeostasis and disease. Histol Histopathol. 2008;23:1417–1424. doi: 10.14670/hh-23.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng AYN, et al. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology. 2002;122:1455–1466. doi: 10.1053/gast.2002.32990. [DOI] [PubMed] [Google Scholar]
- 40.Gregorieff A, et al. The Ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- 42.Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G381–7. doi: 10.1152/ajpgi.00160.2005. [DOI] [PubMed] [Google Scholar]
- 43.Gulig PA, Doyle TJ, Hughes JA, Matsui H. Analysis of host cells associated with the Spv-mediated increased intracellular growth rate of Salmonella typhimurium in mice. Infect Immun. 1998;66:2471–2485. doi: 10.1128/iai.66.6.2471-2485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter PB, Collins FM. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974;9:851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.