Abstract
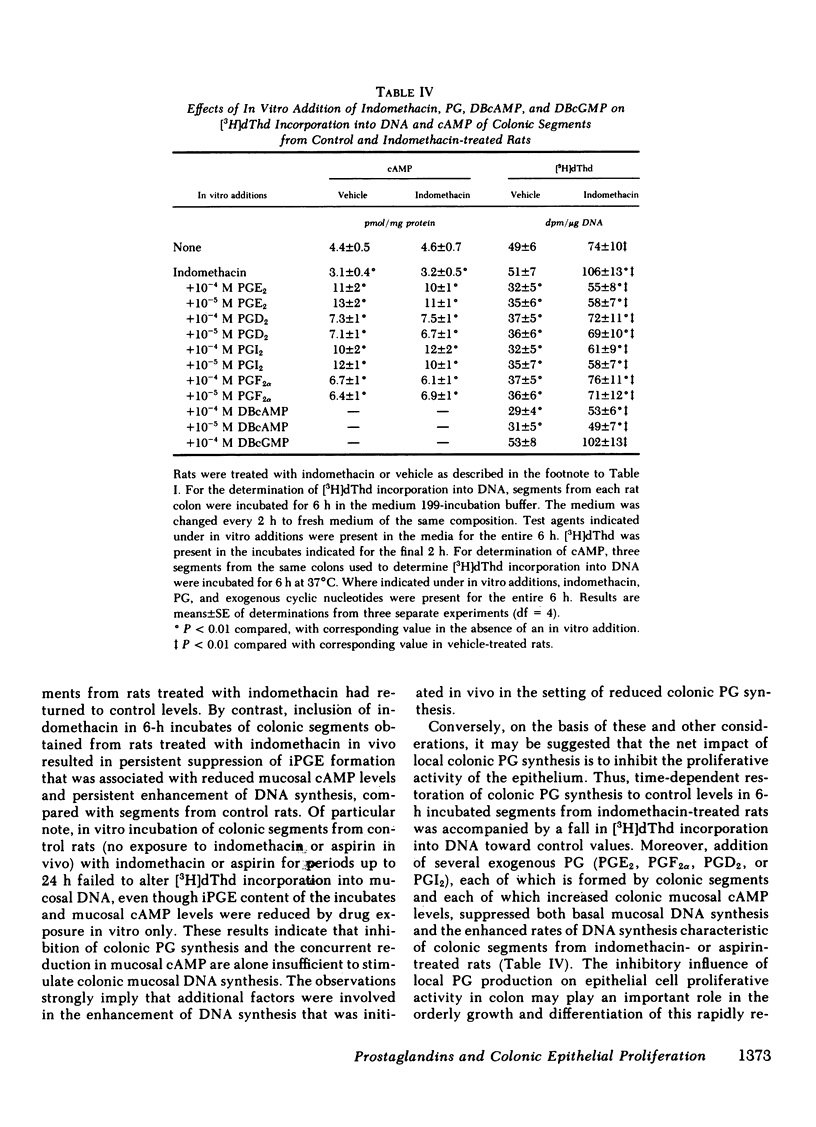
The role of local prostaglandin (PG) synthesis in the modulation of the proliferative activity of colonic epithelium was examined in rat colon. Experimental rats were given either indomethacin (5 mg/kg s.c. every 8 h for three doses) or aspirin (0.5 g/100 g diet for 3 d). In rats treated with indomethacin or aspirin, the incorporation of [3H]thymidine (dThd) into DNA in vivo was increased approximately twofold over control in mucosal scrapings from distal colon, and approximately threefold over control in the proliferating pool of epithelial cells isolated from distal colon. [3H]dThd incorporation into DNA was also examined ex vivo immediately after distal colonic resection. It was approximately twofold higher in mucosa of colonic segments (1-h incubation) from rats treated with indomethacin or aspirin in vivo, compared with corresponding values of segments from control rats. Immunoreactive (i) prostaglandin E (PGE), the dominant PG product of colon segment incubates by high-performance liquid chromatography analysis of [14C]arachidonate metabolites, was markedly (95%) reduced in the media of 1-h colon incubates from indomethacin- or aspirin-treated rats, compared with control rats. Moreover, the cyclic (c)AMP content of mucosa of segments from indomethacin- or aspirin-treated rats was significantly lower than that of control rats. Prolonged incubation (4-24 h) of colonic segments from indomethacin-treated rats, in the absence of indomethacin in vitro, led to an eventual return of [3H]dThd incorporation into DNA, iPGE, and mucosal cAMP to control values. Conversely, inclusion of indomethacin (0.25 mM) in the incubations (6 h) of colonic segments from indomethacin-treated rats resulted in persistent suppression of iPGE and mucosal cAMP, as well as persistent enhancement of [3H]dThd incorporation into mucosal DNA. However, incubation of colonic segments from control rats (no in vivo drug exposure) with indomethacin or aspirin in vitro for periods up to 24 h failed to alter DNA synthesis, despite marked reduction in media iPGE and lower mucosal cAMP. The latter observations suggested that additional in vivo factors initiated the enhancement of DNA synthesis in indomethacin- or aspirin-treated rats. Exogenous PGE2, D2, I2, or F2 alpha, each of which increased the endogenous mucosal cAMP content of incubated colonic segments from control, indomethacin- or aspirin-treated rats, all suppressed [3H]dThd incorporation into mucosal DNA in vitro. Dibutyryl cAMP, but not dibutyryl cGMP, had an analogous suppressive effect on in vitro [3H]dThd incorporation into DNA. Thus, the present observations are consistent with an inhibitory action of endogenous colonic PG synthesis on the proliferative activity of colonic epithelium. This action may be mediated through cAMP.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Philpott G. W. Control of deoxyribonucleic acid synthesis in normal rabbit colonic mucosa. Gastroenterology. 1975 Oct;69(4):951–959. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Cyclic nucleotide metabolism in rat colonic epithelial cells with different proliferative activities. Biochim Biophys Acta. 1981 Aug 17;676(2):155–169. doi: 10.1016/0304-4165(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Effects of vasopressin and urea on Ca2+-calmodulin-dependent renal prostaglandin E. Am J Physiol. 1981 Dec;241(6):F649–F658. doi: 10.1152/ajprenal.1981.241.6.F649. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Stimulation of rat colonic mucosal prostaglandin synthesis by calcium and carbamylcholine: relationship to alterations in cyclic nucleotide metabolism. Prostaglandins. 1981 Jan;21(1):65–81. doi: 10.1016/0090-6980(81)90197-0. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Studer R. K., Derubertis F. R. Renal inner medullary prostaglandin synthesis. A calcium-calmodulin-dependent process suppressed by urea. J Clin Invest. 1981 Sep;68(3):722–732. doi: 10.1172/JCI110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Field J. B. The content and metabolism of cyclic adenosine 3', 5'-monophosphate and cyclic guanosine 3', 5'-monophosphate in adenocarcinoma of the human colon. J Clin Invest. 1976 Mar;57(3):641–649. doi: 10.1172/JCI108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Early alterations in rat colonic mucosal cyclic nucleotide metabolism and protein kinase activity induced by 1,2-dimethylhydrazine. Cancer Res. 1980 Dec;40(12):4589–4598. [PubMed] [Google Scholar]
- Dunn M. J., Zambraski E. J. Renal effects of drugs that inhibit prostaglandin synthesis. Kidney Int. 1980 Nov;18(5):609–622. doi: 10.1038/ki.1980.179. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L. Gastrointestinal epithelial renewal. Gastroenterology. 1977 May;72(5 Pt 1):962–975. [PubMed] [Google Scholar]
- Enochs M. R., Johnson L. R. Trophic effects of gastrointestinal hormones: physiological implications. Fed Proc. 1977 Jun;36(7):1942–1947. [PubMed] [Google Scholar]
- Gal D., Casey M. L., Johnston J. M., MacDonald P. C. Mesenchyme-epithelial interactions in human endometrium. Prostaglandin synthesis in separated cell types. J Clin Invest. 1982 Oct;70(4):798–805. doi: 10.1172/JCI110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gréen K., Hamberg M., Samuelsson B., Frölich J. C. Extraction and chromatographic procedures for purification of prostaglandins, thromboxanes, prostacyclin, and their metabolites. Adv Prostaglandin Thromboxane Res. 1978;5:15–38. [PubMed] [Google Scholar]
- Johansson C., Aly A., Kollberg B., Rubio C., Erikoinen T., Helander H. F. Trophic actions of oral E2 prostaglandins on the rat gastrointestinal mucosa. Adv Prostaglandin Thromboxane Leukot Res. 1983;12:403–407. [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Sweetman B. J., Oates J. A. Synthesis and metabolism of prostaglandins E2, F2alpha and D2 by the rat gastrointestinal tract. Stimulation by a hypertonic environment in vitro. Prostaglandins. 1978 May;15(5):751–757. doi: 10.1016/0090-6980(78)90141-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipkin M. Phase 1 and phase 2 proliferative lesions of colonic epithelial cells in diseases leading to colonic cancer. Cancer. 1974 Sep;34(3):suppl–suppl:888. doi: 10.1002/1097-0142(197409)34:3+<878::aid-cncr2820340715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Proliferative changes in the colon. Am J Dig Dis. 1974 Nov;19(11):1029–1032. doi: 10.1007/BF01255785. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. Autoradiography. Methods Enzymol. 1979;58:279–292. doi: 10.1016/s0076-6879(79)58143-9. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Stevens R. H., Loven D. P., Osborne J. W., Prall J. P., Lawson A. J. Cyclic nucleotide concentrations in 1,2-dimethylhydrazine induced rat colon adenocarcinoma. Cancer Lett. 1978 Jan;4(1):27–33. doi: 10.1016/s0304-3835(78)93192-0. [DOI] [PubMed] [Google Scholar]
- Taub M., Coyne M. J., Bonorris G. G., Chung A., Coyne B., Schoenfield L. J. Inhibition by propranolol of bile acid- and PGE1-stimulated camp and intestinal secretion. Am J Gastroenterol. 1978 Aug;70(2):129–135. [PubMed] [Google Scholar]
- Terragno A., Rydzik R., Terragno N. A. High performance liquid chromatography and UV detection for the separation and quantitation of prostaglandins. Prostaglandins. 1981 Jan;21(1):101–112. doi: 10.1016/0090-6980(81)90200-8. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Effects of cyclic-nucleotide derivatives on the growth of human colonic carcinoma xenografts and on cell production in the rat colonic crypt epithelium. Br J Cancer. 1981 Aug;44(2):182–188. doi: 10.1038/bjc.1981.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Influence of prostaglandin analogues on epithelial cell proliferation and xenograft growth. Br J Cancer. 1980 Jan;41(1):47–51. doi: 10.1038/bjc.1980.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (second of two parts). Mechanisms of control. N Engl J Med. 1978 Jun 29;298(26):1444–1450. doi: 10.1056/NEJM197806292982604. [DOI] [PubMed] [Google Scholar]