Abstract
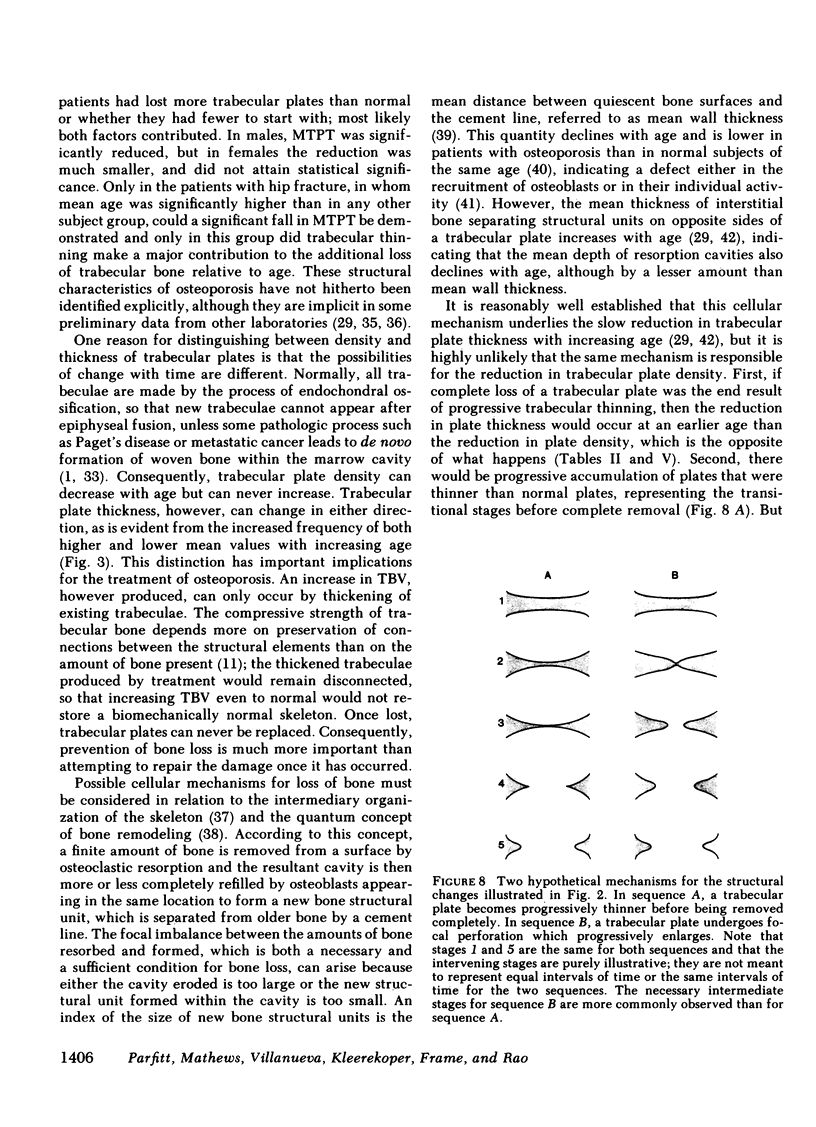
We devised a new method for examining the structural changes that occur in trabecular bone in aging and in osteoporosis. With simultaneous measurement of total perimeter and bone area in thin sections, indirect indices of mean trabecular plate thickness (MTPT) and mean trabecular plate density (MTPD) can be derived, such that trabecular bone volume = MTPD X MTPT. MTPD is an index of the probability that a scanning or test line will intersect a structural element of bone, and is the reciprocal of the mean distance between the midpoints of structural elements, multiplied by pi/2. We applied this method to iliac bone samples from 78 normal subjects, 100 patients with vertebral fracture, and 50 patients with hip fracture. The reduction in trabecular bone volume observed in normal subjects with increasing age was mainly due to a reduction in plate density, with no significant decrease in plate thickness. The further reduction in trabecular bone volume observed in patients with osteoporotic vertebral fracture was mainly due to a further reduction in plate density. There was a relatively smaller reduction in plate thickness that was statistically significant in males but not in females. Only in patients with hip fracture did trabecular thinning contribute substantially to the additional loss of trabecular bone in osteoporosis relative to age. These data indicate that age-related bone loss occurs principally by a process that removes entire structural elements of bone; those that remain are more widely separated and some may undergo compensatory thickening, but most slowly become reduced in thickness. We propose that the process of removal is initiated by increased depth of osteoclastic resorption cavities which leads to focal perforation of trabecular plates; this is followed by progressive enlargement of the perforations with conversion of plates to rods. The resulting structural changes are more severe in osteoporotic patients than in normal subjects, but have been completed in most patients before they develop symptoms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cann C. E., Genant H. K. Precise measurement of vertebral mineral content using computed tomography. J Comput Assist Tomogr. 1980 Aug;4(4):493–500. doi: 10.1097/00004728-198008000-00018. [DOI] [PubMed] [Google Scholar]
- Courpron P. Bone tissue mechanisms underlying osteoporoses. Orthop Clin North Am. 1981 Jul;12(3):513–545. [PubMed] [Google Scholar]
- Crilly R. G., Horseman A., Peacock M., Nordin B. E. The vitamin D metabolites in the pathogenesis and management of osteoporosis. Curr Med Res Opin. 1981;7(5):337–348. doi: 10.1185/03007998109114277. [DOI] [PubMed] [Google Scholar]
- Crilly R. G., Horsman A., Marshall D. H., Nordin B. E. Post-menopausal and corticosteroid-induced osteoporosis. Front Horm Res. 1977;5:53–75. doi: 10.1159/000401985. [DOI] [PubMed] [Google Scholar]
- Dambacher M. A., Langlotz M., Olah A. J., Rüegsegger P. Differentialdiagnose der metabolischen Osteopathien. Orthopade. 1982 Apr;11(2):35–46. [PubMed] [Google Scholar]
- Darby A. J., Meunier P. J. Mean wall thickness and formation periods of trabecular bone packets in idiopathic osteoporosis. Calcif Tissue Int. 1981;33(3):199–204. doi: 10.1007/BF02409438. [DOI] [PubMed] [Google Scholar]
- Delling G. Age-related bone changes. Histomorphometric investigation of the structure of human cancellous bone. Curr Top Pathol. 1973;58:117–147. [PubMed] [Google Scholar]
- Frost H. M. The skeletal intermediary organization. Metab Bone Dis Relat Res. 1983;4(5):281–290. doi: 10.1016/s0221-8747(83)80001-0. [DOI] [PubMed] [Google Scholar]
- Heaney R. P., Recker R. R., Saville P. D. Menopausal changes in bone remodeling. J Lab Clin Med. 1978 Dec;92(6):964–970. [PubMed] [Google Scholar]
- Heaney R. P., Recker R. R., Saville P. D. Menopausal changes in calcium balance performance. J Lab Clin Med. 1978 Dec;92(6):953–963. [PubMed] [Google Scholar]
- Krølner B., Pors Nielsen S. Bone mineral content of the lumbar spine in normal and osteoporotic women: cross-sectional and longitudinal studies. Clin Sci (Lond) 1982 Mar;62(3):329–336. doi: 10.1042/cs0620329. [DOI] [PubMed] [Google Scholar]
- Lips P., Courpron P., Meunier P. J. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res. 1978 Nov 10;26(1):13–17. doi: 10.1007/BF02013227. [DOI] [PubMed] [Google Scholar]
- Lips P., Netelenbos J. C., Jongen M. J., van Ginkel F. C., Althuis A. L., van Schaik C. L., van der Vijgh W. J., Vermeiden J. P., van der Meer C. Histomorphometric profile and vitamin D status in patients with femoral neck fracture. Metab Bone Dis Relat Res. 1982;4(2):85–93. doi: 10.1016/0221-8747(82)90021-2. [DOI] [PubMed] [Google Scholar]
- Melsen F., Melsen B., Mosekilde L., Bergmann S. Histomorphometric analysis of normal bone from the iliac crest. Acta Pathol Microbiol Scand A. 1978 Jan;86(1):70–81. doi: 10.1111/j.1699-0463.1978.tb02014.x. [DOI] [PubMed] [Google Scholar]
- Merz W. A., Schenk R. K. Quantitative structural analysis of human cancellous bone. Acta Anat (Basel) 1970;75(1):54–66. doi: 10.1159/000143440. [DOI] [PubMed] [Google Scholar]
- Nordin B. E., Aaron J., Speed R., Crilly R. G. Bone formation and resorption as the determinants of trabecular bone volume in postmenopausal osteoporosis. Lancet. 1981 Aug 8;2(8241):277–279. doi: 10.1016/s0140-6736(81)90526-2. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Oliver I., Villanueva A. R. Bone histology in metabolic bone disease: the diagnostic value of bone biopsy. Orthop Clin North Am. 1979 Apr;10(2):329–345. [PubMed] [Google Scholar]
- Parfitt A. M. Quantum concept of bone remodeling and turnover: implications for the pathogenesis of osteoporosis. Calcif Tissue Int. 1979 Aug 24;28(1):1–5. doi: 10.1007/BF02441211. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res. 1982;4(1):1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- Pesch H. J., Scharf H. P., Lauer G., Seibold H. Der altersabhängige Verbundbau der Lendenwirbelkörper. Eine Struktur- und Formanalyse. Virchows Arch A Pathol Anat Histol. 1980;386(1):21–41. doi: 10.1007/BF00432642. [DOI] [PubMed] [Google Scholar]
- Pugh J. W., Rose R. M., Radin E. L. Elastic and viscoelastic properties of trabecular bone: dependence on structure. J Biomech. 1973 Sep;6(5):475–485. doi: 10.1016/0021-9290(73)90006-7. [DOI] [PubMed] [Google Scholar]
- Reeve J., Green J. R., Hesp R., Hulme P. Rates of new bone formation in patients with crush fracture osteoporosis. Clin Sci (Lond) 1982 Aug;63(2):153–160. doi: 10.1042/cs0630153. [DOI] [PubMed] [Google Scholar]
- Schwartz M. P., Recker R. R. Comparison of surface density and volume of human iliac trabecular bone measured directly and by applied stereology. Calcif Tissue Int. 1981;33(6):561–565. doi: 10.1007/BF02409492. [DOI] [PubMed] [Google Scholar]
- Wakamatsu E., Sissons H. A. The cancellous bone of the iliac crest. Calcif Tissue Res. 1969;4(2):147–161. doi: 10.1007/BF02279116. [DOI] [PubMed] [Google Scholar]
- Whitehouse W. J. Cancellous bone in the anterior part of the iliac crest. Calcif Tissue Res. 1977 May 31;23(1):67–76. doi: 10.1007/BF02012768. [DOI] [PubMed] [Google Scholar]
- Whitehouse W. J. The quantitative morphology of anisotropic trabecular bone. J Microsc. 1974 Jul;101(Pt 2):153–168. doi: 10.1111/j.1365-2818.1974.tb03878.x. [DOI] [PubMed] [Google Scholar]
- Whyte M. P., Bergfeld M. A., Murphy W. A., Avioli L. V., Teitelbaum S. L. Postmenopausal osteoporosis. A heterogeneous disorder as assessed by histomorphometric analysis of Iliac crest bone from untreated patients. Am J Med. 1982 Feb;72(2):193–202. doi: 10.1016/0002-9343(82)90810-5. [DOI] [PubMed] [Google Scholar]


