Abstract
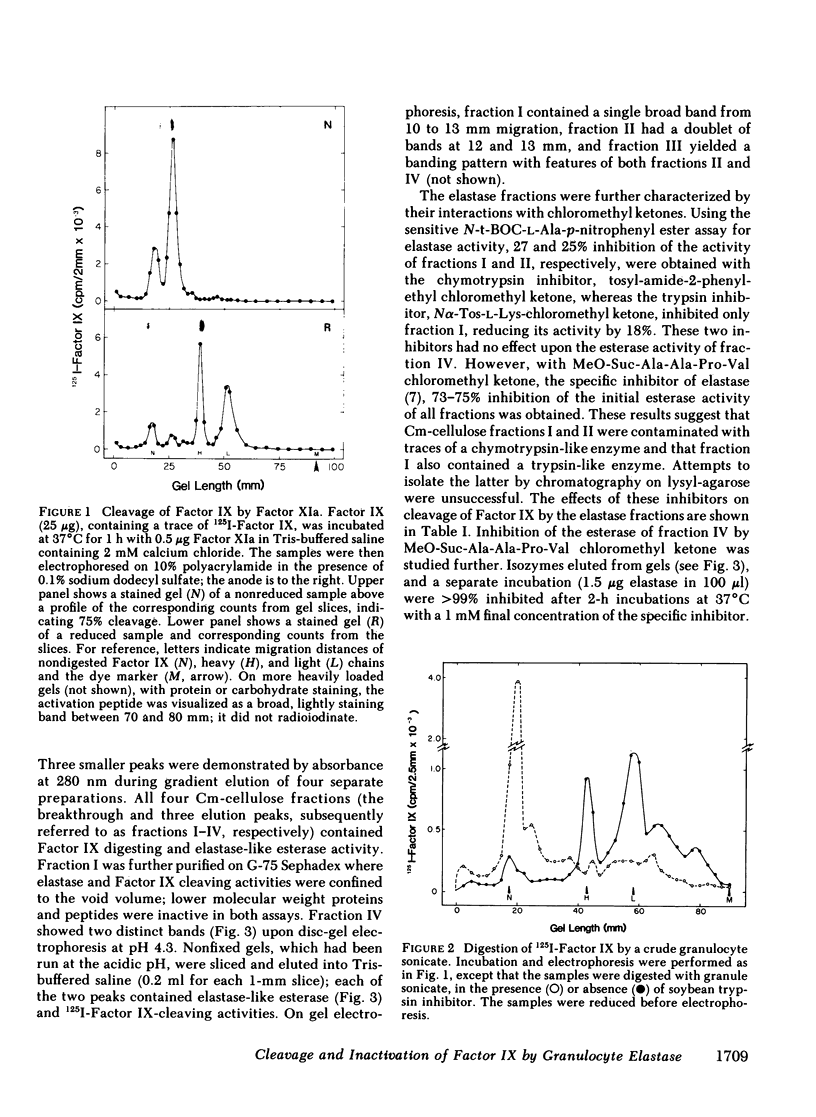
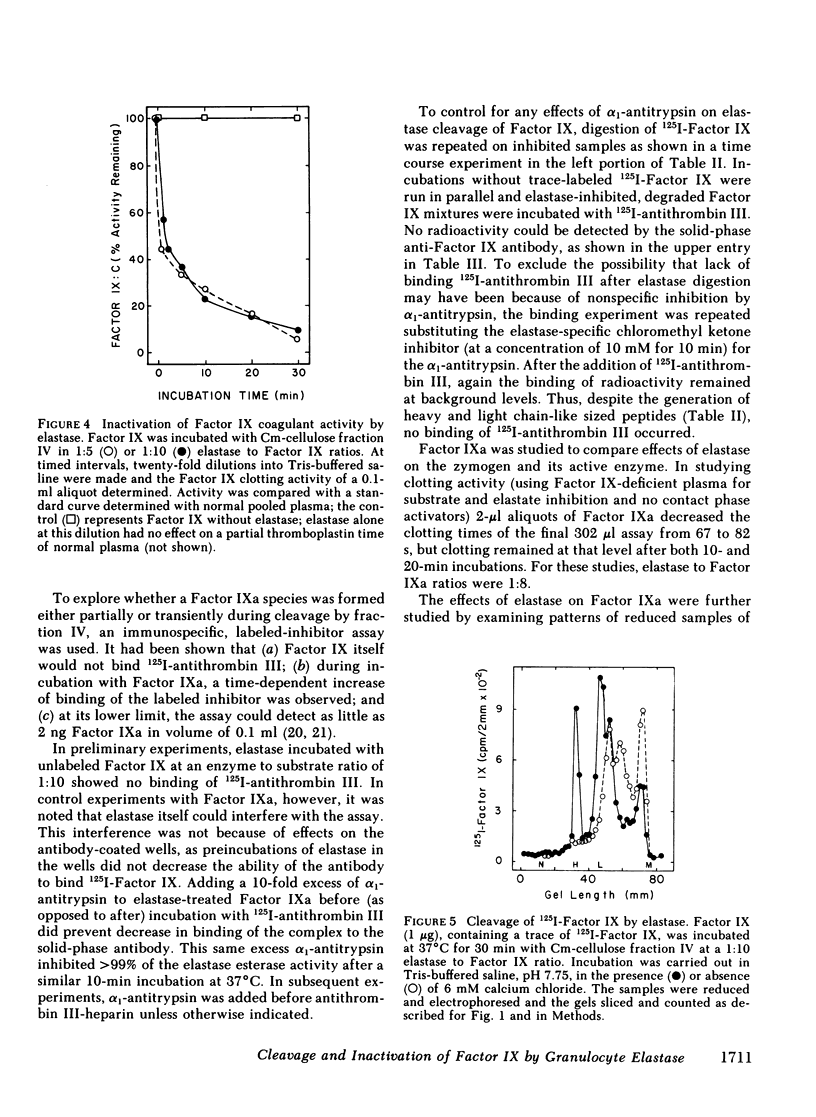
Radioiodinated Factor IX was cleaved by a crude sonicate from leukocytes. In the absence of calcium, fragments of less than 15,000 mol wt were seen from reduced samples on gel electrophoresis. After digestion in 2 mM calcium, however, electrophoresis of reduced samples showed, in addition to low molecular weight fragments, protein bands corresponding in size to heavy and light chains of Factor XIa-activated Factor IX. The cleaving activity in leukocyte sonicates was inhibited by soybean trypsin inhibitor, but only to a small extent by aprotinin. Granulocyte elastase was isolated from purified polymorphonuclear leukocyte granules by affinity chromatography on soybean trypsin inhibitor-agarose and further chromatography on carboxymethyl cellulose. The purified fraction contained two isozymes on acidic gels which cleaved both an ester sensitive to elastase and radiolabeled Factor IX. These two activities were inhibited by elastase-specific chloromethyl ketone. The isolated protease fraction rapidly inactivated apparent Factor IX activity in a coagulant assay system. The degree of inactivation correlated with the amount of intact, radiolabeled Factor IX cleaved. As with the crude sonicate, generation of the larger heavy and light chain-sized fragments was dependent upon calcium. To assess directly the effect of elastase on Factor IX, an immunospecific, active site-directed assay was developed. In this assay, the sample was incubated with solid-phase antibody to Factor IX and the amount of activated product was detected as that which had complexed with radioiodinated antithrombin III. In this system, exposure of Factor IX to Factor XIa showed progressive increase in the ability to bind antithrombin III, whereas after elastase, Factor XIa was unable to generate antithrombin III binding. The elastase-degraded Factor IX did not inhibit activation of additional Factor IX in clotting assays. When Factor IXa was incubated with elastase, binding of antithrombin III was decreased, corresponding to appearance of low molecular weight fragments on parallel samples that were reduced and electrophoresed. These data are consistent with elastase inactivating Factor IX by cleaving bonds near, but distinct from, bonds cleaved by Factor XIa.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Kisiel W., Castellino F. J. The interaction of Ca2+ with human Factor IX. Arch Biochem Biophys. 1981 May;208(2):576–585. doi: 10.1016/0003-9861(81)90546-4. [DOI] [PubMed] [Google Scholar]
- Bajaj S. P. Cooperative Ca2+ binding to human factor IX. Effects of Ca2+ on the kinetic parameters of the activation of factor IX by factor XIa. J Biol Chem. 1982 Apr 25;257(8):4127–4132. [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Brower M. S., Harpel P. C. Alpha-1-antitrypsin-human leukocyte elastase complexes in blood: quantification by an enzyme-linked differential antibody immunosorbent assay and comparison with alpha-2-plasmin inhibitor-plasmin complexes. Blood. 1983 May;61(5):842–849. [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T., Feinman R. D., Landis B. H., Fenton J. W., 2nd Antithrombin reactions with alpha- and gamma-thrombins. Biochemistry. 1979 Jan 9;18(1):113–119. doi: 10.1021/bi00568a018. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Jochum M., Lander S., Heimburger N., Fritz H. Effect of human granulocytic elastase on isolated human antithrombin III. Hoppe Seylers Z Physiol Chem. 1981 Feb;362(2):103–112. doi: 10.1515/bchm2.1981.362.1.103. [DOI] [PubMed] [Google Scholar]
- Kingdon H. S., Herion J. C., Rausch P. G. Cellular activation of factor IX (Christmas factor). Thromb Res. 1978 Sep;13(3):501–507. doi: 10.1016/0049-3848(78)90135-4. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Activation of human factor XI (plasma thromboplastin antecedent) by factor XIIa (activated Hageman factor). Biochemistry. 1977 Dec 27;16(26):5831–5839. doi: 10.1021/bi00645a030. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kárpáti J., Váradi K., Elödi S. Effect of granulocyte proteases on human coagulation factors IX and X. The protective effect of calcium. Hoppe Seylers Z Physiol Chem. 1982 May;363(5):521–525. [PubMed] [Google Scholar]
- Magnusson S., Hofmann T. Inactivation of bovine thrombin by nitrous acid. Can J Biochem. 1970 Apr;48(4):432–437. doi: 10.1139/o70-070. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Kurachi K., Hermodson M. A. Formation and dissociation of the covalent complexes between trypsin and two homologous inhibitors, alpha 1-antitrypsin and antithrombin III. Eur J Biochem. 1980 Apr;105(3):545–552. doi: 10.1111/j.1432-1033.1980.tb04531.x. [DOI] [PubMed] [Google Scholar]
- Osterud B., Bouma B. N., Griffin J. H. Human blood coagulation factor IX. Purification, properties, and mechanism of activation by activated factor XI. J Biol Chem. 1978 Sep 10;253(17):5946–5951. [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F. Leukocyte elastase release during blood coagulation. A potential mechanism for activation of the alternative fibrinolytic pathway. J Clin Invest. 1982 Mar;69(3):564–572. doi: 10.1172/JCI110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., McKenna P. W., Rosenberg R. D. Inhibition of human factor IXa by human antithrombin. J Biol Chem. 1975 Dec 10;250(23):8883–8888. [PubMed] [Google Scholar]
- Schmidt W., Egbring R., Havemann K. Effect of elastase-like and chymotrypsin-like neutral proteases from human granulocytes on isolated clotting factors. Thromb Res. 1975 Apr;6(4):315–329. doi: 10.1016/0049-3848(75)90081-x. [DOI] [PubMed] [Google Scholar]
- Silverstein R. M. The assay of the bromelains using N alpha-CBZ-L-lysine p-nitrophenyl ester and N-CBZ-glycine p-nitrophenyl ester as substrates. Anal Biochem. 1974 Dec;62(2):478–484. doi: 10.1016/0003-2697(74)90180-8. [DOI] [PubMed] [Google Scholar]
- Theodorsson B., Hedner U., Nilsson I. M., Kisiel W. A technique for specific removal of factor IX alloantibodies from human plasma: partial characterization of the alloantibodies. Blood. 1983 May;61(5):973–981. [PubMed] [Google Scholar]
- Thompson A. R. Factor IX antigen by radioimmunoassay. Abnormal factor IX protein in patients on warfarin therapy and with hemophilia B. J Clin Invest. 1977 May;59(5):900–910. doi: 10.1172/JCI108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- West B. C., Rosenthal A. S., Gelb N. A., Kimball H. R. Separation and characterization of human neutrophil granules. Am J Pathol. 1974 Oct;77(1):41–66. [PMC free article] [PubMed] [Google Scholar]
- Zur M., Nemerson Y. Kinetics of factor IX activation via the extrinsic pathway. Dependence of Km on tissue factor. J Biol Chem. 1980 Jun 25;255(12):5703–5707. [PubMed] [Google Scholar]