Abstract
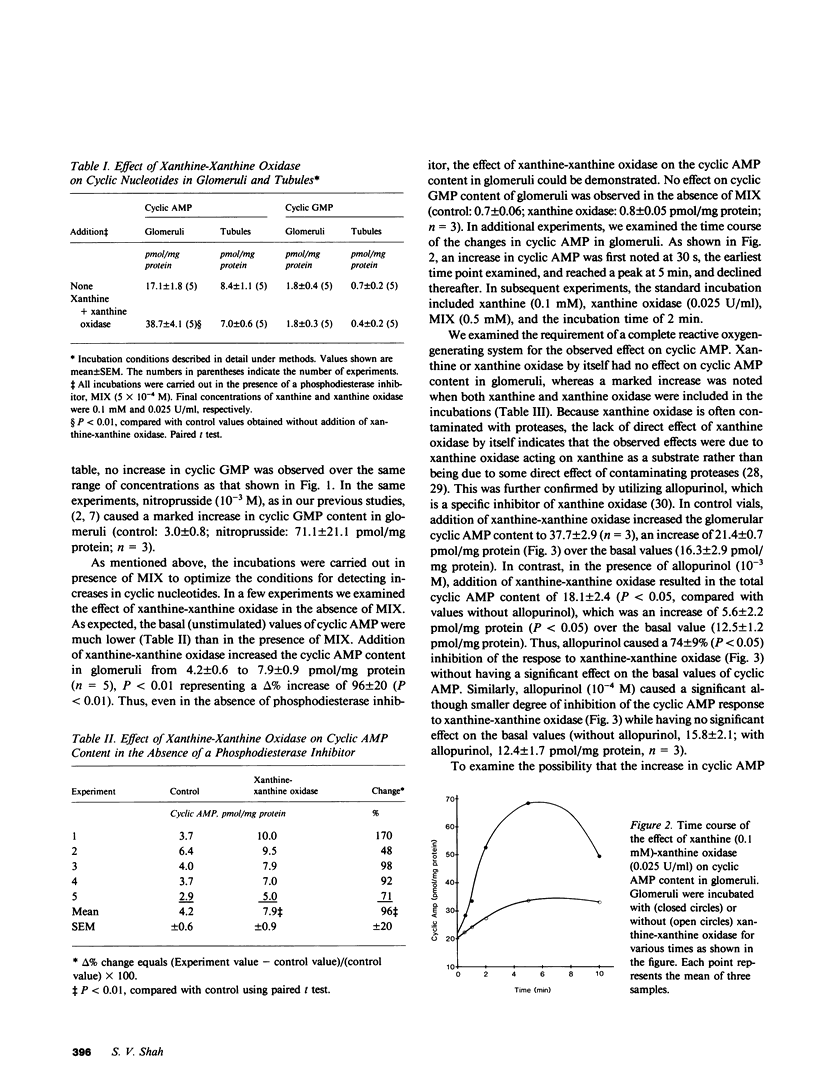
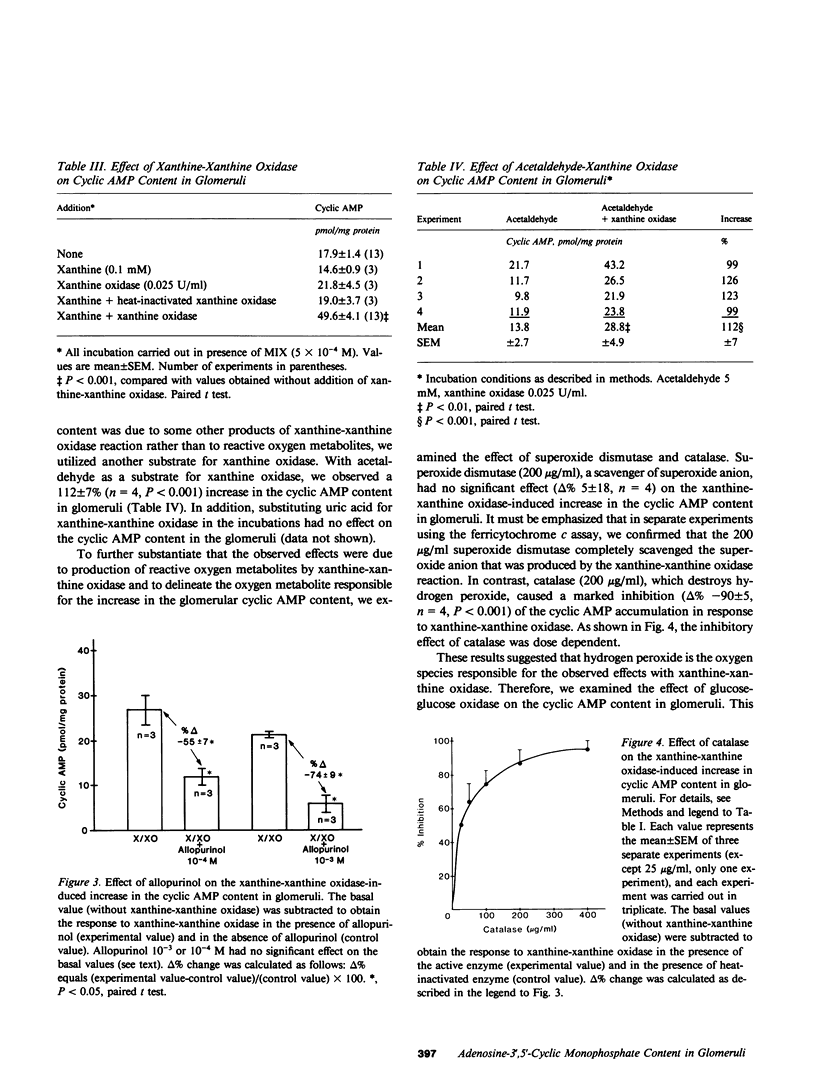
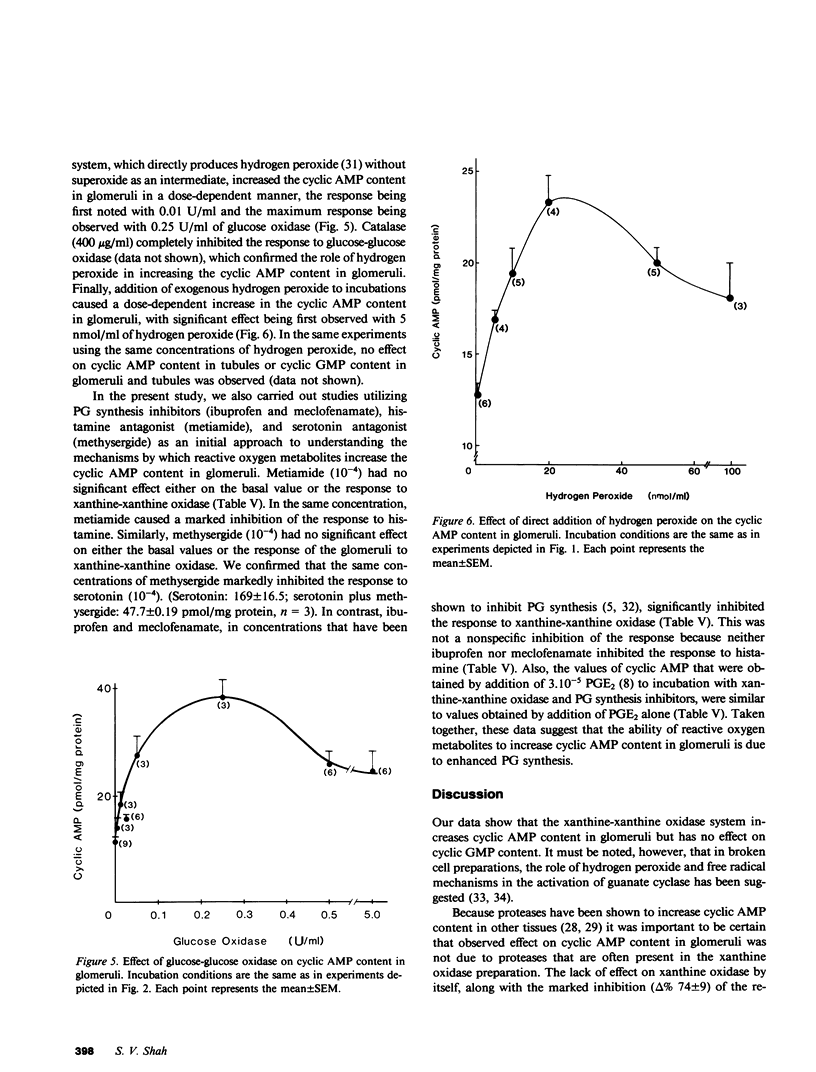
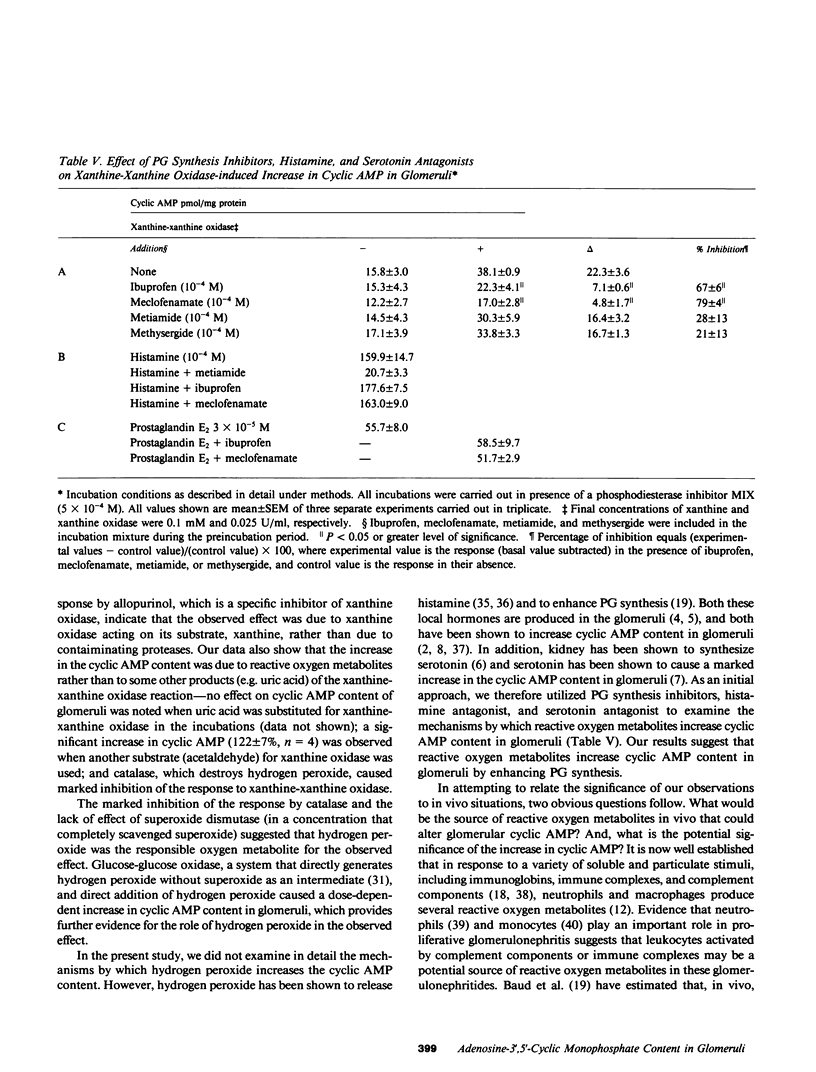
In the present study we examined the effect of reactive oxygen metabolites (generated by the xanthine-xanthine oxidase system), on adenosine-3',5'-cyclic monophosphate (cyclic AMP) and guanosine-3',5'-cyclic monophosphate (cyclic GMP) content in glomeruli and tubules that were isolated from rat renal cortex. Xanthine (0.1 mM)-xanthine oxidase (0.025 U/ml) significantly increased (P less than 0.001) the cyclic AMP content in glomeruli from 18 +/- 1 to 50 +/- 4 pmol/mg protein (n = 13). The response was dose dependent and was markedly inhibited (delta %-74 +/- 9, n = 3) by allopurinol (10(-3), a specific inhibitor of xanthine oxidase. Cyclic AMP content in the tubules, and the cyclic GMP content in glomeruli and tubules, were not altered by the xanthine-xanthine oxidase system. This lack of response was not due to lack of responsiveness of the tissues because parathyroid hormone caused a marked increase in the cyclic AMP content in tubules, and nitroprusside markedly increased the cyclic GMP content in glomeruli. The increase in cyclic AMP in glomeruli was due to generation of reactive oxygen metabolites rather than of other products (e.g. uric acid) of the xanthine-xanthine oxidase reaction--addition of uric acid to incubations had no effect; using another substrate for xanthine oxidase, acetaldehyde significantly increased (delta % 112 +/- 7, n = 4, P less than 0.001) the cyclic AMP content; and catalase that destroys hydrogen peroxide caused a marked inhibition (delta % -90 +/- 5, n = 4) of the response to xanthine-xanthine oxidase. The marked inhibition by catalase, and the lack of effect of superoxide dismutase (in a concentration that completely scavenged superoxide) suggested hydrogen peroxide as the responsible oxygen metabolite for the observed effect. Glucose-glucose oxidase (a system that directly generates hydrogen peroxide), and direct addition of hydrogen peroxide caused a dose-dependent increase in the cyclic AMP content in glomeruli, which further supports the role of hydrogen peroxide as the responsible species for the observed effect. Additional experiments that used prostaglandin synthesis inhibitors and antagonists of serotonin and histamine suggested that hydrogen peroxide increases cyclic AMP content in glomeruli by enhancing prostaglandin synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E., Ou S. L., Velosa J. A., Shah S. V., Dousa T. P. Dynamics of renal histamine in normal rat kidney and in nephrosis induced by aminonucleoside of puromycin. J Clin Invest. 1982 Feb;69(2):327–336. doi: 10.1172/JCI110456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Nivez M. P., Chansel D., Ardaillou R. Stimulation by oxygen radicals of prostaglandin production by rat renal glomeruli. Kidney Int. 1981 Sep;20(3):332–339. doi: 10.1038/ki.1981.143. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R., Thaw H. H., Björk J., Planker M., Arfors K. E. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Dousa T. P., Shah S. V., Abboud H. E. Potential role of cyclic nucleotides in glomerular pathophysiology. Adv Cyclic Nucleotide Res. 1980;12:285–299. [PubMed] [Google Scholar]
- Dworkin L. D., Ichikawa I., Brenner B. M. Hormonal modulation of glomerular function. Am J Physiol. 1983 Feb;244(2):F95–104. doi: 10.1152/ajprenal.1983.244.2.F95. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Kovensky A., Hitchings G. H. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966 Jul;15(7):863–880. doi: 10.1016/0006-2952(66)90163-8. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handin R. I., Karabin R., Boxer G. J. Enhancement of platelet function by superoxide anion. J Clin Invest. 1977 May;59(5):959–965. doi: 10.1172/JCI108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Klebanoff S. J. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980 Aug 1;152(2):265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A., Brachet E. The permeability coefficient of albumin of the isolated rat mesentery. Modification by some mediators of inflammation, cyclic AMP and calcium. Biochim Biophys Acta. 1979 Dec 3;588(2):219–231. doi: 10.1016/0304-4165(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Kahn A., Brachet E. The role of various prostaglandins on the correlation between permeability to albumin and cAMP levels in the isolated mesentery. Prostaglandins. 1978 Dec;16(6):939–944. doi: 10.1016/0090-6980(78)90109-0. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mccord J. M., Wong K., Stokes S. H., Petrone W. F., English D. Superoxide and inflammation: a mechanism for the anti-inflammatory activity of superoxide dismutase. Acta Physiol Scand Suppl. 1980;492:25–30. [PubMed] [Google Scholar]
- Michelakis A. M., Caudle J., Liddle G. W. In vitro stimulation of renin production by epinephrine, norepinephrine, and cyclic AMP. Proc Soc Exp Biol Med. 1969 Mar;130(3):748–753. doi: 10.3181/00379727-130-33647. [DOI] [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R., Pick F. M., Bray R. C. EPR studies on reduction of oxygen to superoxide by some biochemical systems. Biochim Biophys Acta. 1969 Oct 7;192(1):145–148. doi: 10.1016/0304-4165(69)90022-1. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Komoriya K., Azuma A., Kurozumi S., Hashimoto Y. Xanthine oxidase-induced histamine release from isolated rat peritoneal mast cells: involvement of hydrogen peroxide. Biochem Pharmacol. 1979;28(2):333–334. doi: 10.1016/0006-2952(79)90524-0. [DOI] [PubMed] [Google Scholar]
- Parks W. M., Hoak J. C., Czervionke R. L. Comparative effect of ibuprofen on endothelial and platelet prostaglandin synthesis. J Pharmacol Exp Ther. 1981 Nov;219(2):415–419. [PubMed] [Google Scholar]
- Richert N. D., Ryan R. J. Proteolytic enzyme activation of rat ovarian adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4857–4861. doi: 10.1073/pnas.74.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff D., Yoo P., Alpert B. E. Stimulation of adenylate cyclase in isolated rat glomeruli by prostaglandins. Am J Physiol. 1978 Nov;235(5):F458–F464. doi: 10.1152/ajprenal.1978.235.5.F458. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Light emission by isolated rat glomeruli in response to phorbol myristate acetate. J Lab Clin Med. 1981 Jul;98(1):46–57. [PubMed] [Google Scholar]
- Shah S. V., Northrup T. E., Hui Y. S., Dousa T. P. Action of serotonin (5-hydroxytryptamine) on cyclic nucleotides in glomeruli of rat renal cortex. Kidney Int. 1979 May;15(5):463–472. doi: 10.1038/ki.1979.62. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Patt Y., Trainin N. Trypsin-induced increase in intracellular cyclic AMP of lymphocytes. J Immunol. 1976 Dec;117(6):2143–2149. [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Smith R. L., Hunt N. H., Merritt J. E., Evans T., Weidemann M. J. Cyclic nucleotide metabolism and reactive oxygen production by macrophages. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1079–1087. doi: 10.1016/0006-291x(80)90062-5. [DOI] [PubMed] [Google Scholar]
- White A. A., Crawford K. M., Patt C. S., Lad P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J Biol Chem. 1976 Dec 10;251(23):7304–7312. [PubMed] [Google Scholar]


