Abstract
A disproportionate expansion of white adipose tissue and abnormal recruitment of adipogenic precursor cells can not only lead to obesity but also impair glucose metabolism, which are both common causes of insulin resistance and diabetes mellitus. The development of novel and effective therapeutic strategies to slow the progression of obesity, diabetes mellitus and their associated complications will require improved understanding of adipogenesis and glucose metabolism. Klotho might have a role in adipocyte maturation and systemic glucose metabolism. Klotho increases adipocyte differentiation in vitro, and mice that lack Klotho activity are lean owing to reduced white adipose tissue accumulation; moreover, mice that lack the Kl gene (which encodes Klotho) are resistant to obesity induced by a high-fat diet. Knockout of Kl in leptin-deficient Lepob/ob mice reduces obesity and increases insulin sensitivity, which lowers blood glucose levels. Energy metabolism might also be influenced by Klotho. However, further studies are needed to explore the possibility that Klotho could be a novel therapeutic target to reduce obesity and related complications, and to determine whether and how Klotho might influence the regulation and function of a related protein, β-Klotho, which is also involved in energy metabolism.
Introduction
Excess adiposity is associated with the development of insulin resistance and subsequent metabolic disorders, including type 2 diabetes mellitus (T2DM), dyslipidaemia, hypertension and coronary heart disease.1–7 Importantly, obesity-associated disorders, including hypertension and T2DM, can partly be ameliorated by reducing body adipose content. However, inadequate amounts of adipose tissue, such as those seen in patients with lipodystrophy, can also induce the same range of metabolic complications that are observed in patients with obesity—including insulin resistance, T2DM, dyslipidaemia and hepatic steatosis.8 An adequate but not excessive amount of adipose tissue in the body is, therefore, an essential prerequisite for maintaining physiological energy balance.
Despite the identification of different stages and events in adipogenesis and glucose metabolism,9,10 the factors involved in the regulation of energy metabolism are not yet fully defined. A major obstacle for developing an effective therapy to reduce obesity and minimizing its associated complications is that crucial factors involved in energy metabolism have not yet been identified or adequately characterized in an in vivo system. Clarification of the mechanisms underlying energy metabolism is both biologically and clinically important because obesity has been identified as the second most common factor contributing to preventable death (after smoking tobacco).9
A major research focus in the field of energy metabolism over the past decade has been the functional characterization of the peroxisome proliferator-activated receptor (PPAR) family. The nuclear receptors PPAR-α, PPAR-δ and PPAR-γ act as lipid sensors and jointly regulate the expression of several genes that are essential for the regulation of energy metabolism, although only Klotho associations with PPAR-γ have been investigated in depth.10 PPAR-γ has been identified as a key transcriptional regulator of nutrient and energy metabolism.11,12 PPAR-γ also induces expression of Klotho,13 a multifunctional protein14–19 involved in a number of physio logical processes and implicated in a number of diseases (Box 1).20–48
This Review examines the role of Klotho in energy metabolism and considers its contribution to adipogenesis and obesity. The effects of Klotho on glucose control and phosphate metabolism are discussed, along with the potential role of this enzyme in diabetes mellitus. The role of the related protein β-Klotho in FGF-19 and FGF-21 signalling is briefly described in the context of glucose and adipocyte turnover. Other molecular and clinical aspects of β-Klotho function are not discussed further in this article, as they have been extensively reviewed elsewhere.49–52
Klotho proteins
The Klotho proteins (Klotho itself and the related enzyme β-Klotho) exert diverse effects on the physiological regulation of mineral ions (particularly calcium and phosphate) and energy metabolism by influencing the endocrine activities of fibroblast growth factors (FGFs), including FGF-19 and FGF-23.17,19,51–54 The Klotho protein is approximately 130 kDa with a putative signal sequence at the N-terminus, a single transmembrane domain near the C-terminus and a short (10 amino acid) cytoplasmic domain.55,56 The extracellular domain of Klotho consists of two internal repeats of approximately 550 amino acids each that share sequence similarity with β-glucosidase (Figure 1). This similarity gives Klotho a function analogous to that of β-glucosidase (albeit with weaker activity), although whether this activity occurs in vivo is not clear.57 Both secreted and membrane-bound forms of Klotho can be detected in humans and other mammals. The secreted form is generated either by shedding the extracellular domain of the transmembrane protein or as a product of alternative splicing.58 Klotho is expressed primarily in the kidney (distal convoluted tubules), parathyroid gland, brain (choroid plexus epithelium)59 and adipose tissue.60,61
Figure 1.

Schematic diagram of the Klotho protein structure. Klotho is 1,014 amino acids in length and possesses a putative signal sequence at its N-terminus and a transmembrane domain with a short cytoplasmic domain at the C-terminus. The extracellular domain of the Klotho protein consists of two internal repeats (KL1 and KL2) that share sequence homology with β-glucosidase.
Distinguishing between the autocrine, paracrine, and endocrine actions of Klotho is often difficult because this enzyme seems to exert different functions in different cell types in a dependent or, possibly, independent manner. However, mouse genetic studies have led to the identification of a number of in vivo functions of Klotho, including roles in FGF signalling, calcium and phosphate ion transport, and energy metabolism (Box 2).16,17,19,45,61–81
Factors that induce the synthesis of the Klotho protein have not all been identified, although vitamin D seems to be an important regulator. In cells derived from the renal proximal, distal and collecting tubules, expression of Klotho (both membrane-bound and secreted splice variants) is regulated by 1,25-dihydroxyvitamin D.82,83 Candidate vitamin D response elements (VDREs) were identified in the vicinity of mouse and human Klotho genes, and were found to be transcriptionally active sites, as determined by reporter gene assays.83
β-Klotho has 41% amino acid sequence similarity with Klotho and is primarily detected in the liver, pancreas, and adipose tissue.84 β-Klotho regulates bile acid production, and mice lacking activity of this enzyme have a markedly increased synthesis and excretion of bile acids.85 Binding of FGF-15 and FGF-19 to FGF receptor 4 (FGFR-4) is facilitated by β-Klotho, and suppresses the hepatic synthesis of CYP7A1, which in turn triggers a negative-feedback loop to control bile acid metabolism by FGF-15. The overlapping phenotypes of Fgfr4−/−,86 viable Fgf15−/−87 and Klb−/−85 mice suggest that the products of these genes are involved in a common signalling cascade with molecular connections and interactions. In support of this hypothesis, FGF-19 can activate FGF signalling in FGFR-4-expressing hepatocytes to suppress the expression of CYP7A1, which is a rate-limiting enzyme in bile acid synthesis.88
β-Klotho is also involved in FGF-21 signalling.89–91 For example, BaF3 cells (an immortalized murine bone-marrow-derived pro-B-cell line responsive to IL-3) are unresponsive to FGF-21 exposure, but co-expression of β-Klotho and FGFR1c in FGF-21-treated BaF3 cells leads to activation of downstream signalling events resulting in phosphorylation of FGFR substrate 2α (FRS2α), MAPK 2 and MAPK 3.91 However, unlike Fgf15−/− mice, the phenotypes of Fgf21-knockout mice92 differ from those of β-Klotho-knockout mice.85 Importantly, the recombinant FGF-21 protein retains its biological activity in the Klb−/− mice, suggesting the existence of a β-Klotho-independent signalling pathway involving FGF-21.93 However, whether Klotho and β-Klotho can influence each other's functions is not yet clear.
FGF-21 can regulate insulin-independent glucose transport in adipocytes.49 In fact, when differentiated mouse 3T3-L1 adipocytes were treated with FGF-21, uptake of glucose was stimulated, in association with increased expression of GLUT1.94 Furthermore, systemic administration of FGF-21 to diabetic animals led to a significant reduction in blood glucose and triglyceride levels, and Fgf21-transgenic mice, which overexpress FGF-21, were resistant to obesity induced by a high-fat diet.87,95
Adipogenesis regulation
Adipogenesis is the process by which preadipocytes differentiate into mature adipocytes (Figure 2). Over the past decade, a number of important factors have been identified that contribute to the initiation and progression of adipocyte maturation. Dysregulation of these factors results in altered production and distribution of adipose tissue. For example, heterozygous missense mutations in PPAR-γ (a master regulator of adipocyte differentiation) have been linked to the disease phenotype in familial partial lipodystrophy.96,97
Figure 2.
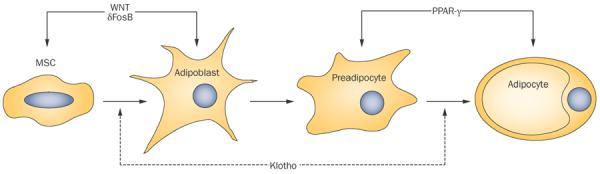
Potential influence of Klotho on adipocyte development. Adipocytes develop from MSCs through two stages of maturation. WNT and δFosB are involved in the differentiation of MSCs to adipoblasts, whereas PPAR-γ drives differentiation of preadipocytes to adipocytes. Klotho could potentially influence both the adipogenic lineage commitment of MSCs and adipocytic maturation, but these roles remain to be confirmed experimentally. Abbreviation: MSC, mesenchymal stem cell.
Klotho can influence in vitro adipose cell maturation by promoting differentiation of preadipocyte cells into adipocyte cells.61 Overexpression of Klotho in 3T3-L1 cells can upregulate adipogenic factors, including PPAR-γ, FABP4 and the CCAAT–enhancer binding proteins, which initiates the maturation process.61 Klotho expression is induced by PPAR-γ,13 and treatment with PPAR-γ agonists (such as thiazolidinediones) increases the synthesis of both Klotho mRNA and protein in renal epithelial cell lines; this induction of Klotho can be blocked by either PPAR-γ antagonists or by silencing of PPAR-γ using small interfering RNAs. Furthermore, a noncanonical PPAR-responsive element was detected within the 5'-flanking region of the human Klotho gene, KL. Importantly, this identified site is functionally active, as demonstrated by increased transcriptional activity of a reporter gene following PPAR-γ agonist (rosiglitazone) treatment.13 This increased activity could be abolished by a PPAR-γ antagonist (GW9662).13 C57BL6 mice treated with thiazolidinediones demonstrated increased renal expression of Klotho,13 and adenovirus-mediated over-expression of PPAR-γ also upregulates Klotho expression in the kidney.13 Similarly, Klotho expression was reduced in the kidneys of Otsuka Long-Evans Tokushima fatty (OLETF) rats, an animal model of metabolic syndrome. However, treatment with troglitazone (an insulin sensitizer and PPAR-γ agonist) induced renal Klotho expression in these rats.98 These in vitro and in vivo results suggest that PPAR-γ increases Klotho expression,13,16,99 but that Klotho can also induce PPAR-γ synthesis during adipocyte maturation.61
Although these experimental observations are suggestive of a potential role of Klotho in adipocyte maturation, the regulatory steps of the adipocyte differentiation process that might be affected by Klotho remain to be identified, and whether Klotho can direct the commitment of multipotent mesenchymal stem cells (MSCs) to an adipogenic lineage still needs to be determined experimentally. Interestingly, stem cell transplantation can alter Klotho levels.100–102 Mice lacking Klotho activity (both Kl−/− and Klkl/k) have less detectable adipose tissue content either in the abdominal cavity or under the skin than wild-type mice.74,75,103
Obesity regulation
Although Klotho can induce the expression of adipogenic factors, exactly how either the soluble or the membrane-bound Klotho protein activates their transcription is not clear. Genetically abolishing Klotho function in mice by knockout or knockdown of Kl resulted in the generation of lean mice with decreased white adipose tissue accumulation, including a reduced subcutaneous adipose tissue layer compared with that in wild-type mice.75,103–105 Despite the obvious reduction in white adipose tissue mass in the Kl-knockdown (Klkl/kl) mice, which retain a very low level of Klotho, no such reduction could be detected in brown adipose tissue mass.73 Moreover, Klkl/kl mice had reduced glycogen content in the liver and decreased insulin content in the pancreas compared with their wild-type littermates.73 Similarly, Klkl/kl mice had decreased lipid content in brown adipose tissues (which can provide heat insulation), along with reduced Ucp1 expression, which was associated with low body temperature.73 In summary, the reduced adipose tissue phenotypes of Kl-mutant Klkl/kl and Kl−/− mice103,105,106 and the in vitro adipocyte-promoting ability of Klotho61 imply that this protein might contribute to either adipocyte maturation or intracellular lipid accumulation. Intracellular lipid accumulation, particularly in skeletal muscle and liver, is usually associated with insulin resistance. Elimination of Klotho function from Lepob/ob mice suppressed hepatic intracellular lipid accumulation in Kl−/−Lepob/ob double-knockout mice,62 which raises the possibility that Klotho has a role in intracellular lipid accumulation. Whether Klotho could increase lipid storage in addition to promoting lipid synthesis remains to be elucidated.
Leptin-deficient Lepob/ob mice are overweight because of increased white adipose tissue deposition; these mice start to gain body weight from 3 weeks of age and are almost three times heavier than their wild-type counterparts by 9 weeks of age. Nonetheless, eliminating Klotho activity in Lepob/ob mice that are then crossed with Kl−/− mice significantly reduces body weight in the resulting Kl−/−Lepob/ob double-knockout mice. Similarly, retro peritoneal, mesenteric and epididymal adipose tissue accumulations are significantly lower in the Kl−/−Lepob/ob double-knockout mice than in the Lepob/ob mice, suggesting a potential role for Klotho in the excessive adipose tissue accumulation characteristic of Lepob/ob mice.62 In addition, a high-fat (60%) diet fed to Kl−/− mice did not lead to any gain in body weight compared with a standard-fat (20%) diet.62 The results of this dietary manipulation study suggest that Kl−/− mice are resistant to obesity induced by a high-fat diet.31 With our current level of understanding, it is not possible to explain the human relevance of such experimental observations. Moreover, the reader should bear in mind that animal models of diet-induced obesity are similar to, but might not always exactly mimic, human obesity. The main difference between Lepob/ob mice and human metabolic disorders is that the Lepob/ob mouse is a monogenic experimental model of obesity, while human metabolic disorders usually have multiple contributing factors, including genetic background, environment and dietary habits. Moreover, the homozygous Lep mutation causes early-onset morbid obesity with diabetes mellitus in mice, whereas in humans such onset could happen later in life.
Importantly, reducing obesity via the genetic elimination of Klotho in Lepob/ob mice subsequently crossed with Kl−/− mice also reduced blood glucose levels in the resulting Kl−/−Lepob/ob mice, suggesting that Klotho might influence glucose metabolism as well as adipogenesis (Figure 3).62
Figure 3.
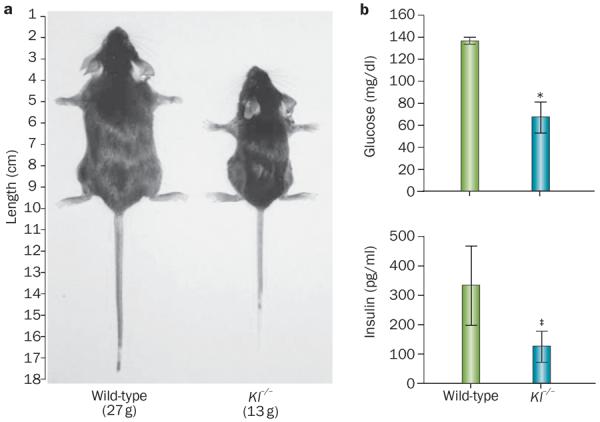
Physiological effects of Klotho inactivation. a | Kl-knockout mice (Kl−/−) are smaller in size and weight than wild-type littermates, partly as a result of a lack of adipose tissue accumulation. b | Kl-knockout mice (Kl−/−) are hypoglycaemic *P <0.001 despite having lower insulin concentrations. ‡P <0.05 than wild-type littermates, indicating that they have increased insulin sensitivity (100 mg/dl glucose = 5.55 mmol/l; 100 pg/ml insulin = 0.02 pmol/l).
Glucose metabolism
The in vivo manipulation of Klotho function affects glucose metabolism;62,72,73Klkl/kl mice have reduced pancreatic insulin content but still develop hypoglycaemia owing to increased insulin sensitivity (Figure 3).72 After insulin injections, blood glucose levels were markedly reduced in the Klkl/kl mice relative to those in wild-type controls.62,72,73
Transgenic mice that overexpress Kl (EFmKL46 and EFmKL48) have biochemical features of insulin resistance.64 Low expression of Klotho in the pancreas of wild-type mice has been reported,103 and whether such expression influences insulin production has not been shown. Furthermore, the hepatic expression of phosphoenolpyruvate carboxykinase, an enzyme that increases gluconeogenesis, was raised in the Klkl/kl mice.73 Non-alcoholic fatty liver disease is considered to be a hepatic manifestation of the metabolic syndrome, and is closely associated with obesity, insulin resistance, T2DM and dyslipidaemia.107–109 Consistent with human studies,107–109 studies in animal models of obesity and T2DM showed features of hepatic fatty changes in those animals that are similar to those seen in Lepob/ob mice (Figure 4).62,110 By contrast, fatty changes were not seen in livers of Kl−/−Lepob/ob mice without Klotho activity. The double-mutant mice had lower fat accumulation in the liver compared with the Lepob/ob mice, which was reflected by reduced blood glucose levels in the double-mutant mice.62
Figure 4.
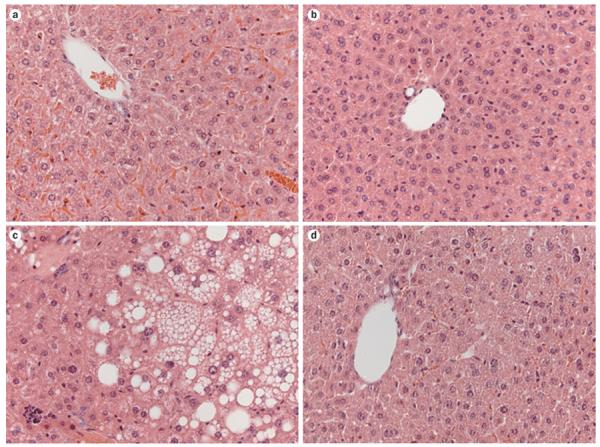
Effects of Klotho on liver structure. Liver sections stained with haematoxylin and eosin (magnification ×40). a | Wild-type mice. b | Kl−/− mice. c | Leptin-deficient Lepob/ob obese mice. d | Kl−/−Lepob/ob double-knockout mice. Steatosis was not seen in liver sections from either of the groups of mice that were lacking Kl.
Despite the intermediate body-weight phenotype of Kl−/−Lepob/ob mice that lacked Klotho activity, hepatic steatosis was completely eliminated.62 Hepatic steatosis is an integral feature of the metabolic syndrome that leads to hepatic insulin resistance; thus, elimination of hepatic steatosis from Kl−/−Lepob/ob mice through suppression of Klotho activity is an important finding that requires further molecular clarification and understanding. Whether the reversal of hepatic steatosis in Kl−/−Lepob/ob mice lacking Klotho activity is a local effect or a systemic consequence is currently unclear. Importantly, Kl−/− mice are resistant to the development of hepatic steatosis induced by a high-fat diet.62
Hepatic glucose synthesis also has a key role in maintaining systemic glucose metabolism. Analyses of glucose tolerance and insulin tolerance show that, compared with Lepob/ob mice, double-mutant Kl−/−Lepob/ob mice have greater insulin sensitivity and better glucose tolerance, which clearly suggests that Klotho has a role in the hyperglycaemia observed in Lepob/ob mice.62 Whether reducing Klotho activity in humans would have beneficial effects similar to those observed in Kl−/−Lepob/ob mice is unclear and warrants further study. A report from the Centers for Disease Control showed that in 2008, approximately 68% of adults with diabetes mellitus were aged 40–64 years at diagnosis, whereas only 17% were diagnosed at age ≥65 years.111 A study of 804 community-dwelling adults showed that serum levels of Klotho decline gradually in individuals aged ≥65 years.112 Whether the reduction in Klotho levels observed in this age group is associated with the markedly reduced incidence of new cases of diabetes mellitus is an important area of future research with clinical and therapeutic importance.
The soluble Klotho protein does not have any effect on IGF-1 production and/or insulin signalling in HEK293, L6 and HepG2 cells,60 and Klotho levels did not correlate with the development of insulin resistance in animal models of metabolic diseases (Wistar rats fed a high-fat diet and obese Zucker rats).60 The mechanisms under lying the potential effects of soluble or membrane-bound Klotho on glucose metabolism require additional experimental clarification. An association between KL genotypes, determined by single nucleotide polymorphism analysis, and fasting levels of either high glucose or low insulin was reported in hospitalized elderly (age >65 years, mean age 79.04 ± 7.14) female patients.113
Phosphate metabolism
Another area that has received very little attention is the association between electrolyte homeostasis (especially of phosphate) and energy metabolism. FGF-23 is a crucial regulator of systemic phosphate metabolism, and requires Klotho to exert its effects.15,19,69,114–120 Serum phosphate levels are reduced through increased urinary excretion, which is induced by FGF-23. The presence of Klotho increases the binding affinity of FGF-23 for its receptors,70,71,121,122 which leads to phosphorylation of FGFR substrate 2, MAPK1 and MAPK2, and activation of downstream signalling events.70,71,121–123 The occurrence of phosphate toxicity in Kl−/− or Klkl/kl mice demonstrates the importance of Klotho in phosphate metabolism in vivo.74,103,124,125 Importantly, mice that lack Phex (which encodes a phosphate-regulating protein), which is homolo gous to the endopeptidase-encoding genes located on the X chromosome, have increased urinary phosphate wasting and severe hypophosphataemia owing to the increased activity of FGF-23.74,126,127 However, the genetic inactivation of Kl in these Phex-mutated mice changed their phenotype to hyperphosphataemia, even though the Kl−/−Phex double-mutant mice have extremely high serum levels of FGF-23, clearly showing an essential requirement of Klotho for in vivo FGF-23 function.17,74 In addition, a point mutation in human KL resulting in a His193Arg amino acid change in a 13-year-old patient with tumoural calcinosis was associated with significantly raised serum levels of phosphate, despite the presence of increased serum levels of FGF-23.45 Our understanding of the critical role of the kidney in the regulation of systemic phosphate metabolism has been improved by the identification of this relationship between FGF-23 and Klotho.19
The kidney also has an important role in maintaining systemic glucose metabolism by influencing gluconeogenesis, partly through controlling glucose filtration and reabsorption.128–130 Further studies are needed to determine whether altered phosphate metabolism can directly affect systemic glucose metabolism and vice-versa. Existing human studies provide some support for this association.131–134 Low serum phosphate levels are associated with reduced insulin activity.135–137 In a study of 298 children and adolescents aged 6–12 years old, in which 190 individuals with obesity and 108 controls without obesity were compared, reduced phosphate serum levels were significantly associated with the development of insulin resistance in children with obesity.138 Moreover, studies in humans show that the glucose infusion rate needed to maintain hyperglycaemia (blood glucose levels 7.0 mmol/l) was 36% lower in individuals with chronic hypophosphataemia but without diabetes mellitus than in controls. Similarly, when exogenous insulin was infused at a constant rate to maintain serum insulin levels at approximately 100 μU/ml above basal levels, the glucose infusion rate required to maintain fasting glucose levels was 43% lower in hypophosphataemic participants than in control individuals.139 These results, therefore, indicate an association between an altered phosphate balance and impaired glucose metabolism in both hyperglycaemic and euglycaemic states.139 Serum phosphate levels were positively correlated with insulin sensitivity (but not with insulin secretion) in a separate study conducted in 881 individuals without diabetes mellitus.140 This correlation was independent of age, sex, proportion of body adipose content, serum calcium and serum creatinine levels.140 Whether low insulin sensitivity and impaired glucose tolerance are the cause or the consequence of hypophosphataemia requires further investigation at the molecular level. Understanding the effects of increased serum phosphate levels on the hypoglycaemic phenotype in Kl-knockout or Kl-knockdown mice could help reveal the effects of phosphate metabolism on glucose metabolism.
Conclusions
The increasing occurrence of obesity and its related complications, including T2DM and cardiovascular anomalies, is alarming and becoming a major public health problem. Over the past decade, numerous in vitro and in vivo experiments have helped to elucidate the various steps of adipose tissue remodelling that are relevant and important to human pathophysiology. Although Klotho expression is restricted to a few organs and circulating levels of this enzyme are quite low, Klotho affects numerous important biological functions, ranging from phosphate metabolism to energy metabolism (Box 2).16,17,19,45,61–81 Dysregulation of these functions has many physiological effects and can lead to disease (Box 1).20–48
The identification of Klotho as a possible adipocyte maturation-promoting factor and its interactions with other known adipogenic factors, such as PPAR-γ and the CCAAT–enhancer binding proteins, suggest a potentially important role for Klotho in adipocyte turnover.13,61 However, additional studies are needed to determine the precise role of Klotho in lipid synthesis. The lean phenotype of the Kl-knockout and Kl-knockdown mice is due to reduced white adipose tissue accumulation, and their resistance to gaining body weight while on a high-fat diet implies a potential in vivo role for Klotho in adipocyte differentiation and maturation. Consistent with this observation, eliminating Klotho activity from obese Lepob/ob mice results in reduced weight gain in double-mutant (Kl−/−Lepob/ob) mice. The in vivo findings that Klotho influences adipose tissue generation and glucose metabolism are likely to serve as the basis for further studies to delineate the biological and therapeutic importance of this enzyme in the control of energy homeostasis.
At present, however, whether Klotho affects the functionality of β-Klotho (which influences energy metabolism by mediating the functions of FGF-19 and FGF-21) is not clearly understood.14,51–53,93 Dissociating the in vivo functions of Klotho and β-Klotho will be a challenging task, but one that could have important clinical benefits, such as enabling the development of therapies to combat obesity and its related complications.
Key points
-
■
Klotho exists in membrane-bound and secreted forms; the secreted form is generated both by alternative splicing and by shedding of the extracellular domain of the transmembrane form
-
■
Klotho increases organ-specific FGF-23 function in the systemic regulation of phosphate metabolism in the kidney
-
■
Klotho might also have a role in energy metabolism
-
■
Reducing or eliminating Klotho activity in mice results in reduced white adipose tissue accumulation
-
■
Kl-mutant mice (both Klkl/kl and Kl−/−) are hypoglycaemic (owing to increased insulin sensitivity) and resistant to obesity induced by a high-fat diet
-
■
Reducing or eliminating Klotho activity from Kl−/−Lepob/ob mice results in mice with decreased obesity and increased insulin sensitivity
Box 1 | Klotho in human physiology and disease.
Physiological processes linked to Klotho
Diseases linked to Klotho
Diseases and physiological factors lacking any confirmed association with Klotho
Box 2 | Functions of Klotho16,17,19,45,61–81.
- ■
-
■
Angiogenesis63
-
■
Antiageing effects64
-
■
Antioxidant effects65
- ■
- ■
- ■
-
■
Insulin signaling64
- ■
-
■
Potassium metabolism76
The above functions were documented in experimental studies.
*The role of Klotho in FGF-23 signalling has also been validated in human studies.45,77–79
Acknowledgements
M. S. Razzaque thanks several members of his research group at the Harvard School of Dental Medicine for technical assistance: Mutsuko Ohnishi, Junko Akiyoshi, Satoko Osuka, Yongguen Hong, Khadijah Turkistani, Ismail Eddafali and Basel Karzoun. Thanks also to Syed Rafi for critically reading the manuscript. M. S. Razzaque acknowledges grant R01-DK077276 from the National Institute of Diabetes and Digestive and Kidney Diseases and institutional support from the Harvard School of Dental Medicine.
Footnotes
Competing interests The author declares no competing interests.
References
- 1.Pan JJ, et al. Prevalence of metabolic syndrome and risks of abnormal serum alanine aminotransferase in Hispanics: a population-based study. PLoS ONE. 2011;6:e21515. doi: 10.1371/journal.pone.0021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin WY, et al. Body mass index and all-cause mortality in a large Chinese cohort. CMAJ. 2011;183:E329–E336. doi: 10.1503/cmaj.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CG, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J. Am. Geriatr. Soc. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon YS, Park HS, Yun KE, Kim SB. Obesity and metabolic syndrome-related chronic kidney disease in nondiabetic, nonhypertensive adults. Metabolism. 2009;58:1737–1742. doi: 10.1016/j.metabol.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Kissebah AH, Freedman DS, Peiris AN. Health risks of obesity. Med. Clin. North Am. 1989;73:111–138. doi: 10.1016/s0025-7125(16)30695-2. [DOI] [PubMed] [Google Scholar]
- 6.Sarafidis PA. Obesity, insulin resistance and kidney disease risk: insights into the relationship. Curr. Opin. Nephrol. Hypertens. 2008;17:450–456. doi: 10.1097/MNH.0b013e328305b994. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, et al. Obesity-associated hypertension and kidney disease. Curr. Opin. Nephrol. Hypertens. 2003;12:195–200. doi: 10.1097/00041552-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Garg A. Acquired and inherited lipodystrophies. N. Engl. J. Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 9.Mokdad AH, Marks JD, Stroup DF, Gerberding JL. Actual causes of death in the United States. JAMA. 2004;29:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 10.Berger J, Moller DE. The mechanism of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 11.Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell. Res. 2010;20:124–137. doi: 10.1038/cr.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai M, Rosen CJ. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, et al. Klotho is a target gene of PPAR-γ. Kidney Int. 2008;74:732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 14.Kuro-o M. Endocrine FGFs and Klothos: emerging concepts. Trends Endocrinol. Metab. 2008;19:239–245. doi: 10.1016/j.tem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Razzaque MS. Phosphate toxicity: new insights into an old problem. Clin. Sci. (Lond.) 2011;120:91–97. doi: 10.1042/CS20100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Zheng F. PPAR-γ and aging: one link through Klotho? Kidney Int. 2008;74:702–704. doi: 10.1038/ki.2008.382. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am. J. Physiol. Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger RH. Klotho-induced insulin resistance: a blessing in disguise? Nat. Med. 2006;12:56–57. doi: 10.1038/nm0106-56. [DOI] [PubMed] [Google Scholar]
- 19.Razzaque MS. The FGF23–Klotho axis: endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arking DE, et al. Association of human aging with a functional variant of Klotho. Proc. Natl Acad. Sci. USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the Klotho gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ. Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 22.Nzietchueng R, et al. Klotho KL-VS genotype is involved in blood pressure regulation. Clin. Chim. Acta. 2011;412:1773–1777. doi: 10.1016/j.cca.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Shimoyama Y, Nishio K, Hamajima N, Niwa T. Klotho gene polymorphisms G-395A and C1818T are associated with lipid and glucose metabolism, bone mineral density and systolic blood pressure in Japanese healthy subjects. Clin. Chim. Acta. 2009;406:134–138. doi: 10.1016/j.cca.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Oguro R, et al. Association of carotid atherosclerosis with genetic polymorphisms of the Klotho gene in patients with hypertension. Geriatr. Gerontol. Int. 2010;10:311–318. doi: 10.1111/j.1447-0594.2010.00612.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang HL, et al. A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin. Chim. Acta. 2010;411:386–390. doi: 10.1016/j.cca.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Riancho JA, et al. Association of the F352V variant of the Klotho gene with bone mineral density. Biogerontology. 2007;8:121–127. doi: 10.1007/s10522-006-9039-5. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of the androgen receptor and Klotho genes with bone mineral density in Japanese women. J. Mol. Med. 2005;83:50–57. doi: 10.1007/s00109-004-0578-4. [DOI] [PubMed] [Google Scholar]
- 28.Zarrabeitia MT, et al. Klotho gene polymorphism and male bone mass. Calcif. Tissue Int. 2007;80:10–14. doi: 10.1007/s00223-006-0233-x. [DOI] [PubMed] [Google Scholar]
- 29.Ogata N, et al. Association of Klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–42. doi: 10.1016/s8756-3282(02)00786-x. [DOI] [PubMed] [Google Scholar]
- 30.Kawano K, et al. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J. Bone Miner. Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- 31.Rhee EJ, et al. Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the Klotho gene with glucose metabolism and cardiovascular risk factors in Korean women. J. Endocrinol. Invest. 2006;29:613–618. doi: 10.1007/BF03344160. [DOI] [PubMed] [Google Scholar]
- 32.Shimoyama Y, et al. Klotho gene polymorphisms G-395A and C1818T are associated with low-density lipoprotein cholesterol and uric acid in Japanese hemodialysis patients. Am. J. Nephrol. 2009;30:383–388. doi: 10.1159/000235686. [DOI] [PubMed] [Google Scholar]
- 33.Wolf I, et al. Functional variant of Klotho: a breast cancer risk modifier among BRCA1 mutation carriers of Ashkenazi origin. Oncogene. 2010;29:26–33. doi: 10.1038/onc.2009.301. [DOI] [PubMed] [Google Scholar]
- 34.Arking DE, et al. Klotho allele status and the risk of early-onset occult coronary artery disease. Am. J. Hum. Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee EJ, et al. The differential effects of age on the association of Klotho gene polymorphisms with coronary artery disease. Metabolism. 2006;55:1344–1351. doi: 10.1016/j.metabol.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Jo SH, et al. Klotho gene polymorphism is associated with coronary artery stenosis but not with coronary calcification in a Korean population. Int. Heart J. 2009;50:23–32. doi: 10.1536/ihj.50.23. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, et al. Klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci. Lett. 2006;407:189–194. doi: 10.1016/j.neulet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 38.Majumdar V, Nagaraja D, Christopher R. Association of the functional KL-VS variant of Klotho gene with early-onset ischemic stroke. Biochem. Biophys. Res. Commun. 2010;403:412–416. doi: 10.1016/j.bbrc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 39.Telci D, et al. Klotho gene polymorphism of G395A is associated with kidney stones. Am. J. Nephrol. 2011;33:337–343. doi: 10.1159/000325505. [DOI] [PubMed] [Google Scholar]
- 40.Friedman DJ, et al. Klotho variants and chronic hemodialysis mortality. J. Bone Miner. Res. 2009;24:1847–1855. doi: 10.1359/JBMR.090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, et al. Polymorphism in the promoter region of the Kotho gene (G.-395A) is associated with early dysfunction in vascular access in hemodialysis patients. Korean J. Intern. Med. 2008;23:201–207. doi: 10.3904/kjim.2008.23.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, et al. Association between Klotho gene and hand osteoarthritis in a female Caucasian population. Osteoarthritis Cartilage. 2007;15:624–629. doi: 10.1016/j.joca.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Brownstein CA, et al. A translocation causing increased α-Klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl Acad. Sci. USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nolan VG, et al. Association of single nucleotide polymorphisms in Klotho with priapism in sickle cell anaemia. Br. J. Haematol. 2005;128:266–272. doi: 10.1111/j.1365-2141.2004.05295.x. [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa S, et al. A homozygous missense mutation in human Klotho causes severe tumoral calcinosis. J. Clin. Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tangri N, Alam A, Wooten EC, Huggins GS. Lack of association of Klotho gene variants with valvular and vascular calcification in Caucasians: a candidate gene study of the Framingham Offspring Cohort. Nephrol. Dial. Transplant. 2011;26:3998–4002. doi: 10.1093/ndt/gfr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F, et al. Lack of association between leukocyte telomere length and genetic variants in two ageing-related candidate genes. Mech. Ageing Dev. 2007;128:415–422. doi: 10.1016/j.mad.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Freathy RM, et al. The functional “KL-VS” variant of Klotho is not associated with type 2 diabetes in 5028 UK Caucasians. BMC Med. Genet. 2006;7:51. doi: 10.1186/1471-2350-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kharitonenkov A. FGFs and metabolism. Curr. Opin. Pharmacol. 2009;9:805–810. doi: 10.1016/j.coph.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Kurosu H, Kuro-o M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol. Cell Endocrinol. 2008;299:72–78. doi: 10.1016/j.mce.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 51.Long YC, Kharitonenkov A. Hormone-like fibroblast growth factors and metabolic regulation. Biochim. Biophys. Acta. 2011;1812:791–795. doi: 10.1016/j.bbadis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Itoh N. Hormone-like (endocrine) FGFs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010;342:1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha J, et al. β-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G996–G1003. doi: 10.1152/ajpgi.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Li Y. Role of FGF19 induced FGFR4 activation in the regulation of glucose homeostasis. Aging (Albany NY) 2009;1:1023–1027. doi: 10.18632/aging.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nabeshima Y. The discovery of α-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis. Cell. Mol. Life Sci. 2008;65:3218–3230. doi: 10.1007/s00018-008-8177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nabeshima Y, Imura H. α-Klotho: a regulator that integrates calcium homeostasis. Am. J. Nephrol. 2008;28:455–464. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- 57.Tohyama O, et al. Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J. Biol. Chem. 2004;279:9777–9784. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 58.Nabeshima Y. Discovery of α-Klotho unveiled new insights into calcium and phosphate homeostasis. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 2009;85:125–141. doi: 10.2183/pjab/85.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumura Y, et al. Identification of the human Klotho gene and its two transcripts encoding membrane and secreted Klotho protein. Biochem. Biophys. Res. Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 60.Lorenzi O, et al. Evidence against a direct role of Klotho in insulin resistance. Pflugers Arch. 2010;459:465–473. doi: 10.1007/s00424-009-0735-2. [DOI] [PubMed] [Google Scholar]
- 61.Chihara Y, et al. Klotho protein promotes adipocyte differentiation. Endocrinology. 2006;147:3835–3842. doi: 10.1210/en.2005-1529. [DOI] [PubMed] [Google Scholar]
- 62.Ohnishi M, Kato S, Akiyoshi J, Atfi A, Razzaque MS. Dietary and genetic evidence for enhancing glucose metabolism and reducing obesity by inhibiting Klotho functions. FASEB J. 2011;25:2031–2039. doi: 10.1096/fj.10-167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimada T, et al. Angiogenesis and vasculogenesis are impaired in the precociousaging Klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 64.Kurosu H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto M, et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J. Biol. Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imura A, et al. α-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 67.Razzaque MS. Klotho and Na+, K+-ATPase activity: solving the calcium metabolism dilemma? Nephrol. Dial. Transplant. 2008;23:459–461. doi: 10.1093/ndt/gfm702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang Q, et al. The β-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 69.Razzaque MS. Osteo-renal regulation of systemic phosphate metabolism. IUBMB Life. 2011;63:240–247. doi: 10.1002/iub.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by Klotho. J. Biol. Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 72.Utsugi T, et al. Decreased insulin production and increased insulin sensitivity in the Klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–1123. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 73.Mori K, et al. Disruption of Klotho gene causes an abnormal energy homeostasis in mice. Biochem. Biophys. Res. Commun. 2000;278:665–670. doi: 10.1006/bbrc.2000.3864. [DOI] [PubMed] [Google Scholar]
- 74.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of Klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakatani T, et al. In vivo genetic evidence for Klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cha SK, et al. Regulation of renal outer medullary potassium channel and renal K+ excretion by Klotho. Mol. Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 78.Shimada T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonsson KB, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 80.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr. Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang CL. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2010;77:855–860. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 82.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 83.Forster RE, et al. Vitamin D receptor controls expression of the anti-aging Klotho gene in mouse and human renal cells. Biochem. Biophys. Res. Commun. 2011;414:557–562. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito S, et al. Molecular cloning and expression analyses of mouse β-Klotho, which encodes a novel Klotho family protein. Mech. Dev. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 85.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking β-Klotho. J. Clin. Invest. 2005;115:2202–2208. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu C, et al. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 2000;275:15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 87.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell. Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Kurosu H, et al. Tissue-specific expression of β-Klotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogawa Y, et al. β-Klotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kharitonenkov A, Shanafelt AB. Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs. 2008;22:37–44. doi: 10.2165/00063030-200822010-00004. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki M, et al. β-Klotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hotta Y, et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 93.Tomiyama K, et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl Acad. Sci. USA. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPAR-α and is a key mediator of hepatic lipid metabolism in ketotic states. Cell. Metab. 2007;5:426–347. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARγ F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 97.Francis GA, et al. Peroxisomal proliferator activated receptor-γ deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3) BMC Med. Genet. 2006;7:3. doi: 10.1186/1471-2350-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamagishi T, et al. Troglitazone improves endothelial function and augments renal Klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertens. Res. 2001;24:705–709. doi: 10.1291/hypres.24.705. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Sun Z. Current understanding of Klotho. Ageing Res. Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Min D, et al. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamaza T, et al. Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood. 2009;113:2595–2604. doi: 10.1182/blood-2008-10-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Izbeki F, et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J. Physiol. 2010;588:3101–3117. doi: 10.1113/jphysiol.2010.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuro-o M, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 104.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of Klotho knockout mice by deletion of vitamin D 1α-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin D levels. Circ. Cardiovasc. Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver—the link between adipocytes and hepatocytes. Digestion. 2011;83:124–133. doi: 10.1159/000318741. [DOI] [PubMed] [Google Scholar]
- 109.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat. Rev. Endocrinol. 2011;7:456–465. doi: 10.1038/nrendo.2011.72. [DOI] [PubMed] [Google Scholar]
- 110.Haluzik M, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 111.US Department of Health and Human Services CDC Diabetes Data and Trends. 2008 [online] http://www.cdc.gov/diabetes/statistics/age/fig1.htm.
- 112.Semba RD, et al. Plasma Klotho and mortality risk in older community-dwelling adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:794–800. doi: 10.1093/gerona/glr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paroni G, et al. Klotho locus, metabolic traits, and serum hemoglobin in hospitalized older patients: a genetic association analysis. Age (Dordr.) doi: 10.1007/s11357-011-9273-x. http://dx.doi.org/10.1007/s11357-011-9273-x. [DOI] [PMC free article] [PubMed]
- 114.Razzaque MS. Therapeutic potential of Klotho-FGF23 fusion polypeptides: WO2009095372. Expert Opin. Ther. Pat. 2010;20:981–985. doi: 10.1517/13543771003774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng CY, Kuro-o M, Razzaque MS. Molecular regulation of phosphate metabolism by fibroblast growth factor-23–Klotho system. Adv. Chronic Kidney Dis. 2011;18:91–97. doi: 10.1053/j.ackd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Berndt T, Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda) 2009;24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J. Nephrol. 2010;23(Suppl. 16):S136–S144. [PMC free article] [PubMed] [Google Scholar]
- 119.Razzaque MS. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol. Dial. Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goetz R, et al. Molecular insights into the Klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goetz R, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR–Klotho complex formation. Proc. Natl Acad. Sci. USA. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Medici D, et al. FGF-23–Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J. Cell Biol. 2008;182:459–465. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osuka S, Razzaque MS. Can features of phosphate toxicity appear in normophosphatemia? J. Bone Miner. Metab. 2012;30:10–18. doi: 10.1007/s00774-011-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohnishi M, Kato S, Razzaque MS. Genetic induction of phosphate toxicity significantly reduces the survival of hypercholesterolemic obese mice. Biochem. Biophys. Res. Commun. 2011;415:434–438. doi: 10.1016/j.bbrc.2011.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu S, et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 127.Sitara D, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am. J. Kidney Dis. 2009;53:875–883. doi: 10.1053/j.ajkd.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 129.Mather A, Pollock C. Glucose handling by the kidney. Kidney Int. 2011;(Suppl.):S1–S6. doi: 10.1038/ki.2010.509. [DOI] [PubMed] [Google Scholar]
- 130.Mitrakou A. Kidney: its impact on glucose homeostasis and hormonal regulation. Diabetes Res. Clin. Pract. 2011;93(Suppl. 1):S66–S72. doi: 10.1016/S0168-8227(11)70016-X. [DOI] [PubMed] [Google Scholar]
- 131.Ravenscroft AJ, Valentine JM, Knappett PA. Severe hypophosphataemia and insulin resistance in diabetic ketoacidosis. Anaesthesia. 1999;54:198. doi: 10.1046/j.1365-2044.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 132.Paula FJ, Plens AE, Foss MC. Effects of hypophosphatemia on glucose tolerance and insulin secretion. Horm. Metab. Res. 1998;30:281–284. doi: 10.1055/s-2007-978884. [DOI] [PubMed] [Google Scholar]
- 133.Bohannon NJ. Large phosphate shifts with treatment for hyperglycemia. Arch. Intern. Med. 1989;149:1423–1425. [PubMed] [Google Scholar]
- 134.Kim H, Kalkhoff RK, Costrini NV, Cerletty JM, Jacobson M. Plasma insulin disturbances in primary hyperparathyroidism. J. Clin. Invest. 1971;50:2596–2605. doi: 10.1172/JCI106760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fadda GZ, Massry SG. Impaired glucose-induced calcium signal in pancreatic islets in chronic renal failure. Am. J. Nephrol. 1991;11:475–478. doi: 10.1159/000168362. [DOI] [PubMed] [Google Scholar]
- 136.Simonson D, DeFronzo RA. Hypophosphatemia and glucose intolerance. Adv. Exp. Med. Biol. 1982;151:217–228. doi: 10.1007/978-1-4684-4259-5_28. [DOI] [PubMed] [Google Scholar]
- 137.Zhou XJ, Fadda GZ, Perna AF, Massry SG. Phosphate depletion impairs insulin secretion by pancreatic islets. Kidney Int. 1991;39:120–128. doi: 10.1038/ki.1991.15. [DOI] [PubMed] [Google Scholar]
- 138.Celik N, Andiran N. The relationship between serum phosphate levels with childhood obesity and insulin resistance. J. Pediatr. Endocrinol. Metab. 2011;24:81–83. doi: 10.1515/jpem.2011.116. [DOI] [PubMed] [Google Scholar]
- 139.DeFronzo RA, Lang R. Hypophosphatemia and glucose intolerance: evidence for tissue insensitivity to insulin. N. Engl. J. Med. 1980;303:1259–1263. doi: 10.1056/NEJM198011273032203. [DOI] [PubMed] [Google Scholar]
- 140.Haap M, et al. Association of serum phosphate levels with glucose tolerance, insulin sensitivity and insulin secretion in non-diabetic subjects. Eur. J. Clin. Nutr. 2006;60:734–739. doi: 10.1038/sj.ejcn.1602375. [DOI] [PubMed] [Google Scholar]


