Abstract
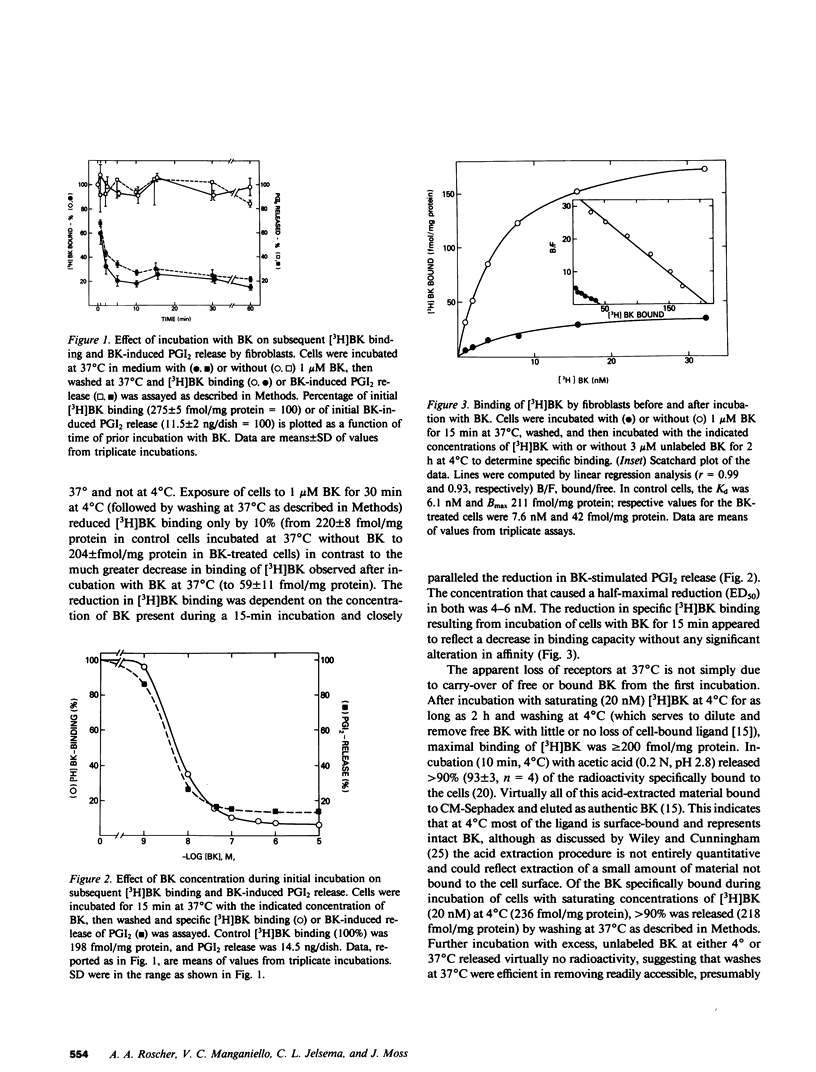
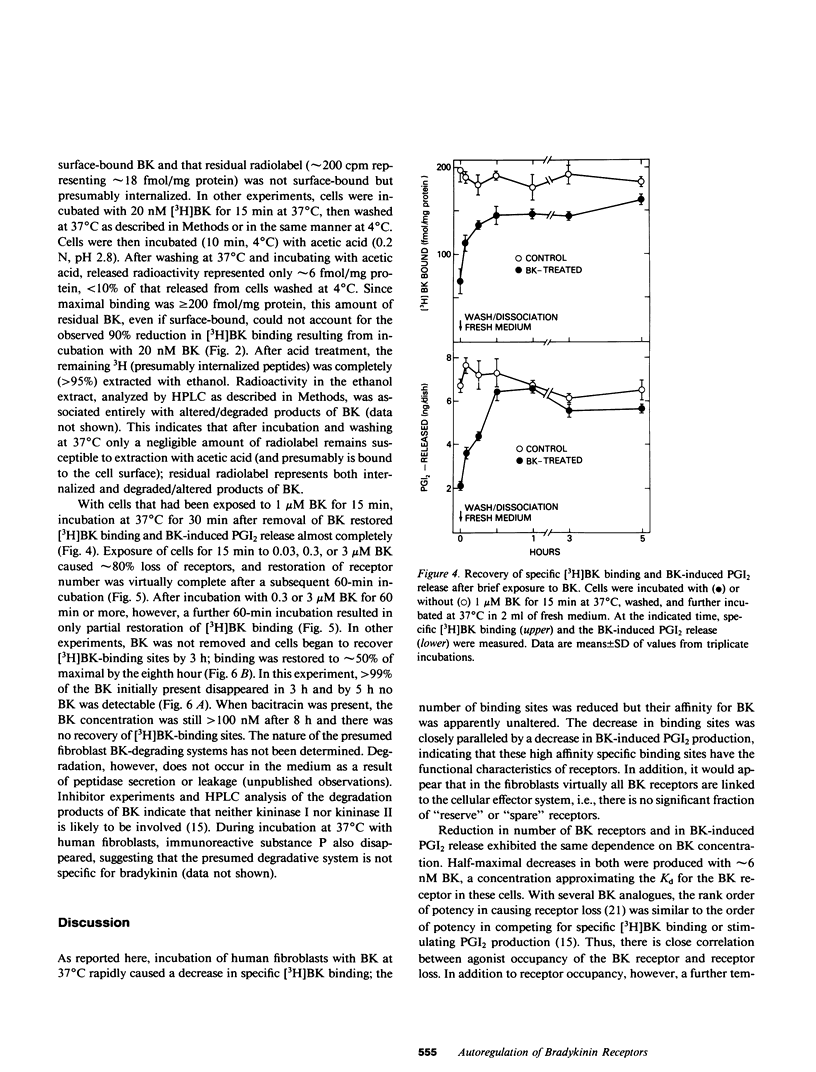
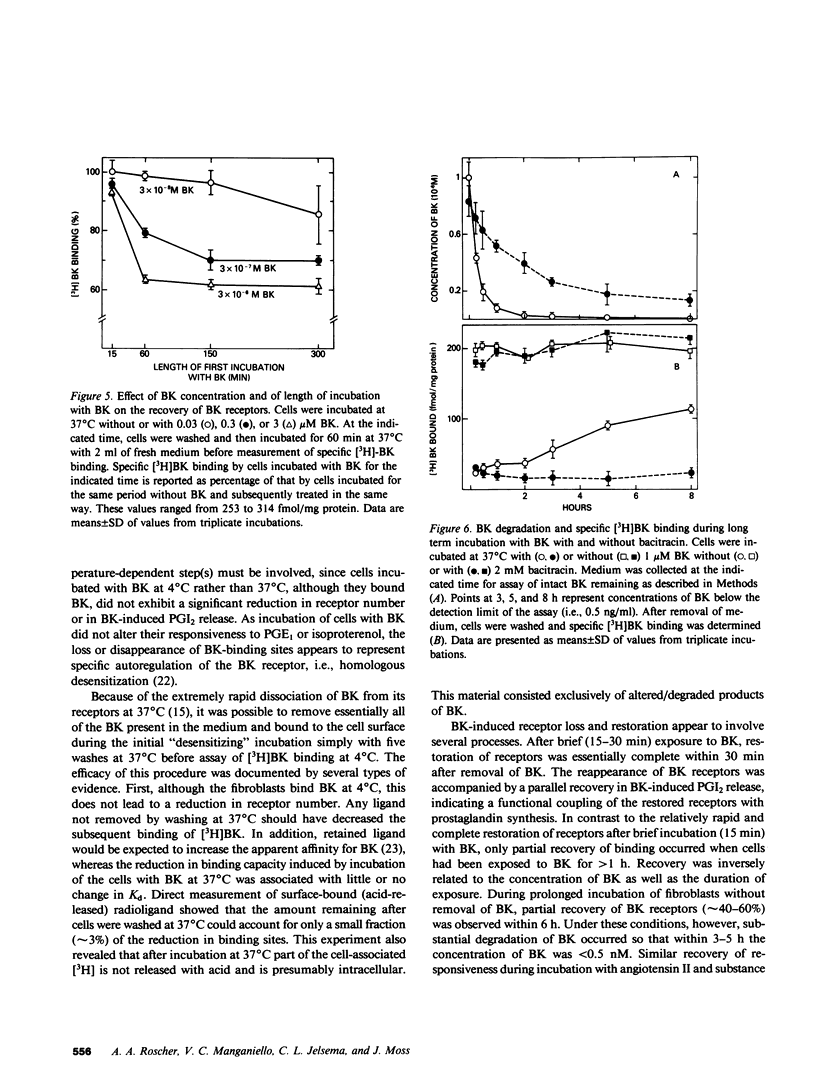
The interaction of bradykinin (BK) with its specific receptors on intact cultured human fibroblasts results in production of prostaglandins, including prostacyclin (PGI2), and accumulation of cyclic AMP. Incubation of cells with 1 microM BK for 5 min at 37 degrees C led to a marked reduction (75-90%) in BK-induced PGI2 release and in total number of [3H]BK-binding sites with no change in dissociation constant (6.1 and 7.6 nM for control and BK-treated cells, respectively). The decrease in receptor number did not result from BK transferred from the first incubation into the binding assay. BK-induced receptor loss was temperature dependent; exposure of cells to BK at 4 degrees C had little or no effect on receptor number. After incubation with BK for approximately equal to 15 min, further incubation in the absence of BK for 30 min at 37 degrees C almost completely restored both receptor number and BK-induced PGI2 release. With more prolonged exposure to BK (greater than 1 h), restoration of receptors was inversely related to the length of exposure and the concentration of BK. Recovery was unaffected by cycloheximide. During prolonged incubation without removal of BK, cells began to recover receptors by 5 h; greater than 99% of the bradykinin initially present disappeared by 3 h. Bacitracin greatly retarded BK disappearance and totally prevented recovery. These observations provide direct evidence that the number of BK receptors on cultured human fibroblasts can be regulated by BK itself. In addition, it appears that BK-degrading systems, by influencing local concentrations of the peptide, may play an important role in the autoregulation of BK receptors. The presence of highly active degradation systems might serve to protect target tissues from developing chronic insensitivity to BK and, perhaps, similar peptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bareis D. L., Manganiello V. C., Hirata F., Vaughan M., Axelrod J. Bradykinin stimulates phospholipid methylation, calcium influx, prostaglandin formation, and cAMP accumulation in human fibroblasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2514–2518. doi: 10.1073/pnas.80.9.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Baenziger N. L., Majerus P. W. Bradykinin-stimulated release of arachidonate from phosphatidyl inositol in mouse fibrosarcoma cells. Prostaglandins. 1980 Aug;20(2):269–274. doi: 10.1016/s0090-6980(80)80045-1. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Corrêa F. M., Innis R. B., Uhl G. R., Snyder S. H. Bradykinin-like immunoreactive neuronal systems localized histochemically in rat brain. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1489–1493. doi: 10.1073/pnas.76.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Margolius H. S. Kinins stimulate net chloride secretion by the rat colon. Br J Pharmacol. 1982 Apr;75(4):587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J. N., St-Pierre S. A., Regoli D. Receptors for bradykinin and kallidin. Can J Physiol Pharmacol. 1979 Apr;57(4):375–379. doi: 10.1139/y79-056. [DOI] [PubMed] [Google Scholar]
- Fahey J. V., Ciosek C. P., Jr, Newcombe D. S. Human synovial fibroblasts: the relationships between cyclic AMP, bradykinin, and prostaglandins. Agents Actions. 1977 Jul;7(2):255–264. doi: 10.1007/BF01969984. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Fan J. Y., Orci L. Receptor mediated endocytosis of polypeptide hormones: mechanism and significance. Metabolism. 1982 Jul;31(7):664–669. doi: 10.1016/0026-0495(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hong S. L., Levine L. Stimulation of prostaglandin synthesis by bradykinin and thrombin and their mechanisms of action on MC5-5 fibroblasts. J Biol Chem. 1976 Sep 25;251(18):5814–5816. [PubMed] [Google Scholar]
- Jaffe B. M., Smith J. W., Newton W. T., Parker C. W. Radioimmunoassay for prostaglandins. Science. 1971 Feb 5;171(3970):494–496. doi: 10.1126/science.171.3970.494. [DOI] [PubMed] [Google Scholar]
- Juan H., Lembeck F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn Schmiedebergs Arch Pharmacol. 1974;283(2):151–164. doi: 10.1007/BF00501142. [DOI] [PubMed] [Google Scholar]
- Karliner J. S., Motulsky H. J., Insel P. A. Apparent "down-regulation" of human platelet alpha 2-adrenergic receptors is due to retained agonist. Mol Pharmacol. 1982 Jan;21(1):36–43. [PubMed] [Google Scholar]
- Lefkowitz R. J., Wessels M. R., Stadel J. M. Hormones, receptors, and cyclic AMP: their role in target cell refractoriness. Curr Top Cell Regul. 1980;17:205–230. doi: 10.1016/b978-0-12-152817-1.50011-0. [DOI] [PubMed] [Google Scholar]
- Manganiello V. C., Breslow J. Effects of prostaglandin E1 and isoproterenol on cyclic AMP content of human fibroblasts modified by time and cell density in subculture. Biochim Biophys Acta. 1974 Oct 8;362(3):509–520. doi: 10.1016/0304-4165(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Manning D. C., Snyder S. H., Kachur J. F., Miller R. J., Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982 Sep 16;299(5880):256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- McGiff J. C., Itskovitz H. D., Terragno A., Wong P. Y. Modulation and mediation of the action of the renal kallikrein-kinin system by prostaglandins. Fed Proc. 1976 Feb;35(2):175–180. [PubMed] [Google Scholar]
- Newcombe D. S., Fahey J. V., Ishikawa Y. Hydrocortisone inhibition of the bradykinin activation of human synovial fibroblasts. Prostaglandins. 1977 Feb;13(2):235–244. doi: 10.1016/0090-6980(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Roscher A. A., Manganiello V. C., Jelsema C. L., Moss J. Receptors for bradykinin in intact cultured human fibroblasts. Identification and characterization by direct binding study. J Clin Invest. 1983 Aug;72(2):626–635. doi: 10.1172/JCI111012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscher A. A., Manganiello V. C., Jelsema C. L., Moss J., Vaughan M. Regulation by bradykinin of its receptor and of receptor-mediated prostacyclin formation in cultured human fibroblasts. Trans Assoc Am Physicians. 1983;96:175–181. [PubMed] [Google Scholar]
- Stoner J., Manganiello V. C., Vaughan M. Effects of bradykinin and indomethacin on cyclic GMP and cyclic AMP in lung slices. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3830–3833. doi: 10.1073/pnas.70.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeten D. H., Kerr C. B., Kerr L. P., Prior J. C., Dalakos T. G. Hyperbradykininism: a new orthostatic syndrome. Lancet. 1972 Nov 18;2(7786):1048–1053. doi: 10.1016/s0140-6736(72)92337-9. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- Williams G. H., Hollenberg N. K. Accentuated vascular and endocrine response to SQ 20881 in hypertension. N Engl J Med. 1977 Jul 28;297(4):184–188. doi: 10.1056/NEJM197707282970404. [DOI] [PubMed] [Google Scholar]
- Yasujima M., Matthews P. G., Johnston C. I. Regulation of uterine smooth muscle bradykinin receptors by bradykinin levels and angiotensin converting enzyme inhibitor in the rat. Clin Exp Pharmacol Physiol. 1981 Sep-Oct;8(5):515–518. doi: 10.1111/j.1440-1681.1981.tb00759.x. [DOI] [PubMed] [Google Scholar]


