Abstract
Impaired O2 transport to skeletal muscle potentially contributes to the decline in aerobic capacity with aging. Thus, we examined whether (1) skeletal muscle oxidative capacity decreases with age and (2) O2 availability or mitochondrial capacity limits the maximal rate of mitochondrial ATP synthesis in vivo in sedentary elderly individuals. We used 31P-magnetic resonance spectroscopy (31P-MRS) to examine the PCr recovery kinetics in six young (26 ± 10 years) and six older (69 ± 3 years) sedentary subjects following 4 min of dynamic plantar flexion exercise under different fractions of inspired O2 (FiO2, normoxia 0.2; hyperoxia 1.0). End-exercise pH was not significantly different between old (7.04 ± 0.10) and young (7.05 ± 0.04) and was not affected by breathing hyperoxia (old 7.08 ± 0.08, P > 0.05 and young 7.05 ± 0.03). Likewise, end-exercise PCr was not significantly different between old (19 ± 4 mM) and young (24 ± 5 mM) and was not changed in hyperoxia. The PCr recovery time constant was significantly longer in the old (36 ± 9 s) compared to the young in normoxia (23 ± 8 s, P < 0.05) and was not significantly altered by breathing hyperoxia in both the old (35 ± 9 s) and young (29 ± 10 s) groups. Therefore, this study reveals that the muscle oxidative capacity of both sedentary young and old individuals is independent of O2 availability and that the decline in oxidative capacity with age is most likely due to limited mitochondrial content and/or mitochondrial dysfunction and not O2 availability.
Keywords: 31P-MRS, PCr recovery, Muscle energetics, O2 availability, Mitochondrial capacity, Aging
Introduction
Reductions in exercise tolerance and maximal O2 consumption during whole-body exercise are among the most consistent hallmarks of aging (Poole et al. 2006). Although the mechanistic basis for this age-related decline in physical performance is complex, alterations in the capacity to transport, exchange, and utilize O2 are believed to play a major role. Indeed, it is well known that aging is associated with reduced maximal heart rate (Fleg et al. 1995) and cardiac output (Hossack and Bruce 1982), as well as peripheral hemodynamic alterations such as reduced limb blood flow, and vascular conductance (Lawrenson et al. 2003; Proctor and Parker 2006; Russell et al. 2003). In combination, these age-related changes likely alter both convective and diffusive components of O2 transport to skeletal muscle and contribute to a reduction in exercise capacity (Poole et al. 2003).
Unlike O2 transport, the question whether skeletal muscle oxidative capacity itself declines as a consequence of aging is still unclear. For instance, several studies have documented that in vivo measurements of PCr recovery rate, an index of muscle oxidative capacity, using 31P magnetic resonance spectroscopy (31P-MRS) was either slowed (Taylor et al. 1997; McCully et al. 1993; Conley et al. 2000) or similar (Wray et al. 2009a; Lanza et al. 2005; Kent-Braun and Ng 2000) in young and elderly subjects, suggesting impaired or preserved muscle oxidative capacity with age. In light of these studies, it is interesting to note that PCr recovery kinetics can be very sensitive to O2 availability (Haseler et al. 1999; Haseler et al. 2007) and, likely as a consequence of the aforementioned reduction in O2 transport, the postexercise O2 partial pressure in the microvasculature appears to be lower with age (Hirai et al. 2009). Therefore, in terms of in vivo assessment of muscle oxidative capacity, questions remain as to the relative role of O2 supply and the capacity of the mitochondria to generate ATP adequately in older subjects. Interestingly, the combination of PCr recovery measurements with 31P-MRS under different fractions of inspired O2 (FiO2) has previously been successfully applied to differentiate O2 supply limitation from the limited mitochondrial capacity to generate ATP in young sedentary and exercise-trained subjects (Haseler et al. 1999, 2007) and could, therefore, provide new insight into the role of these potential limitations with age.
Based upon previous research, there are two equally plausible hypotheses regarding the role of O2 supply and demand in limiting metabolic capacity in the elderly. Specifically, as O2 supply appears to slow pulmonary O2 consumption kinetics at the onset of contractions in older individuals (DeLorey et al. 2007), it could be hypothesized that O2 availability will also limit the recovery kinetics of PCr. Therefore, unlike their sedentary younger counterparts (Haseler et al. 2004), an improved O2 availability induced by breathing a hyperoxic gas mixture could accelerate the PCr recovery time constant in the older sedentary subjects. Alternatively, it could be hypothesized that, if despite the reduction in O2 transport, intracellular PO2 in older adults is maintained above a critical value (Wilson et al. 1977; Haseler et al. 2007), PCr recovery kinetics could be limited by mitochondrial capacity to generate ATP such that hyperoxia will not affect the PCr recovery time constant. The validity of these two, quite different, conceptual scenarios has yet to be tested.
Therefore, the purpose of the present study was to utilize 31P-MRS to explore skeletal muscle PCr recovery kinetics following plantar flexion exercise in both normoxia (FiO2 0.21) and hyperoxia (FiO2 1.0) in a group of old and young sedentary subjects. Altough the literature is mixed on this topic, we hypothesized that (1) PCr recovery time constant in normoxia will be slower in the elderly compared to young sedentary individuals and (2) based upon previous work from our group, revealing substantial metabolic reserve in young sedentary individuals (Haseler et al. 2007), hyperoxia will not alter PCr recovery in either young or old sedentary subjects. This would indicate that the decline in oxidative capacity with age is most likely a consequence of limited mitochondrial content and/or mitochondrial dysfunction rather than O2 availability.
Method
Subjects
Six young (mean ± SD, age 26 ± 10 years; height, 176 ± 6 cm; weight, 76 ± 4 kg) and six older male subjects (age 69 ± 3 years; height, 180 ± 3 cm; weight, 83 ± 4 kg) volunteered to participate in this study and gave written informed consent. The subjects were recruited based upon no evidence of regular or occasional physical activity above that required for activities of daily living (self-report and interview). All the subjects included in the young and older groups were male, nonsmoker, and free of diabetes and known cardiovascular, peripheral vascular, neuromuscular, or pulmonary disease. Additionally, all the subjects were not taking any medications known to affect muscle function or blood flow. The study was approved by the Human Research Protection Program of the University of California, San Diego.
Exercise protocol
Subjects were familiarized with supine plantar flexion exercise in the whole-body MRI system (GE 1.5 T Medical Systems, Milwaukee, WI). Individual maximum work rate (WRmax) was determined by performing incremental dynamic plantar flexion exercise until exhaustion. On a separate day, subjects performed constant-load submaximal plantar flexion at ~50 % of WRmax (frequency of 1 Hz) under condition of normoxia (FiO2 0.21) or hyperoxia (FiO2 1.0) in the scanner. Specifically, after 5 min of rest, subjects exercised for 4 min followed by 5 min of recovery. The sequence of the two treatments, which were separated by 45 min, was varied to minimize any potential ordering effects and the subjects were blinded to the treatment.
31P MRS
MRS was performed using a clinical 1.5 T General Electric Signa system (LX 8.3 version) operating at 25.86 MHz for 31P. 31P MRS data were acquired with a dual frequency flexible array spectroscopy coil (Medical Advances Inc., Milwaukee, Wisconsin) positioned around the calf at its maximum diameter. The phosphorus coil was an 11.5-cm square, centered between two 14 × 15.5 cm Helmholtz-type proton coils. The centering of the coil around the leg was confirmed by T1-weighted 1 H localizing images obtained in the axial plane and the coil was repositioned if the majority of the gastrocnemius muscle was not encompassed. For all subjects, a similar ratio between the volumes of gastrocnemius/soleus muscles was maintained within the coil. Magnetic field homogeneity was optimized by shimming on the proton signal from tissue water and the 31P MRS signal was optimized by prescan transmitter gain adjustment. Then, MR data were acquired throughout the graded exercise protocol with the following parameters (radiofrequency hard pulse duration = 500 μs, repetition time = 4 s, number of excitation = 1, sweep width = 2.5 kHz, data points = 1,024, nominal flip angle = 90°) so that the corresponding time resolution was 4 s.
Data analysis
Data were processed using SAGE/IDL software on a Silicon Graphics Indigo workstation. Each FID was processed with 5 Hz exponential line broadening prior to zero filling and Fourier transformation. All spectra were manually phased using zero- and first-order phase corrections. The levels of PCr determined from the intensity of that peak were normalized to 100 % using the average value obtained from the last 40 s of rest acquired for each subject as a reference. Muscle intracellular pH was calculated from the chemical shift difference (δ) of the Pi peak relative to the PCr peak using the following equation: 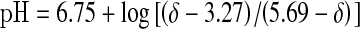 (Taylor et al. 1983).
(Taylor et al. 1983).
The PCr recovery kinetics were determined by fitting the PCr time-dependent changes during the recovery period to a single exponential curve described by the following equation:
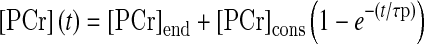 |
where [PCr]end is the concentration of [PCr] measured at end-of-exercise and [PCr]cons refers to the amount of PCr consumed at the end of the exercise session. The initial rate of PCr resynthesis (ViPCr) was calculated as follows:
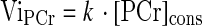 |
in which [PCr]cons indicates the amount of PCr consumed at the end of exercise and the rate constant, k = 1/τ.
Model variables were determined with an iterative process by minimizing the sum of squared residuals (RSS) between the fitted function and the observed values. Goodness of the fit was assessed by visual inspection of the residual plot and the frequency plot distribution of the residuals, Chi-square values, and the coefficient of determination (r2) calculated as follows (Motulsky and Christopoulos 2003):
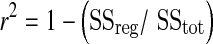 |
with SSreg, the sum of squares of the residuals from the fit and SStot, the sum of squares of the residuals from the mean.
Statistical analysis
Difference in PCr recovery time constant between young and old in normoxia was analyzed using the Mann–Whitney test (Statsoft, version 5.5; Statistica, Tulsa, Oklahoma). The effect of hyperoxia on the PCr recovery time constant within each group was analyzed using the Wilcoxon test. Statistical significance was accepted at P < 0.05. Results are presented as mean ± SD in the tables and for clarity, mean ± SEM are illustrated in the figures.
Results
Phosphorylated compounds and intracellular pH
End-exercise pH following constant load exercise was not significantly different from baseline in normoxia and hyperoxia for both groups (Table 1). Similarly, end-exercise PCr, Pi, and ADP were not significantly different in normoxic and hyperoxic conditions within either group (Table 1). With the exception of ADP, which was significantly higher in old subjects compared to young in normoxia (P < 0.05), end-exercise PCr, Pi, and pH were not significantly between these groups.
Table 1.
End-exercise phosphorylated compounds and intracellular pH
| Old | Young | |||
|---|---|---|---|---|
| Normoxia | Hyperoxia | Normoxia | Hyperoxia | |
| PCr (mM) | 19 ± 4 | 19 ± 4 | 24 ± 5 | 25 ± 5 |
| Pi (mM) | 16 ± 4 | 15 ± 3 | 12 ± 3 | 12 ± 3 |
| ADP (mM) | 67 ± 14* | 79 ± 27 | 44 ± 18 | 38 ± 15 |
| pH | 7.04 ± 0.10 | 7.08 ± 0.08 | 7.05 ± 0.04 | 7.05 ± 0.03 |
All values are expressed as mean ± SD
*P < 0.05 (statistically significant difference from young)
PCr offset kinetics
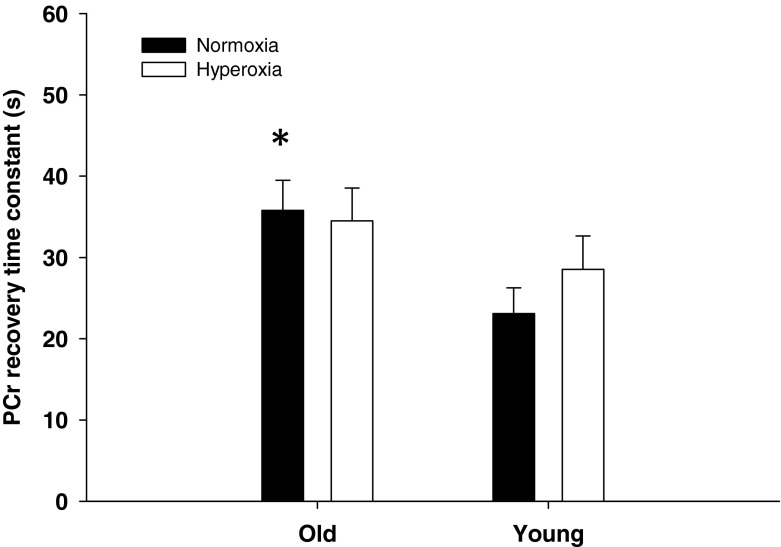
As illustrated with exemplary data in Fig. 1, the PCr recovery time constant was significantly longer in old subjects compared to young in normoxia (Table 2). The group mean PCr recovery responses in normoxia and hyperoxia for both old and young are illustrated in Fig. 2. During the postexercise period, there was no difference in the normoxic and hyperoxic PCr recovery time constant (Fig. 3) and the corresponding amplitude (Table 2) for either the old or the young groups. As a result, the initial PCr resynthesis rate was also not significantly different in normoxia and hyperoxia in either group (Table 2). Likewise, the minimum intracellular pH reached during recovery was not significantly different under conditions of normoxia or hyperoxia within or between the groups (Table 2).
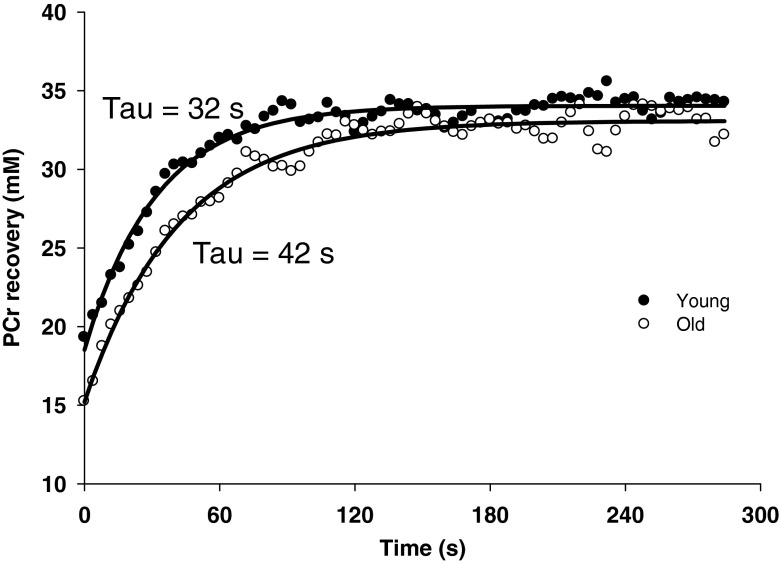
Fig. 1.
Example of young (filled circle) and old (unfilled circle) PCr recoveries with respect to time following exercise in normoxia
Table 2.
PCr recovery kinetics parameters
| Old | Young | |||
|---|---|---|---|---|
| Normoxia | Hyperoxia | Normoxia | Hyperoxia | |
| Amplitude (mM) | 14 ± 4 | 14 ± 3 | 9 ± 4 | 8 ± 4 |
| Tau (s) | 36 ± 9* | 35 ± 10 | 23 ± 8 | 29 ± 10 |
| IC 95 | 1.2 ± 0.4 | 1.0 ± 0.5 | 2.3 ± 1.7 | 2.6 ± 2.4 |
| Vi (mM min−1) | 24 ± 6 | 24 ± 4 | 23 ± 7 | 24 ± 4 |
All values are expressed as mean ± SD. Amplitude refers to the amount of PCr resynthesized during the recovery
Vi initial PCr resynthesis rate, IC 95 95 % confidence interval
*P < 0.05 (statistically significant difference from young)
Fig. 2.
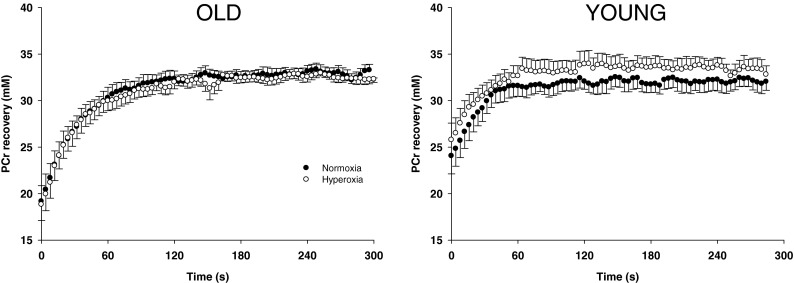
PCr resynthesis kinetics in old (left panel) and young humans (right panel) following submaximal plantar flexion exercise in normoxia and hyperoxia
Fig. 3.
PCr recovery time constants, an index of maximal oxidative ATP synthesis, in old and young humans following submaximal plantar flexion exercise in normoxia and hyperoxia. Asterisk indicates significant difference from young in normoxia (P < 0.05)
Discussion
We sought to examine whether skeletal muscle oxidative capacity in vivo was limited by O2 supply or the mitochondrial capacity to generate ATP in untrained humans with a special interest in the effect of age. The main findings were that (1) the PCr recovery time constant in normoxia was slower in the elderly group compared to young sedentary subjects and (2) the PCr recovery time constant was unaffected by breathing a hyperoxic gas mixture in both groups of sedentary individuals. Together, these findings indicate that the documented decline in maximal rate of mitochondrial ATP synthesis in the skeletal muscle with age is likely due to reduced mitochondrial content and/or mitochondrial dysfunction rather than limited O2 supply.
Evidence of age-related decline in oxidative capacity
An important finding of this study was the ~50 % slower PCr recovery kinetics in the elderly compared to the young subjects in normoxic conditions (Fig. 3), documenting a reduced muscle oxidative capacity with age. This decrease in oxidative capacity is of a similar magnitude to previous values reported in the plantar flexor muscles of elderly individuals (~46–48 %) (Taylor et al. 1997; McCully et al. 1993). However, unlike the present study, the groups of older and younger subjects in these prior studies were not sedentary and/or included subjects who were late–middle aged. It should, however, be noted that the current findings do contrast with other studies from our group (Wray et al. 2009b; Wray et al. 2009c), documenting a preserved oxidative capacity in the plantar flexor muscles of older subjects. Unfortunately, it is unclear to what extent the contrasting level of end-exercise PCr between young and older individuals, which may affect the PCr recovery kinetics (Roussel et al. 2000), could have affected these prior results by Wray et al. (2009b; 2009c). In contrast, in the present study, the end-exercise conditions were remarkably similar between groups, which rules out any confounding effects of pH or PCr level on our conlusions.
Evidence that mitochondrial ATP production is not O2 supply-dependent in normoxia
In agreement with our hypothesis, the PCr recovery time constant was unchanged when breathing hyperoxia in both young and old sedentary subjects (Fig. 2). These results are consistent with the unaltered postexercise PCr recovery kinetics (Haseler et al. 2004) and leg VO2max (Cardus et al. 1998) in hyperoxia compared to normoxia previously reported in young sedentary subjects and extend these findings to the elderly. These findings, therefore, reveal that the maximal rate of mitochondrial ATP synthesis in the calf muscle is determined by mitochondrial capacity rather than O2 supply in both young and elderly sedentary individuals. Somewhat in contrast to the present study, Wray et al. (2009b) has recently reported that enhanced tissue perfusion induced by acute antioxidant administration in elderly subjects improved oxidative capacity, again assessed by 31P-MR spectroscopy. However, this apparent discrepancy is likely related to the quite different methods used to improve O2 availability (hyperoxia versus enteral antioxidant administration resulting in hyperemia). Indeed, while hyperoxia enhanced O2 availability by increasing blood O2 content, antioxidant administration induced an increase in muscle blood flow by enhanced scavenging of free radicals and a subsequent increase in nitric oxide (NO) bioavailability in the elderly subjects (Wray et al. 2011). Although such an intervention has consistently been documented to increase muscle blood flow in old sedentary subjects by restoring endothelium-dependent vasodilation (Donato et al. 2006; Kirby et al. 2009), an acute increase in NO bioavailability has also been recognized to decrease O2 cost (Larsen et al. 2010) and improve mitochondrial efficiency (Larsen et al. 2011). Therefore, while an improved O2 supply may contribute to some extent to the faster PCr recovery in the study of Wray et al. (2009b), other factors related to mitochondrial function could also play a role when antioxidant administration alters NO bioavailability.
The unchanged PCr recovery time constant from normoxia to hyperoxia in elderly individuals (Fig. 3) also suggests that, in normoxia, the diffusion of O2 from capillary to mitochondria is able to compensate for the reduction in convective O2 delivery commonly associated with aging (Proctor and Parker 2006). If true, this increased O2 diffusion during exercise in the elderly would then result in a preserved intracellular O2 pressure (iPO2), which drives mitochondrial respiration (Wilson et al. 1977; Richardson et al. 1999; Richardson et al. 1995). In line with this possibility, O2 diffusing capacity appears to be well preserved in the elderly as the capillary to fiber area ratios is maintained (Chilibeck et al. 1997; Proctor et al. 1995) and may actually be increased when capillary to fiber area is normalized for mitochondrial volume (Mathieu-Costello et al. 2005). In addition, the reduction in muscle blood flow does not necessarily translate into a reduced O2 supply to the mitochondria as, in this scenario, the erythrocyte transit time through the capillary is prolonged, which allows higher O2 extraction by the tissues (Richardson 1998). Accordingly, it has previously been reported that O2 extraction was increased in the elderly during steady-state submaximal cycling exercise (Poole et al. 2003; Lawrenson et al. 2003; Proctor et al. 2003). Likewise, during the transition from rest to cycling exercise, duManoir et al. (2010) recently documented a greater O2 extraction, inferred from near-infrared spectroscopy measurements, to compensate for the slower adjustment of muscle blood flow to exercise in older people (duManoir et al. 2010). Thus, there may be significant age-related peripheral adaptations that tend to offset the potential consequences of attenuated O2 delivery to skeletal muscle.
An alternative explanation for the unaltered PC recovery kinetics in conditions of hyperoxia in elderly sedentary subjects is that, despite the probable reduction in convective O2 transport, iPO2 does not reach a critical level, such that O2 supply still exceeds mitochondrial capacity for ATP synthesis. Indeed, it has been demonstrated both in vitro (Wilson et al. 1977) and in vivo (Richardson et al. 1999; Richardson et al. 1995) that the relationship between respiration rate and iPO2 is not linear, but hyperbolic. For this reason, mitochondrial ATP synthesis is independent of changes in O2 supply unless intracellular iPO2 falls below ~2–5 mmHg during exercise (Richardson et al. 1999; Richardson et al. 1995). Given the concomitant reduction in mitochondrial capacity with age (see section below), it is possible that some reduction in convective O2 delivery and iPO2 in elderly individuals may take place before O2 supply limits mitochondrial respiration rate. This possible scenario is also further supported by our prior finding that O2 supply was in excess relative to mitochondrial capacity in young sedentary subjects during normoxia and even mild hypoxia (Haseler et al. 2007).
One could notice that while the PCr recovery time constant was almost similar in normoxia and hyperoxia in elderly subjects, young subjects exhibited a nonsignificant increase in hyperoxia (~29 s) compared to normoxia (~23 s). A potential explanation for this slower PCr recovery in the young is the development of hyperoxic vasoconstriction with the use of high fraction of inspired O2. Indeed, by using a high O2 gas mixture, we reasoned that it would provide a larger O2 gradient between red blood cell and mitochondria thereby facilitating O2 diffusion. This increase in the PO2 gradient is induced by a greater arterial O2 saturation and O2 delivery, provided that muscle blood flow is unchanged. However, it has been suggested that hyperoxia can reduce muscle blood flow during exercise thereby reducing O2 delivery to the muscle to a level similar to normoxic conditions (Welch et al. 1977; Pedersen et al. 1999). For instance, Pedersen et al. (1999) reported diminished leg blood flow and a trend for a lower maximal O2 consumption during one-leg knee extension exercise in hyperoxia compared to normoxia (Pedersen et al. 1999). Interestingly, similar to the present study, Haseler et al. (2004) also reported a slight increase in the PCr recovery time constant in hyperoxia compared to normoxia in young untrained individuals, although this difference was less marked (Haseler et al. 2004). It cannot be ruled out that breathing hyperoxic gas mixture during our plantar flexion exercise may have resulted in hyperoxic vasoconstriction, thus compromising to some extent O2 availability in the young untrained subjects. However, this explanation is unlikely to be accurate as it has been demonstrated that O2 availability had to be dramatically reduced before affecting the PCr recovery kinetics (Haseler et al. 2007). A more likely explanation is that the parameters of the PCr recovery kinetics typically exhibit some variability (~10–20 %), due to the procedure of fitting and biological variability (Layec et al. 2009), which can probably explain most of this nonsignificant difference between the normoxic and hyperoxic results in the young.
Mechanism of age-related decline in muscle oxidative capacity
Together, the impaired oxidative capacity in elderly subjects (Fig. 3) and unchanged PCr recovery rate in hyperoxia (Fig. 2) indicate that this was likely the result of a decrease in mitochondrial capacity rather than O2 supply limitation. Possible mechanisms accounting for this decrease in mitochondrial capacity in older individuals include a reduction mitochondrial content and/or mitochondrial dysfunction. For instance, there is some evidence that mitochondrial density in skeletal muscle declines with age as measured by electron microscopy (Conley et al. 2000; Bailey et al. 2010) or citrate synthase activity (Short et al. 2005; Coggan et al. 1993; McCully et al. 1993). However, it has been suggested that physical inactivity rather than aging per se may be an underlying cause of the reduced mitochondrial density (Russ and Lanza 2012) as these effects can be reversed by exercise training (Coggan et al. 1993; Short et al. 2003; Konopka et al. 2010). Interestingly, both young and elderly subjects in the present study were not involved in any regular physical activity. Therefore, if we assume no mitochondrial dysfunction, our findings that oxidative capacity was reduced in the sedentary elderly compared to the sedentary young could be interpreted to suggest that the activities of daily living were not sufficient to maintain mitochondrial capacity in the older group.
Using a combination of in vivo and in vitro measurements, Conley et al. (2000) previously reported a 50 % lower oxidative capacity in adults compared to elderly subjects, a result similar to the current study. This group interpreted their results to indicate that half of this impairment was due to reduced mitochondrial density and the remaining half due to reduced mitochondrial function (Conley et al. 2000). In particular, these authors documented a greater in vivo respiration uncoupling (P/O) at rest with age in muscles with high type II fiber content, indicative of an intrinsic defect in mitochondrial machinery (Amara et al. 2007). In this line, we observed that despite similar PCr resynthesis rate in normoxic conditions, the old subjects displayed a higher [ADP] at the end of exercise in comparison to the young subjects suggestive of a disturbance in the metabolic control of respiration rate by ADP (Chance and Williams 1955; Erecinska and Wilson 1982). Given its dimension, the coil used in the current study interrogated both the soleus, mostly composed of type I fibers, and the gastrocnemius, which is comprised of the same proportion of type I and type II fibers in the human adult (Johnson et al. 1973). Therefore, despite the progressive loss of type II fibers typically observed in older individuals (Lexell 1995), it cannot be ruled out that part of the reduction in oxidative capacity reported here was induced by greater respiration uncoupling during exercise in the elderly subjects.
Conclusion
In summary, this study has revealed that elderly subjects exhibit an impaired muscle oxidative capacity in comparison to sedentary young controls. PCr recovery kinetics were unaffected by breathing a hyperoxic gas mixture in both groups of sedentary individuals, thereby indicating that the maximal rate of mitochondrial ATP synthesis was limited by mitochondrial capacity rather than O2 supply. Together, these findings reveal that reduced mitochondrial content and/or mitochondrial dysfunction rather than limited O2 supply likely contributes to the decline in muscle oxidative capacity of sedentary elderly individuals.
References
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA. 2007;104(3):1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010;109(2):449–456. doi: 10.1152/japplphysiol.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardus J, Marrades RM, Roca J, Barbera JA, Diaz O, Masclans JR, Rodriguez-Roisin R, Wagner PD. Effects of F(I)O2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc. 1998;30(5):697–703. doi: 10.1097/00005768-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem. 1955;217(1):429–438. [PubMed] [Google Scholar]
- Chilibeck PD, Paterson DH, Cunningham DA, Taylor AW, Noble EG. Muscle capillarization O2 diffusion distance, and VO2 kinetics in old and young individuals. J Appl Physiol. 1997;82(1):63–69. doi: 10.1152/jappl.1997.82.1.63. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Abduljalil AM, Swanson SC, Earle MS, Farris JW, Mendenhall LA, Robitaille PM. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol. 1993;75(5):2125–2133. doi: 10.1152/jappl.1993.75.5.2125. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Paterson DH, Kowalchuk JM. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metabol (Physiologie Appliquee, Nutrition et Metabolisme) 2007;32(6):1251–1262. doi: 10.1139/H07-121. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol. 2006;290(1):H272–278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Differences in exercise limb blood flow and muscle deoxygenation with age: contributions to O2 uptake kinetics. Eur J Appl Physiol. 2010;110(4):739–751. doi: 10.1007/s00421-010-1546-z. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Wilson DF. Regulation of cellular energy metabolism. J Membr Biol. 1982;70(1):1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- Fleg JL, O’Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78(3):890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol. 1999;86(6):2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97(3):1077–1081. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Lin A, Hoff J, Richardson RS (2007) Oxygen availability and PCr recovery rate in untrained human calf muscle: evidence of metabolic limitation in normoxia. Am J Physiol Regul Integr Comp Physiol [DOI] [PubMed]
- Hirai DM, Copp SW, Herspring KF, Ferreira LF, Poole DC, Musch TI. Aging impacts microvascular oxygen pressures during recovery from contractions in rat skeletal muscle. Respir Physiol Neurobiol. 2009;169(3):315–322. doi: 10.1016/j.resp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: comparison of age-related changes. J Appl Physiol. 1982;53(4):799–804. doi: 10.1152/jappl.1982.53.4.799. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18(1):111–129. doi: 10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89(3):1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587(Pt 9):1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Douglass MD, Kaminsky LA, Jemiolo B, Trappe TA, Trappe S, Harber MP. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol. 2010;65(11):1201–1207. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99(5):1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48(2):342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol. 2003;285(3):H1023–1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Layec G, Bringard A, Le Fur Y, Vilmen C, Micallef JP, Perrey S, Cozzone PJ, Bendahan D. Reproducibility assessment of metabolic variables characterizing muscle energetics in vivo: a 31P-MRS study. Magn Reson Med. 2009;62(4):840–854. doi: 10.1002/mrm.22085. [DOI] [PubMed] [Google Scholar]
- Lexell J (1995) Human aging, muscle mass, and fiber type composition. The journals of gerontology 50 Spec No:11-16 [DOI] [PubMed]
- Mathieu-Costello O, Ju Y, Trejo-Morales M, Cui L. Greater capillary–fiber interface per fiber mitochondrial volume in skeletal muscles of old rats. J Appl Physiol. 2005;99(1):281–289. doi: 10.1152/japplphysiol.00750.2004. [DOI] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75(2):813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Christopoulos A (2003) Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. San Diego
- Pedersen PK, Kiens B, Saltin B. Hyperoxia does not increase peak muscle oxygen uptake in small muscle group exercise. Acta Physiol Scand. 1999;166(4):309–318. doi: 10.1046/j.1365-201x.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol. 2003;284(4):H1251–1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Poole D, Behnke B, Musch T. Capillary hemodynamics and oxygen pressures in the aging microcirculation. Microcirculation. 2006;13(4):289–299. doi: 10.1080/10739680600618793. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13(4):315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78(6):2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol. 2003;94(5):1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Richardson RS. Oxygen transport: air to muscle cell. Med Sci Sports Exerc. 1998;30(1):53–59. doi: 10.1097/00005768-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Investig. 1995;96(4):1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O(2) consumption in trained human skeletal muscle. J Appl Physiol. 1999;87(1):325–331. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta. 2000;1457(1–2):18–26. doi: 10.1016/s0005-2728(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Russ DW, Lanza IR (2012) The impact of old age on skeletal muscle energetics: supply and demand. Current aging science [DOI] [PubMed]
- Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI. Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol. 2003;285(1):H251–258. doi: 10.1152/ajpheart.01086.2002. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1(1):77–94. [PubMed] [Google Scholar]
- Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem. 1997;174(1–2):321–324. doi: 10.1023/A:1006802602497. [DOI] [PubMed] [Google Scholar]
- Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol. 1977;42(3):385–390. doi: 10.1152/jappl.1977.42.3.385. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Drown C, Silver IA. Effect of oxygen tension on cellular energetics. Am J Physiol. 1977;233(5):C135–140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol. 2009;64(9):968–974. doi: 10.1093/gerona/glp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol. 2009;297(5):H1870–1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 2009;116(5):433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Donato AJ, Carlier P, Bailey DM, Uberoi A, Richardson RS. The paradox of oxidative stress and exercise with advancing age. Exerc Sport Sci Rev. 2011;39(2):68–76. doi: 10.1097/JES.0b013e31820d7657. [DOI] [PMC free article] [PubMed] [Google Scholar]