Abstract
Long-term exercise prior to brain ischemia enhances the activities of antioxidant enzymes and leads to a significant reduction in brain damage and neurological deficits in rats subjected to transient middle cerebral artery occlusion. However, it has not been established whether relatively short-term exercise generates similar results following middle cerebral artery occlusion. We aimed to determine whether short-term exercise could reduce oxidative damage and prevent sensori-motor dysfunction. Male Wistar rats were subjected to perform daily exercise on a treadmill for 30 min at a speed of 15 m/min for 3 weeks, followed by a 90-min middle cerebral artery occlusion. Animals were assessed after middle cerebral artery occlusion for neurological deficits and sensori-motor function. Brain tissues were processed to evaluate infarct volume and oxidative damage. Oxidative stress was assessed using immunohistochemistry for 4-hydroxy-2-nonenal-modified proteins and 8-hydroxy-2'-deoxyguanosine. Antioxidant enzymes were evaluated using immunohistochemistry for thioredoxin and activity assay for superoxide dismutase. Exercise for 3 weeks decreased the severity of paralysis and impairment in forelimb motor coordination. Furthermore, exercise had effect on superoxide dismutase and reduced the infarct volume and the number of cells immunopositive for 4-hydroxy-2-nonenal-modified proteins and 8-hydroxy-2'-deoxyguanosine. Our results suggest that pre-conditioning treadmill exercise for 3 weeks is useful for ameliorating ischemia-induced brain injury.
Keywords: middle cerebral artery occlusion, pre-conditioning exercise, oxidative stress, sensori-motor function, rat
Introduction
Brain infarction ranks as the third-leading cause of death in Western countries.(1) Therefore, major efforts on prevention should be focused on modifiable risk factors, which, among others, include physical inactivity.(2)
Oxidative stress induced by reactive oxygen species exacerbates histological damage during brain ischemia-reperfusion. During this process, each component of the cell is damaged, as revealed by an increase in cells with immunopositivity for 4-hydroxy-2-nonenal-modified proteins (HNE-modified proteins, a marker for lipid peroxidation) and 8-hydroxy-2'-deoxyguanosine (8-OHdG, a marker for oxidative DNA damage) in the ischemic brain.(3–6) Previous studies have reported that infarct volume was larger in thioredoxin (TRX)-deficient mice compared with wild-type mice.(7) These reports indicate that oxidative stress is closely associated with adverse effects on ischemic brain tissue.
Long-term exercise enhances antioxidant activities in the brain.(8) It was reported that adequate training results in lower levels of free radicals,(9) thus lowering peroxidation levels of lipids or proteins in the rat brain.(10) Furthermore, regular exercise for 7.5 to 8 weeks increased the levels of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase, or TRX.(11–13) These findings suggest that long-term exercise prevents oxidative stress by increasing antioxidant action.
Pre-conditioning exercise for 2 to 4 weeks prior to stroke was reported to significantly reduce brain damage and neurological deficits in the transient middle cerebral artery occlusion (MCAO) model in rats.(14–16) However, it remains unknown whether relatively short term exercise can enhance antioxidant activities in the brain. Thus, we aimed to study whether short-term exercise can reduce oxidative stress following MCAO.
Materials and Methods
Animals
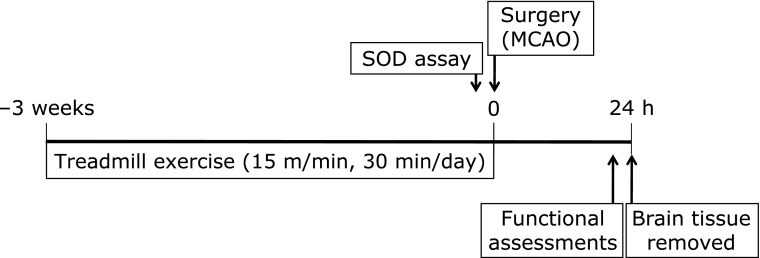
We used 35 male Wistar rats (5 weeks old, Japan SLC, Hamamatsu, Japan), weighing 120–140 g. Animals were housed at 25°C with a 12 h light/dark cycle, with food (CE-2, CLEA Japan, Tokyo, Japan) and water available ad libitum throughout the experimental protocol, which is shown in Fig. 1. Rats were randomly assigned to groups with or without treadmill exercise (Ex). Each group was then divided into 2 subgroups; sham operation (Sham) or MCAO. Therefore, 4 groups were included in this study: Sham (n = 11), Ex + Sham (n = 10), MCAO (n = 11) and Ex + MCAO (n = 10). The animal experiment committee of Nagoya University Graduate School of Medicine approved this study. All efforts were made to minimize the number and suffering of animals used.
Fig. 1.
Experimental protocol. Animals in the exercise groups (Ex + Sham, exercise and sham operation; Ex + MCAO, exercise and transient middle cerebral artery occlusion) were forced to run on a motorized treadmill at a speed of 15 m/min for 30 min each day for 3 weeks prior to surgery. Neurological and motor function assessments were examined at 24 h after reperfusion, and the brains were then removed for histological assessments.
Treadmill exercise
Animals in the exercise groups (Ex + Sham and Ex + MCAO) were forced to run on a motorized treadmill at a speed of 15 m/min for 30 min every day for three weeks, as previously described.(17) Each rat ran 450 m per day. Animals in the no-exercise groups (Sham or MCAO) were left on an unmoving treadmill for the same period as the exercise groups. Body weights were measured every day to monitor each animal’s condition. One animal was excluded from this experiment because we could not train the animal to run.
Transient middle cerebral artery occlusion model
Twenty-four hours after exercising on the treadmill for 3 weeks, the MCAO surgery was performed using the following method. Animals were anesthetized using an injection of 4% chloral hydrate (10 ml/kg, i.p.) and were given further doses as necessary to maintain adequate anesthesia during surgery. Throughout the surgical procedure, rectal temperature was monitored and maintained at 37°C using a circulating heating pad. Rats were subjected to a 90-min left-MCA occlusion using an intraluminal filament, as previously described.(18) Briefly, a monofilament (4-0 nylon suture, with a blunted tip and 16 mm in length) was inserted through the left common carotid artery (CCA) and passed up into the lumen of the internal carotid artery (ICA) into the intracranial circulation and finally lodged into the narrow proximal anterior cerebral artery, thus blocking the MCA at its origin. Following 90 min of MCA occlusion, reperfusion was established by withdrawing the filament. For the sham operation, the left CCA and the ICA were exposed following general anesthesia, as for the stroke induction, but the MCA was not occluded. All of the animals were then placed in a warm environment until fully recovered from the anesthesia.
Neurological and sensori-motor function assessments
Neurological and sensori-motor function assessments were performed 24 h after the reperfusion.
Neurological deficits
Neurological deficits were scored using a modified scoring system developed by Zea Longa et al.(18) as follows: 0) no deficits, failure to extend left forepaw fully; 1) mild, unable to extend the contralateral forelimb; 2) moderate, circling to the left; 3) severe, falling to the left; and 4) severe, unable to walk spontaneously, with a depressed level of consciousness.
Beam walking test
Rats were tested for beam-walking ability using their hind limbs by traversing a beam (2.4 cm-wide; 80 cm-long).(19) Briefly, performance was graded as 0) rat fell off the beam within 10 s; 1) rat remained on the beam for more than 10 s but could not place the affected limb on the beam; 2) rat was unable to cross but could place the affected limb on the beam and maintain balance; 3) rat traversed beam while dragging the affected limb; 4) rat crossed the beam and placed the affected limb on the beam at least once; 5) rat crossed with more than 50% foot slips with the affected limb; 6) rat crossed with fewer than 50% foot slips with affected limb; or 7) rat crossed with 2 or fewer foot slips. Animals were trained three times per day for 3 days prior to the surgery. Their performance was expressed as a mean score of three trials.
Ladder test
The ladder test was performed to quantitatively examine forelimb grip function and coordination.(20) Rats were trained to cross a 1 m-long horizontal ladder, which was set at 30 cm above the ground. Rungs were set irregularly; the distance between rungs varied from 1 to 5 cm. The test was conducted three times per rat. All tests were video recorded and evaluated using a scoring system as follows: 0) total miss, deep fall after limb missed the rung; 1) deep slip, deep fall after the limb slipped off the rung; 2) slight slip, slight fall after the limb slipped off the rung; 3) replacement, the limb was replaced from one rung to another; 4) correction, the limb aimed for one rung but was placed on another or the limb position on the same rung was corrected; 5) partial placement, the limb was placed on the rung at the position of either the digits or wrist; and 6) correct placement, the midportion of the limb was placed on the rung.(20) For the analysis, the success rate (ratio of 6 points for all steps) and fall rate (ratio of points 0, 1, 2 for all steps) were quantified from three trials and then averaged. Animals were trained three times per day for 3 days prior to surgery.
Limb placing test
Limb placing, an evaluation of sensori-motor function, is used to test whether a rat responds to sensory stimulation with forelimb placement. Rats were tested as described previously, with some modifications.(21) Briefly, visual placing in the forward and sideways directions, tactile placing of the dorsal and lateral paw surfaces and proprioceptive placing of the forelimb or hindlimb were tested. When normal rats receive visual, tactile or proprioceptive stimulation at one edge of the table, they reach, stretch and place both forepaws on the table top. For each test, limb placing scores were 0, no placing; 1, incomplete and/or delayed (>2 s) placing including interspersed flailing; or 2, immediate and complete placing. The performance was expressed as the sum of the scores of the individual tests ranging from 0 (maximum deficit) to 12 (no deficit).
Infarct volume
Twenty-four hours after surgery, the animals were placed under deep anesthesia using 4% chloral hydrate (10 ml/kg, i.p.) and perfused transcardially with 0.9% saline. The brain was removed carefully and immediately cut into six 2-mm-thick coronal sections from its frontal apex, using a brain slicer. The fresh brain slices were then immersed in a solution of 2,3,5-triphenyltetrazolium chloride (TTC, 2% in saline) at 37°C for 5 min. The TTC-stained sections were used to determine the infarct volume in the ischemic rat brain. The infarct region was defined as the area with reduced or no staining. To minimize the error introduced by edema, an indirect method for calculating infarct volume was used.(22) Infarct volume was calculated using the following formula: corrected percentage of infarct volume = 100 × (contralateral hemispheric volume - ipsilateral non-infarcted volume) / contralateral hemispheric volume.
Immunohistochemical analyses
The above-mentioned TTC-stained sections were fixed immediately in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C overnight, dehydrated, embedded in paraffin, and processed for immunohistochemical analyses. Coronal sections (5 µm) were processed to assess whether exercise affected oxidative stress and the antioxidant protein after MCAO. Immunohistochemistry for HNE-modified proteins and for 8-OHdG was performed to evaluate oxidative stress, and TRX was selected as an antioxidant protein. The avidin-biotin complex method was used. Paraffin-embedded sections were de-waxed in xylene for 20 min and then rehydrated in decreasing concentrations of ethanol (100%, 90% and 70%) for 5 min at each concentration. Immunosaver (Nissin EM, Tokyo, Japan) was used for antigen retrieval (98°C for 45 min). Immunohistochemical analyses were performed using mouse monoclonal antibodies for HNE-modified proteins (HNE-J-2), 8-OHdG (N45.1) or a rabbit polyclonal antibody for TRX, as previously described.(23–25) Two sections, at 0.3 mm and 3.8 mm caudal to the bregma, were selected. Ten defined areas (1.4 mm × 1.0 mm) of the bilateral somato-sensory cortex (5 areas per hemisphere) of each section were evaluated. We counted cells positive for HNE-modified proteins or 8-OHdG and also quantified the density, in gray scale, of TRX-stained sections, using a computer-assisted image analyzer (BioRevo BZ-9000, Keyence, Osaka, Japan).
SOD activity assay
SOD activity of Sham groups (Ex + Sham: n = 3, Sham: n = 4) was quantified to evaluate antioxidant enzyme activity. Twenty-four hrs after exercising on the treadmill for three weeks, the animals were placed under deep anesthesia and perfused transcardially with 0.9% saline. Left somato-sensory cortex was removed and assayed SOD activity using SOD assay kit- WST (Dojindo, Kumamoto, Japan).
Statistical analysis
Statistical analyses were performed using SPSS for Windows, ver. 16.0 (SPSS Inc., Chicago, IL). Mann-Whitney U tests were used to analyze neurological deficits, beam walking and limb placing. Student’s t tests were used for ladder test, infarct volume and SOD activity comparisons. For the statistical analysis of immunopositive cell numbers and gray scale, one-way ANOVAs followed by Tukey’s post-hoc tests were used. All data are the mean ± SEM, and the criterion for significance was set at p<0.05.
Results
The body weights of all the animals increased gradually until surgery and decreased thereafter, with no significant differences between groups.
Neurological and sensori-motor functions
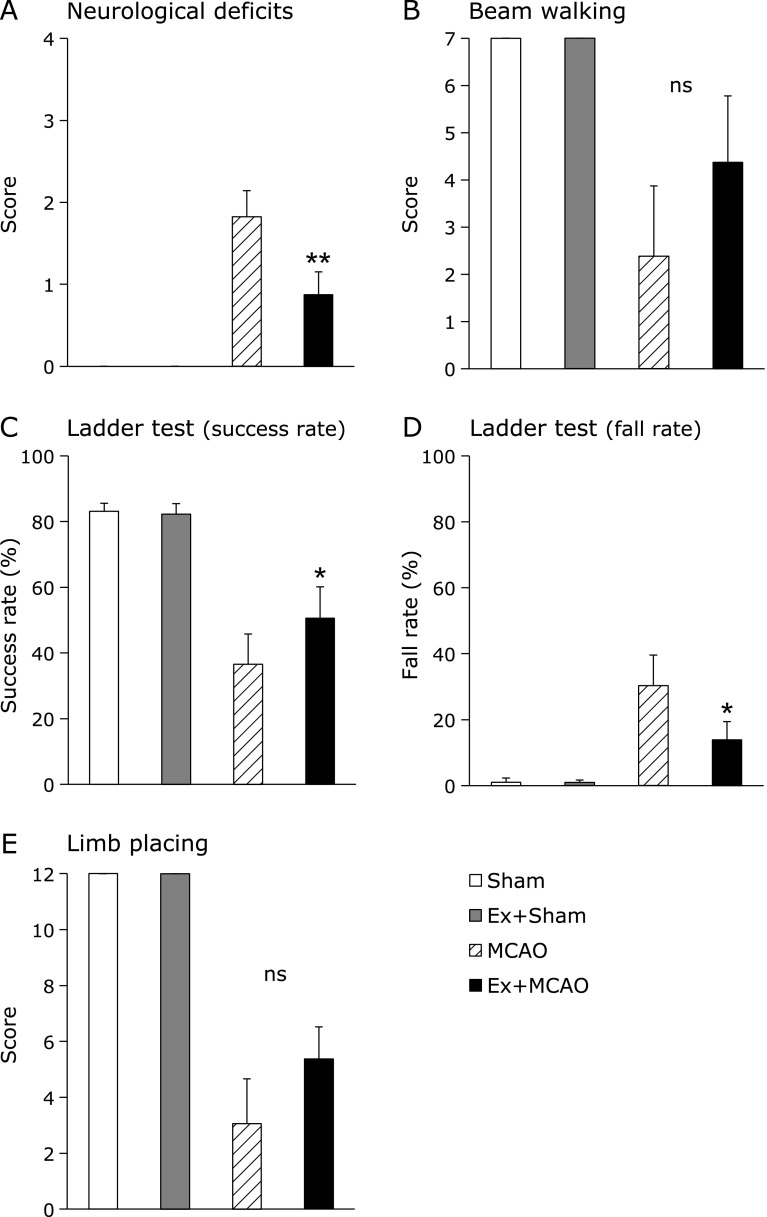
Neurological deficits were significantly ameliorated in the Ex + MCAO group (n = 11) compared with the MCAO only group (n = 10) at 24 h after MCA occlusion (Ex + MCAO: 0.8 ± 0.2, MCAO: 1.8 ± 0.3, p<0.01, Fig. 2A). There was no difference in beam walking deficits between groups (Ex + MCAO: 2.5 ± 1.5, MCAO: 4.2 ± 1.4, Fig. 2B). The success rate in the ladder test was significantly improved in the Ex + MCAO group compared with the MCAO group (Ex + MCAO: 50.6 ± 9.6%, MCAO: 34.3 ± 10.0%, p<0.05, Fig. 2C), and the fall rate showed a similar pattern (Ex + MCAO: 13.9 ± 5.5%, MCAO: 28.4 ± 9.7%, p<0.05, Fig. 2D). There were no differences between groups in the limb placing test (Ex + MCAO: 5.4 ± 1.2, MCAO: 3.1 ± 1.7, Fig. 2E).
Fig. 2.
Neurological deficits and sensori-motor functions. Neurological deficits and sensori-motor functions at 24 h after MCAO. Neurological deficits (A), beam walking (B), success rate (C) and fall rate (D) of the ladder test, and limb placing (E). Neurological deficits and the ladder test were significantly improved in the Ex + MCAO group when compared with the MCAO group. Beam walking and limb placing deficits were not different between groups. Values represent the mean ± SEM. **p<0.01 vs MCAO, Mann-Whitney U test; *p<0.05 vs MCAO, Student’s t test.
Infarct volume
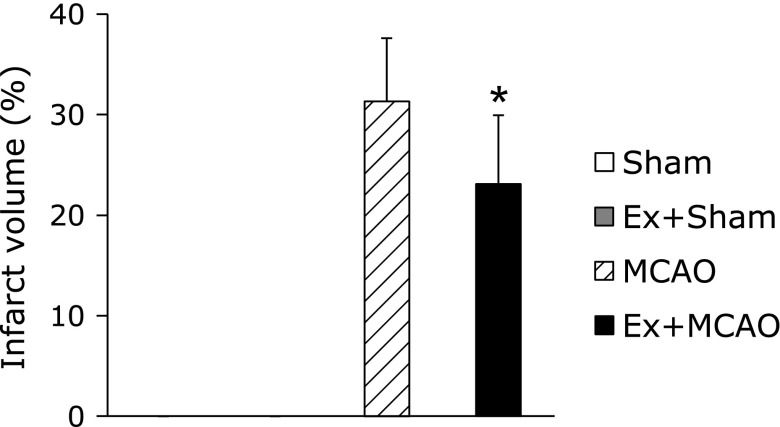
The infarct volume in the Ex + MCAO group was decreased significantly when compared with the MCAO only group (Ex + MCAO: 23.1 ± 1.7%, MCAO: 29.8 ± 3.3%, p<0.05, Fig. 3). The infarct lesion included primarily the forelimb and barrel regions of the somatosensory and visual cortex, and when the animals had larger infarctions, it extended further through the trunk area of the somatosensory or auditory cortex or striatum.
Fig. 3.
Infarct volume. Infarct volume of all groups, indicating a significant reduction in brain damage in the Ex + MCAO group. Values represent the mean ± SEM. *p<0.05 vs MCAO, Student’s t test.
Oxidative stress and antioxidant enzymes
Oxidative stress
In the ipsilateral cortex, the number of HNE-modified proteins or 8-OHdG-positive cells was increased markedly by MCAO occlusion (p<0.01). Pre-conditioning exercise suppressed these increases significantly (p<0.01, Fig. 4C and 5C). In the contralateral cortex, the number of these cells in the MCAO only group was significantly higher than in the three other groups (p<0.001, Fig. 4F and 5F).
Fig. 4.
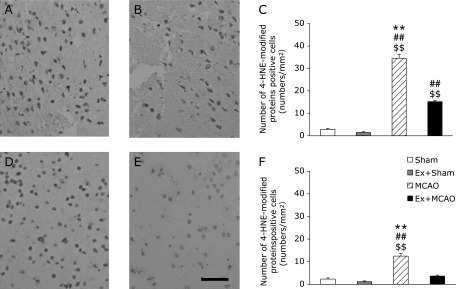
The number of cells positive for HNE-modified proteins. Photomicrographs showing cells containing 4-hydroxy-2-nonenal (HNE)-modified proteins. Immunohistochemistry in the sensory cortex at 24 h after MCAO. MCAO group (A, D); Ex + MCAO group (B, E); the number of cells positive for HNE-modified proteins in the sensory cortex (C, F) (Scale bar in E; 200 µm). Ipsilateral (A, B, C); contralateral cortex (D, E, F). Graphs indicate a significant reduction in the number of cells positive for HNE-modified proteins in the Ex + MCAO group. Values represent the mean ± SEM. $$p<0.01 vs Sham, ##p<0.01 vs Ex + Sham, **p<0.01 vs Ex + MCAO, One-way factorial ANOVA followed by Tukey’s post hoc test.
Fig. 5.
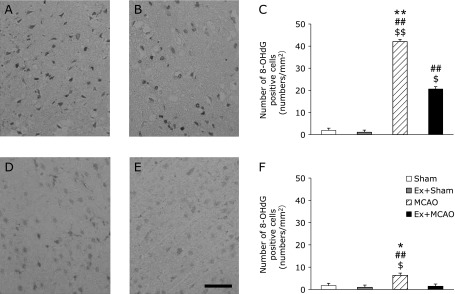
The number of 8-OHdG-positive cells. Photomicrographs showing 8-hydroxy-2'-deoxyguanosine (8-OHdG) positive cells. Immunohistochemistry in the sensory cortex at 24 h after MCAO. MCAO group (A, D); Ex + MCAO group (B, E); the number of 8-OHdG-positive cells in the sensory cortex (C, F) (Scale bar in E; 200 µm). (A, B, C) ipsilateral; (D, E, F) contralateral cortex. Graphs indicate a significant reduction in the number of 8-OHdG-positive cells in the Ex + MCAO group. Values represent the mean ± SEM. $p<0.05 vs Sham, $$p<0.01 vs Sham, ##p<0.01 vs Ex + Sham, *p<0.05 vs Ex + MCAO, **p<0.01 vs Ex + MCAO, One-way factorial ANOVA followed by Tukey’s post hoc test.
Antioxidant enzymes
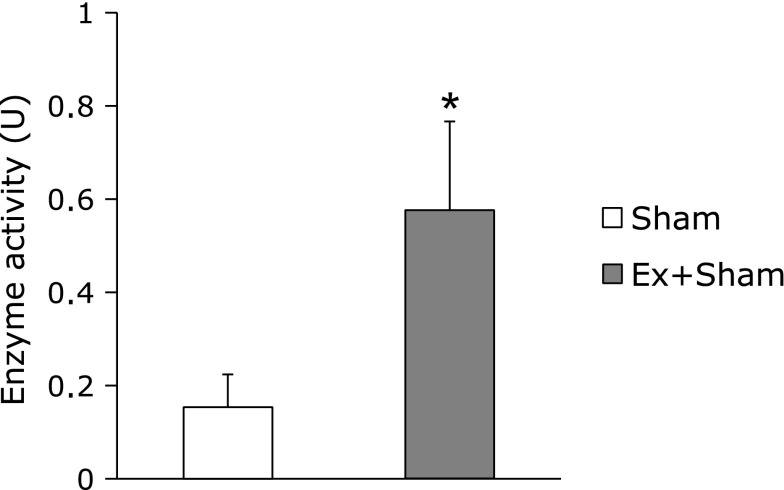
There were no significant differences in TRX gray scale integration in either the ipsilateral or contralateral cortex between groups (Fig. 6). On the other hand, SOD activity in the Ex + Sham group was significantly higher than in the Sham group (p<0.05, Fig. 7).
Fig. 6.
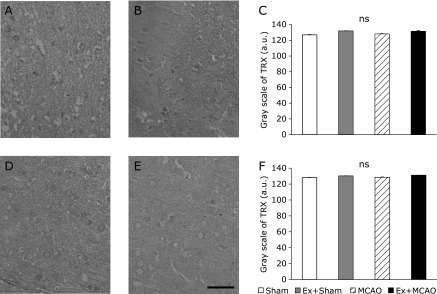
The gray scale of TRX. Photomicrographs showing thioredoxin (TRX) immunohistochemistry in the sensory cortex at 24 h after MCAO. MCAO group (A, D); Ex + MCAO group (B, E); the gray scale of TRX in the sensory cortex (C, F) (Scale bar in E; 200 µm). (A, B, C) ipsilateral; (D, E, F) contralateral cortex. Graphs indicate no significant differences in TRX gray scale values in either the ipsilateral or contralateral cortex between groups. Values represent the mean ± SEM. One-way factorial ANOVA followed by Tukey’s post hoc test.
Fig. 7.
SOD assay. Superoxide dismutase (SOD) assay, indicating significant increase in the SOD activity in the Ex + Sham group. Values represent the mean ± SEM. *p<0.05 vs Sham, Student’s t test.
Discussion
In this study, we asked whether a pre-conditioning exercise program for three weeks could reduce brain oxidative stress and sensori-motor dysfunction after MCAO and reperfusion. Our data revealed that treadmill exercise attenuated motor dysfunction and ameliorated brain damage, consistent with previous studies.(15–17) In addition to reducing the infarct volume, treadmill exercise decreased the number of cells immunopositive for HNE-modified proteins and 8-OHdG in the somatosensory cortex of both hemispheres. These findings suggest that aerobic pre-conditioning exercise for three weeks ameliorates motor dysfunction and brain damage, and these improvements were closely associated with a reduction in oxidative brain damage.
Our exercise conditions were 15 m/min, as described previously,(17) every day for 3 weeks. The efficacy of different periods of exercise has varied. Exercise for 1 week did not induce protection, but exercise for more than 2 weeks did.(26) These results suggest that a few weeks of exercise are necessary to obtain protection. Therefore, the training term in this study would be sufficient to induce this effect. With regard to running speed, previous studies reported that the mean anaerobic threshold was 26.4 ± 3.75 m/min (with a 10% incline of the treadmill) in normal rats.(27) Accordingly, our speed enabled aerobic exercise in our animals. Moreover, this protocol induced less stress because there was no difference in weight between groups. Thus, treadmill exercise at a speed of 15 m/min for 3 weeks produced a protective effect following MCAO without inducing excessive stress.
To evaluate motor function in detail, we used not only neurological deficits but also beam walking, the ladder test and limb placing. Our results showed that neurological deficits and ladder test deficits after MCAO were improved by the pre-conditioning exercise. It has been previously reported that the severity of paralysis (neurological deficits) and impairment in forelimb motor coordination (Grid-walking test) were correlated with infarct volume.(28) Furthermore, suppression of oxidative stress by exercise improved the functional behavioral score by maintaining normal cell function because oxidative stress evokes cell dysfunction even if it is not lethal to the cells. Therefore, pre-conditioning exercise is likely to improve not only the severity of paralysis but also the impairment in forelimb motor coordination after MCAO.
This study is the first report to examine the effect of three weeks of pre-conditioning exercise on oxidative stress following MCAO. Previous study has reported no change in oxidative stress following regular exercise in normal rats,(29) whereas other has demonstrated that exercise inhibited a stress-induced increase in lipid peroxidation in restraint-stressed mice.(10) In this study, we confirmed that there was no exercise-induced change in oxidative stress in the Sham group, but exercise inhibited the ischemia-induced increase in lipid and DNA oxidation. These results indicate that exercise inhibits the event that induces the oxidative stress. In addition, we showed inhibition of oxidative stress in the contralateral cortex, which is likely to prevent indirect damage. Because the contralateral hemisphere to ischemia engages motor function on the ipsilateral side, this protection may contribute to the functional improvement.
In contrast, opposing data show that exercise increases oxidative stress, e.g., 2,3-dihydroxybenzonic acid was increased following a one-shot treadmill exercise (20 m/min,for 15 min).(30) This report indicated that a single exercise bout was insufficient to enhance antioxidant action and instead increased oxidative stress. Because there were no significant differences in the number of cells immunopositive for HNE-modified proteins or 8-OHdG between the Sham group and Ex + Sham group, the exercise condition in this study may not increase oxidative stress.
We showed that the levels of thioredoxin did not change with exercise. It was probably because we measured TRX using a semi-quantitative method, with gray scale integration of immunohistochemical staining. However, we found that significantly high SOD activity in the Ex + Sham group. This result indicated that exercise in our protocol enhanced SOD activity and it provided the protective effect on oxidative damage following MCAO. We need the further studies to confirm the total antioxidant environment induced by our protocol.
Other factors that inhibit oxidative stress may include angiogenesis or the exercise-induced up-regulation of neuroprotective factors. Exercise increases mRNA levels of vascular endothelial growth factor and angiopoietin 1/2 and then strengthens the brain microvascular integrity in rats exposed to MCAO.(14,31) Angiogenesis is expected to reduce oxidative stress indirectly by ensuring cerebral blood flow during ischemia. Brain-derived neurotrophic factor (BDNF) and endothelial NO synthase (eNOS), which produce neuroprotective effects during stroke, were increased following pre-conditioning exercise.(32,33) Up-regulation of BDNF may reduce oxidative stress because BDNF improves mitochondrial function, and mitochondria produce a large amount of oxidative stress when exposed to ischemia.(34) eNOS provides antioxidant action by decreasing the superoxide anion, and it also induces a vasodepressor effect during ischemia. Therefore, eNOS may directly or indirectly inhibit oxidative stress. Accordingly, further study is warranted to understand the mechanism underlying the prevention of oxidative stress following three weeks of exercise.
In conclusion, we showed that a relatively short term exercise program prior to brain ischemia and reperfusion enhanced antioxidant activity and reduced oxidative stress in the brain. Therefore, the pre-conditioning exercise ameliorated brain damage and attenuated motor dysfunction following MCAO. This evidence could help develop health promotion programs and bring clinical benefit by preventing complications of human stroke.
Acknowledgments
This study was supported by the JSPS Grant-in-Aid for Scientific Research (22500456).
Abbreviations
- CCA
common carotid artery
- Ex
exercise
- HNE
4-hydroxy-2-nonenal
- ICA
internal carotid artery
- MCAO
transient middle cerebral artery occlusion
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- PBS
phosphate-buffered saline
- SOD
superoxide dismutase
- TRX
thioredoxin
- TTC
2,3,5-triphenyltetrazolium chloride
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.National Center for Health Statistics (US) Public Health Service. 2010. Health, United States, 2010: With Special Feature on Death and Dying. [PubMed] [Google Scholar]
- 2.Bronner LL, Kanter DS, Manson JE. Primary prevention of stroke. N Engl J Med. 1995;333:1392–1400. doi: 10.1056/NEJM199511233332106. [DOI] [PubMed] [Google Scholar]
- 3.Uchida K. Protein-bound 4-hydroxy-2-nonenal as a marker of oxidative stress. J Clin Biochem Nutr. 2005;36:1–10. [Google Scholar]
- 4.McCracken E, Valeriani V, Simpson C, Jover T, McCulloch J, Dewar D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J Cereb Blood Flow Metab. 2000;20:1529–1536. doi: 10.1097/00004647-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 5.McKracken E, Graham DI, Nilsen M, Stewart J, Nicoll JA, Horsburgh K. 4-Hydroxynonenal immunoreactivity is increased in human hippocampus after global ischemia. Brain Pathol. 2001;11:414–421. doi: 10.1111/j.1750-3639.2001.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Won MH, Kang TC, Jeon GS, et al. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70–78. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- 7.Takagi Y, Mitsui A, Nishiyama A, et al. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci USA. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab. 2007;32:942–946. doi: 10.1139/H07-081. [DOI] [PubMed] [Google Scholar]
- 9.Radak Z, Toldy A, Szabo Z, et al. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49:387–392. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Radák Z, Kaneko T, Tahara S, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 11.Somani SM, Ravi R, Rybak LP. Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol Biochem Behav. 1995;50:635–639. doi: 10.1016/0091-3057(94)00357-2. [DOI] [PubMed] [Google Scholar]
- 12.Somani SM, Husain K. Interaction of exercise training and chronic ethanol ingestion on antioxidant system of rat brain regions. J Appl Toxicol. 1997;17:329–336. doi: 10.1002/(sici)1099-1263(199709)17:5<329::aid-jat452>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Lappalainen Z, Lappalainen J, Oksala NK, et al. Diabetes impairs exercise training-associated thioredoxin response and glutathione status in rat brain. J Appl Physiol. 2009;106:461–467. doi: 10.1152/japplphysiol.91252.2008. [DOI] [PubMed] [Google Scholar]
- 14.Ding YH, Luan XD, Li J, et al. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004;1:411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- 15.Ding YH, Young CN, Luan X, et al. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- 16.Guo M, Cox B, Mahale S, et al. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience. 2008;151:340–351. doi: 10.1016/j.neuroscience.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y, Li J, Luan X, et al. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124:583–591. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.MacLellan CL, Auriat AM, McGie SC, et al. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J Cereb Blood Flow Metab. 2006;26:1031–1042. doi: 10.1038/sj.jcbfm.9600255. [DOI] [PubMed] [Google Scholar]
- 20.Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 21.De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- 22.Swanson RA, Morton MT, Tsao Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 23.Toyokuni S, Miyake N, Hiai H, et al. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995;359:189–191. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 24.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 25.Tanaka T, Nishiyama Y, Okada K, et al. Induction and nuclear translocation of thioredoxin by oxidative damage in the mouse kidney: independence of tubular necrosis and sulfhydryl depletion. Lab Invest. 1997;77:145–155. [PubMed] [Google Scholar]
- 26.Wang RY, Yang YR, Yu SM. Protective effects of treadmill training on infarction in rats. Brain Res. 2001;922:140–143. doi: 10.1016/s0006-8993(01)03154-7. [DOI] [PubMed] [Google Scholar]
- 27.Pilis W, Zarzeczny R, Langfort J, Kaciuba-Uscilko H, Nazar K, Wojtyna J. Anaerobic threshold in rats. Comp Biochem Physiol. 1993;106:285–289. doi: 10.1016/0300-9629(93)90513-4. [DOI] [PubMed] [Google Scholar]
- 28.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 29.Cechetti F, Fochesatto C, Scopel D, et al. Effect of a neuroprotective exercise protocol on oxidative state and BDNF levels in the rat hippocampus. Brain Res. 2008;1188:182–188. doi: 10.1016/j.brainres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Ohkuwa T, Sato Y, Naoi M. Glutathione status and reactive oxygen generation in tissues of young and old exercised rats. Acta Physiol Scand. 1997;159:237–244. doi: 10.1046/j.1365-201X.1997.576351000.x. [DOI] [PubMed] [Google Scholar]
- 31.Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006;28:184–189. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- 32.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]
- 33.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 34.El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]