Abstract
The patient population of borderline personality disorder (BPD) is heterogeneous; many different combinations of BPD symptoms can lead to a BPD diagnosis. We investigated to what extent the covariance among four main components of BPD is explained by shared genetic and environmental factors. Using an extended twin design, multivariate genetic models were applied to the scales of the PAI-BOR, a self-report questionnaire tapping four main features of BPD (affective instability, identity problems, negative relationships, and self-harm). Data on the four BPD scales were available for 5,533 twins and 1,202 siblings from the Netherlands, Belgium, and Australia. The correlations among the scales ranged from 0.23 to 0.50 and were best explained by a genetic common pathway model. This model specifies that genes and environment influence the covariance between four main features of BPD in qualitatively similar ways, through a single latent factor representing the BPD construct. The heritability of the latent BPD factor was 51% and the remainder of its variance was explained by unique environmental influences. For each BPD scale, except self-harm, around 50% of its variance was explained by the latent BPD factor. The remaining variance for each of the four scales was explained by genetic (4% for affective instability to 20% for self-harm) and environmental (38% for negative relationships to 67% for self-harm) factors that were specific to each scale.
Borderline personality disorder (BPD) is one of the most studied personality disorders (Blashfield & Mcelroy, 1987; Blashfield & Intoccia, 2000). However, when compared to research on disorders such as depression or other psychiatric disorders, studies on the genetic factors that influence the development of BPD are surprisingly sparse (Crowell et al., 2009). BPD is complex, as symptoms contributing to a BPD diagnosis are very heterogeneous. The Diagnostic and Statistical Manual for mental disorders (American Psychiatric Association, 2000) describes nine symptoms of BPD of which at least five must be present to warrant a BPD diagnosis. The presence of five or more out of nine symptoms, however, results in many possible combinations of symptoms leading to a BPD diagnosis.
At the population level, the clustering of symptoms of BPD has frequently been studied. Results of factor analytic studies of the DSM-III (Clarkin, Hull, & Hurt, 1993; Rosenberger & Miller, 1989; Sanislow, Grilo, & McGlashan, 2000; Becker, McGlashan, & Grilo, 2006) and DSM-IV (Fossati et al., 1999; Blais, Hilsenroth, & Castlebury, 1997; Johansen, Karterud, Pedersen, Gude, & Falkum, 2004; Benazzi, 2006; Taylor & Reeves, 2007) criteria for BPD show evidence for two (Rosenberger & Miller, 1989; Benazzi, 2006), three (Clarkin et al., 1993; Blais et al., 1997; Sanislow et al., 2000; Taylor & Reeves, 2007) or four (Becker et al., 2006) underlying factors. Important similarities between the structures identified in clinical and nonclinical samples were found (Taylor & Reeves, 2007). The factor structure found depends on the sample and instrument used. In our own study using data from twins and siblings from three countries and using the Personality Assessment Inventory Borderline features scale (Morey, 1991) to assess BPD features, we found that a four-factor structure best described the data (De Moor, Distel, Trull, & Boomsma, 2009). These four components of BPD are: affective instability, identity problems, negative relationships, and self-harm. Affective instability refers to the highly reactive moods of individuals with BPD in response to stimuli from the individual’s environment. The basic mood often shifts between periods of anger, panic, anxiety, or despair and is rarely relieved by periods of well-being or satisfaction. Identity problems involve a poorly defined concept of self. The self-image of persons with BPD may shift a lot, including sudden changes in opinions, sexual identity, types of friends, or career plans. The third factor, impulsivity, often results in self-damaging behavior. Common forms of impulsive behavior are excessive spending, reckless driving, binge eating, substance abuse, and promiscuity. Unstable and stormy relationships and feelings of loneliness reflect the fourth factor of the PAI-BOR: unstable relationships. This four-factor structure resembles the four scales of the PAI-BOR as proposed by Morey (Morey, 1991).
In the present study we explore why these features of BPD, represented by the four scales of the PAI-BOR, co-occur in the population by conducting genetic factor analyses. We test two models that represent different ways in which genes and environment might affect the four scales of the PAI-BOR. The first model, the independent pathway model, specifies direct paths from one or more genetic factors and one or more environmental factors common to all PAI-BOR scales as well as paths from unique genetic and environmental factors specific to each scale. In this model, genes and environment can influence the covariance between the four scales through different pathways. The second model, the single factor common pathway model, is based on the assumption that the covariation among the four BPD scales is determined by a single latent factor (the BPD construct) whose variance is determined by genetic and environmental influences. In this model, genes and environment influence the covariance between the four scales in similar ways. In both models, there may be genetic and environmental influences specific to each scale, but these influences do not affect the co-occurrence of the four scales (Neale & Cardon, 1992; Kendler, Heath, Martin, & Eaves, 1987).
Data from twins and their siblings were available from the Netherlands Twin Register (Boomsma et al., 2006), the East Flanders Prospective Twin Survey (Derom et al., 2006) and the Australian Twin Register (Jardine, Martin, & Henderson, 1984). Data from twins allow the identification of genetic and environmental factors, as monozygotic (MZ) twins share (nearly) 100% of their genetic material and dizygotic (DZ) twins and non-twin siblings share on average 50% of their segregating genes. By comparing the covariance structure in MZ and DZ twins, the relative influence of genetic and environmental factors on the variance in the four scales and on the covariance between them can be estimated and different multivariate genetic factor models can be tested.
METHOD
PARTICIPANTS
Data were collected as part of an international project on BPD features in Dutch, Belgian, and Australian twin cohorts. Twins and siblings were approached by mail and invited to participate in the study by completing a questionnaire. The Dutch sample consisted of 3,951 twins (1,209 complete pairs) and 1,202 siblings from 2,931 families registered with the Netherlands Twin Register (Boomsma et al., 2006). The Belgian sample consisted of 908 twins (242 complete pairs) from 595 families recruited through the East Flanders Prospective Twin Survey (Derom et al., 2006). A total of 674 twins (275 complete pairs) from 399 families were drawn from the Australian Twin Register (Jardine et al., 1984). Six months after the first questionnaire was sent 199 twins, siblings and parents (1 per family) from the Netherlands completed a retest survey. Details on response rates, demographic characteristics of the samples and zygosity determination procedures can be found elsewhere (Derom & Derom, 2005; Distel et al., 2007; Nyholt, 2006).
MEASURES
BPD features were measured by the 24-item Personality Assessment Inventory-Borderline Features scale (PAI-BOR; Morey, 1991). The PAI-BOR consists of four subscales each composed of six items that are rated on a four-point scale (0 to 3; false, slightly true, mainly true, very true). The four subscales are affective instability (AI; e.g., stability of mood and affect, emotionally responsiveness, and anger control), identity problems (IP; e.g., self image, concept of self, and feelings of emptiness), negative relationships (NR; e.g., intense and unstable relationships and loneliness) and self harm (SH; e.g., impulsivity, self-harm, and recklessness). Several studies have shown the PAI-BOR to be a reliable and valid measure of BPD features, and support the usefulness of the PAI-BOR in assessing BPD features in the general population as well as BPD in clinical settings (Kurtz, Morey, & Tomarken, 1993; BellPringle, Pate, & Brown, 1997; Stein, Pinkster-Aspen, & Hilsenroth, 2007; Trull, 1995). For example, Stein et al. (2007) showed that the PAI-BOR differentiates between patients diagnosed with BPD and patients without borderline personality pathology with 73% accuracy. Receiver operating character analysis showed that the PAI-BOR performs reasonably well in discriminating BPD patients and non-BPD depressed psychiatric patients, supporting the validity of PAI-BOR scores (Distel, Hottenga, Trull, & Boomsma, 2008). In the Netherlands and in Belgium, the Dutch translation of the PAI-BOR was used. The English PAI-BOR was translated into Dutch and translated back into English by a native English-speaking translator. The Dutch translation of the PAI-BOR was reviewed and approved by the test author and publishing company (Psychological Assessment Resources). Multigroup confirmatory factor analysis showed that the Dutch version of the PAI-BOR is measurement invariant across sex and age (De Moor et al., 2009). The PAI-BOR was scored according to the test manual, which states that at least 80% of the items must be answered to calculate a sum score and that missing and ambiguous answers should be substituted by a zero score (Morey, 1991).
ANALYSES
In twin-family studies, the different degree of genetic relatedness of monozygotic (MZ) and dizygotic (DZ) twin pairs and other first-degree relatives such as siblings is used to identify the relative contribution of genes and environment to the phenotypic variation of a trait. MZ twins share (nearly) all their genes while DZ twins and siblings share on average 50% of their segregating genes (Boomsma, Busjahn, & Peltonen, 2002a). For a (univariate) phenotype (P) in a single individual we can express P as:
| (1) |
where i refers to an individual and A, D, C, and E represent additive genetic, nonadditive genetic, common environmental and unique environmental factor scores respectively. A refers to the additive effects of alleles at all genomic loci contributing to the phenotype, D to nonadditive (dominance) effects of alleles, C to the effects of common environment shared by individuals growing up in the same family and E to nonshared environment (which also includes measurement error). The lower case letters a, d, c, and e are regression coefficients on the latent variables A, C, D, and E which are assumed to be independent of (uncorrelated with) each other. The expectation for the phenotypic variation may be written as:
| (2) |
Broad-sense heritability (h2) is the proportion of phenotypic variance that is attributable to genotypic variance (h2 = (V(A) + V(D)/V(P)); narrow-sense heritability is the proportion of variation explained by additive genetic factors (hn2 = V(A)/V(P)). Based on data from only MZ and DZ twins and siblings, this model is not identified and a choice for an ADE or ACE model needs to be made. This choice may be based on the pattern of correlations in MZ and DZ twins. When the DZ correlation is more than half the MZ correlation, there is evidence for environmental effects shared by twins from the same family (C) but when the DZ correlation is less than half the MZ correlation, there is evidence for nonadditive genetic effects (D). In the present study an ADE model was fitted to the data (see results section). Identification of the ADE model is achieved because, based on quantitative genetic theory, the correlation among the latent factors influencing the phenotype are known. For MZ twin pairs correlations between A1 and A2 (where A1 and A2 refer to the additive genetic factor score in twin 1 and twin 2) and between D1 and D2 is one. For DZ pairs, these correlations are 0.5 and 0.25, respectively. Correlations between E1 and E2 are zero in MZ and DZ pairs (e.g., Falconer & Mackay, 1996; Boomsma & Molenaar, 1986).
Multivariate genetic analyses can be applied to determine to what extent the covariation between traits can be explained by genetic and environmental factors. The comparison of MZ and DZ cross-twin cross-trait correlations provides a first indication about the shared etiology between traits. If a significant cross-twin cross-trait correlation is present it suggests that there is a familial influence on the etiology of the correlation between the two traits. If the MZ cross-twin cross-trait correlation exceeds the DZ cross-twin cross-trait correlation it suggests that the familial influence on the correlation is at least partly genetic in origin. Equations 1 and 2 can be generalized to multivariate phenotypes by writing:
| (3) |
where i refers to individual and j to trait.
| (4) |
where Σ has dimension j × j and consists of variances on the diagonal and covariances on the off-diagonal. If, for example, Σ(E) is a diagonal matrix, then environmental correlations among traits are zero and the traits are only influenced by trait specific environmental factors (Martin & Eaves, 1977; Polderman et al., 2007; Boomsma, Molenaar, & Orlebeke, 1990).
Qualitative and quantitative sex differences in genetic architecture can arise in all parameters of the model. A first impression of such differences is obtained by inspection of twin correlations in male and female MZ and DZ twin pairs. If, for example, heritability is larger in men, we expect MZ males > MZ females and DZ males > DZ females. Qualitative sex differences are suggested if correlations in DZ twins of opposite sex (DOS) cannot be predicted based on the pattern of correlations in same-sex twin pairs. Testing for quantitative sex differences in the importance of A, D/C, and E can be achieved by testing the equality of correlations in male-male and female-female twin pairs by constraining the correlations between men and women within zygosity to be equal. To test whether the same genes influence BPD features in men and women (qualitative differences) DOS correlations are predicted from DZ same-sex correlations.
We first fitted a saturated multivariate model that estimated means, variances, and covariances (among family members and among scales). Data from the three countries were analyzed simultaneously in a multigroup analysis. For each scale an effect of sex and age was modeled and tested for significance. These effects were included as a regression of sex (coded as 0 for males and 1 for females) and age (in years) on each scale. By constraining the regression coefficients to equal zero and examining the change in log-likelihood we tested the significance of these effects. Significant effects of sex and age were retained in subsequent genetic analyses.
All correlations between MZ and DZ twin and sibling pairs within and between scales were initially estimated as a function of zygosity and sex. By constraining within-scale and cross-scale correlations to be equal for men and women within the zygosity groups qualitative and quantitative sex differences were tested.
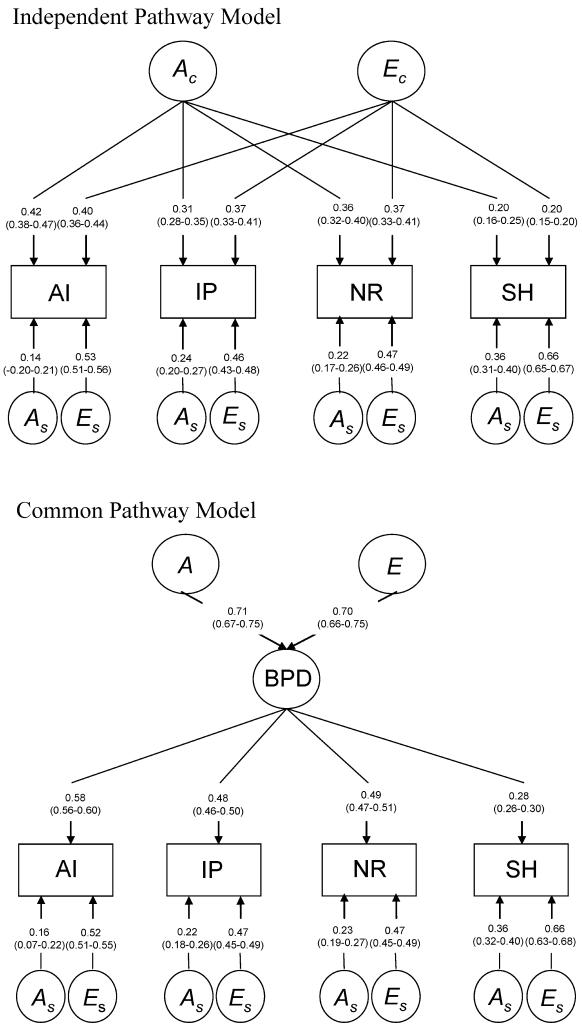
We fitted three multivariate genetic models to the data (Figure 1 provides graphical representations of the three models):
A Cholesky (or triangular) decomposition (model 1) decomposes the covariance matrix among the four scales into genetic and environmental covariance matrices (e.g., Σ(A) and Σ(E)). The Cholesky decomposition is a fully parameterized, descriptive model and yields the best fit of a variance components model to the data. It imposes no underlying structure on the genetic and environmental influences and can be fitted to the data as depicted in Figure 1 for an AE model, (i.e., if there are 4 scales there are four A and four E factors). The order of the variables in a Cholesky decomposition is arbitrary in that either order would produce the same fit to the data. However, with sex limitation this is not the case. It is therefore important to explore whether qualitative and quantitative sex differences are present before fitting a Cholesky model to the data (Neale, Roysamb, & Jacobson, 2006). The full Cholesky model serves as a baseline model to which more restricted factor models can be compared; in our case the independent pathway and the common pathway models.
The independent pathway model (model 2) specifies direct paths from genetic and environmental factors common to all scales as well as paths from genetic and environmental factors specific to each scale. Loadings on the common genetic factor contribute to the within-person cross-scale and to the cross-person cross-scale correlations. Loadings on the common environmental factor contribute to the within-person cross-scale correlation but not to the cross-person cross-scale correlation. The same genetic and environmental factors thus influence scores on all four scales of the PAI-BOR, although the magnitude of the effects can differ per scale. Loadings on the scale specific genetic factors contribute to the correlation between persons for a specific scale, but not to correlations across scales. Loadings on the scale specific environmental factors do not contribute to correlations between scales or between family members. Based on the results of the saturated model, parameter estimates were constrained to be equal between the countries, when possible.
The common pathway model (model 3) is a more stringent version of the independent pathway model and tests the assumption that the covariation among the scales is determined by one or more latent factors (common pathways) whose variance is determined by a genetic and an environmental factor. However, under the common pathway model, genetic and environmental factors affect the trait by both acting on the same latent variable (Neale & Cardon, 1992).
FIGURE 1.
Graphical representations of the Cholesky, independent pathway and the common pathway models. AI = affective instability; IP = identity problems; NR = negative relationships; SH = self harm; BPD = borderline personality disorder; A1 through A4 = additive genetic factors, E1 through E4 = unique environmental factors, Ac = additive genetic factor common to multiple traits; Ec = unique environmental factor common to multiple traits; As = specific genetic factors; Es = specific unique environmental factors; a, e, and f = factor loadings; k = latent factor A or E; j = phenotype AI, IP, NR, or SH. All latent A and E factors have unit variance.
Model fitting was performed using the structural equation modelling software package Mx (Neale, Boker, Xie, & Maes, 2003). Comparison of models was done by means of likelihood-ratio tests, by subtracting the negative log likelihood (−2LL) for the more restricted models (models 2 and 3) from the −2LL for the general model (model 1). This yields a statistic that is distributed as χ2 with degrees of freedom (df) equal to the difference in the number of parameters in the two models. If the χ2-test yields a p-value higher than 0.01, the constrained model is deemed not significantly worse than the unconstrained model and is therefore the most parsimonious model. In addition, Akaike’s Information Criterion (AIC; Akaike, 1987), calculated as χ2−2df, was evaluated because it reflects both the goodness of fit and the parsimony of the model. The lower the AIC value, the better the fit of the model relative to the number of parameters estimated (Lubke & Neale, 2006; Markon & Krueger, 2004). Finally, Bayesian information criterion (BIC; Schwarz, 1978), calculated as 0.5, −2LL−df *ln(N), is reported.
Because the data on all four scales showed a somewhat skewed distribution, a square root transformation was performed.
RESULTS
DESCRIPTIVES
Table 1 shows the number of participants from complete and incomplete twin pairs, mean age, standard deviation, and age range per zygosity in each country. In total, 5,533 twins (1,879 complete twin pairs) and 1,202 siblings from 3,925 families took part in the study. The 6-month test-retest correlation of the Dutch PAI-BOR scales were 0.75, 0.69, 0.60, and 0.53 for AI, IP, NR, and SH, respectively. The internal consistencies (Cronbach’s alpha) of the scales AI, IP, NR, and SH were 0.71, 0.62, 0.56, and 0.64 in the Dutch sample, 0.66, 0.67, 0.59, and 0.67 in the Belgian sample and 0.78, 0.68, 0.70, and 0.73 in the Australian sample, respectively.
TABLE 1.
Number (N) of Participants from Complete/Incomplete Twin Pairs, Mean Age, Standard Deviation (SD) and Age Range per Zygosity in Each Country
| Zygosity | N | Mean Age | SD | Range |
|---|---|---|---|---|
| Dutch MZ males | 378/190 | 35.8 | 13.0 | 19–76 |
| Dutch DZ males | 156/151 | 35.0 | 11.6 | 19–75 |
| Dutch MZ females | 1,146/384 | 35.7 | 12.2 | 19–86 |
| Dutch DZ females | 484/273 | 35.0 | 10.9 | 19–74 |
| Dutch males from DZ opposite-sex pairs | 209/107 | 33.4 | 10.5 | 20–75 |
| Dutch females from DZ opposite-sex pairs | 209/264 | 32.9 | 9.5 | 19–75 |
| Dutch brothers | 449 | 38.7 | 14.2 | 18–90 |
| Dutch sisters | 753 | 38.2 | 11.3 | 18–84 |
| Belgian MZ males | 118/45 | 27.5 | 6.1 | 18–40 |
| Belgian DZ males | 32/33 | 28.3 | 5.7 | 18–40 |
| Belgian MZ females | 246/72 | 29.7 | 6.9 | 18–48 |
| Belgian DZ females | 88/58 | 29.1 | 7.2 | 18–46 |
| Belgian males from DZ opposite-sex pairs | 71/14 | 25.5 | 6.1 | 18–39 |
| Belgian females from DZ opposite-sex pairs | 71/60 | 27.2 | 6.7 | 18–40 |
| Australian MZ males | 100/36 | 23.2 | 3.7 | 18–33 |
| Australian DZ males | 58/18 | 22.2 | 2.6 | 18–29 |
| Australian MZ females | 170/23 | 23.4 | 3.9 | 18–33 |
| Australian DZ females | 96/12 | 24.5 | 4.0 | 18–32 |
| Australian males from DZ opposite-sex pairs | 63/15 | 22.1 | 3.6 | 18–32 |
| Australian females from DZ opposite-sex pairs | 63/20 | 22.2 | 3.6 | 18–32 |
Note. MZ = monozygotic; DZ = dizygotic
TESTS OF FIXED EFFECTS ON MEAN STRUCTURE
Sex effects on the means were significant in the Dutch sample for AI (χ2 = 66.5, p < 0.001), IP (χ2 = 64.1, p < 0.001), and NR (χ2 = 28.2, p < 0.001); with women scoring higher than men. The same direction of effect was seen for AI (χ2 = 2.5, p = 0.112), IP (χ2 = 4.0, p = 0.045), and NR (χ2 = 1.3, p = 0.261) and SH (χ2 = 0.4, p = 0.530) in the Belgian data and for AI (χ2 = 5.3, p = 0.022), IP (χ2 = 4.9, p = 0.026), and NR (χ2 = 4.5, p = 0.034) in the Australian data, but these effects were not significant. Men from the Netherlands (χ2 = 0.13, p = 0.720) and Australia (χ2 = 3.9, p = 0.049) had higher scores on the subscale SH than women, but these effects were not significant. BPD features decreased significantly with age (all p < 0.01) except for NR in the Dutch sample (χ2 = 5.5, p = 0.019) and AI (χ2 = 1.01, p = 0.313) and NR (χ2 = 1.1, p = 0.290) in the Australian sample.
CORRELATION STRUCTURE
Phenotypic correlations (within-person, cross-scales) and twin and sibling correlations (cross-persons, within-scale) did not differ significantly across the three countries. Cross correlations (cross-persons, cross-scales) also did not differ significantly. Based on these results, in subsequent analyses all correlations were constrained to be equal between countries. Table 2 summarizes the correlation structure for males, females, and opposite-sex pairs. The first four columns show the phenotypic correlations for men and women. Constraining the phenotypic correlations to be equal for men and women did not result in a significant deterioration of model fit (χ2 = 14.28, p = 0.027). The diagonals of the other 4 × 4 correlation matrices show the within-scale correlations, the off-diagonals show the cross-scales correlations. The within-scale and cross-scales correlations did not differ significantly between MZ male and female twin pairs (χ2(4) = 3.57, p = 0.467 and χ2(6) = 4.41, p = 0.622), nor between DZ twin and sibling male and female pairs (χ2(4) = 7.25, p = 0.123 and χ2(6) = 8.95, p = 0.177). This indicates that the heritability for the four scales of the PAI-BOR is the same for men and women and that the same genetic structure explains the covariance between the four scales in men and women. The within-scale and cross-scales correlations for opposite-sex DZ twin and sibling pairs did not differ from same-sex DZ twin and sibling pairs (χ2(4) = 4.62, p = 0.328 and χ2(6) = 5.59, p = 0.471) indicating that the same set of genes influences BPD features in men and women. All correlations were thus equal across sex. Estimates of the MZ and DZ twin/sibling correlations were 0.34 and 0.12 for AI, 0.33 and 0.13 for IP, 0.37 and 0.14 for NR, and 0.31 and 0.08 for SH. All MZ twin correlations were more than twice as large as those for DZ twins and siblings, suggesting that the genetic effects that contribute to individual differences may be partly nonadditive. All MZ cross-scales correlations exceeded the DZ cross-scales correlations, suggesting that factors influencing all four scales of the PAI-BOR are at least partly genetic. Based on the correlation structure, ADE models were fitted in subsequent analyses. The absence of evidence for sex-limitation in these data circumvents the problems with the Cholesky model noted by Neale et al (2006).
TABLE 2.
MZ and DZ Male and Female within Person, Within-Scale, and Cross-Scale Correlations for Affective Instability (AI), Identity Problems (IP), Negative Relationships (NR), and Self-Harm (SH)
| Within scale (diagonal) and cross scale (off diagonals) correlations |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within person correlation |
Male pairs |
Female pairs |
Opposite sex pairs |
||||||||||||||||
| AI | IP | NR | SH | AI | IP | NR | SH | AI | IP | NR | SH | AI | IP | NR | SH | ||||
| AI | Males | 1 | AI | MZ | 0.30 | 0.36 | — | ||||||||||||
| Females | 1 | DZ | 0.09 | 0.16 | 0.08 | ||||||||||||||
| IP | Males | 0.48 | 1 | IP | MZ | 0.28 | 0.38 | 0.25 | 0.32 | — | — | ||||||||
| Females | 0.50 | 1 | DZ | 0.12 | 0.12 | 0.12 | 0.15 | 0.06 | 0.11 | ||||||||||
| NR | Males | 0.50 | 0.44 | 1 | NR | MZ | 0.25 | 0.23 | 0.34 | 0.29 | 0.22 | 0.38 | — | — | — | ||||
| Females | 0.50 | 0.47 | 1 | DZ | 0.09 | 0.09 | 0.15 | 0.15 | 0.13 | 0.17 | 0.08 | 0.08 | 0.12 | ||||||
| SH | Males | 0.30 | 0.26 | 0.28 | 1 | SH | MZ | 0.15 | 0.10 | 0.13 | 0.29 | 0.13 | 0.11 | 0.14 | 0.31 | — | — | — | — |
| Females | 0.23 | 0.22 | 0.24 | 1 | DZ | 0.11 | 0.05 | 0.09 | 0.23 | 0.01 | 0.02 | 0.05 | 0.07 | 0.05 | 0.05 | 0.06 | 0.05 | ||
MULTIVARIATE GENETIC MODELING
In the multivariate genetic models nonadditive genetic effects could be removed from the model without a significant deterioration in the fit of the model (χ2(10) = 20.1, p = 0.029). Thus, variance in AI, IP, NR, and SH and their covariance can be explained by additive genetic and unique environmental factors. Results of the Cholesky decomposition are depicted in Table 3. In addition to the phenotypic correlations between scales, the genetic and environmental correlations are given. Their impact on the phenotypic correlation is weighted by the heritabilities (h2) and environmentalities (e2) of the scales. The heritability estimates for AI, IP, NR, and SH were 31% (95% CI 27%–35%), 31% (95% CI 26%–35%), 35% (95% CI 31%–39%), and 26% (95% CI 22%–30%), respectively, and the remainder of the variance was explained by e2. The genetic risk factors for AI, IP, and NR were strongly correlated (rg 0.67 to 0.81) while the genetic risk factors for SH and the other three scales were moderately correlated (rg 0.37 to 0.46). The same pattern was seen for the environmental correlations. The phenotypic correlations between the four scales were explained half by genetic effects and half by unique environmental effects.
TABLE 3.
Estimates of Phenotypic (rp), Genetic (rg) and Environmental (re), Correlations and the Percentage of Correlation Explained by Genetic and Environmental Factors (95% Confidence Intervals)
| rp | rg | % of correlation explained by genetic factors |
re | % of correlation explained by environmental factors |
|
|---|---|---|---|---|---|
| AI-IP | 0.50 | 0.77 (0.76–0.84) | 48% (42%–55%) | 0.37 (0.33–0.41) | 52% (45%–57%) |
| AI-NR | 0.50 | 0.81 (0.75–0.88) | 54% (48%–60%) | 0.34 (0.30–0.38) | 46% (39%–52%) |
| AI-SH | 0.25 | 0.43 (0.32–0.54) | 49% (36%–62%) | 0.18 (0.14–0.22) | 51% (38%–64%) |
| IP-NR | 0.46 | 0.67 (0.59–0.74) | 48% (40%–54%) | 0.36 (0.33–0.40) | 52% (46%–59%) |
| IP-SH | 0.23 | 0.37 (0.25–0.48) | 45% (30%–59%) | 0.18 (0.15–0.22) | 55% (41%–70%) |
| NR-SH | 0.25 | 0.46 (0.35–0.56) | 55% (42%–68%) | 0.16 (0.12–0.20) | 45% (32%–58%) |
Note. AI = affective instability; IP = identity problems; NR = negative relationships; SH = self-harm
Next we fitted two models with different theoretical implications on this pattern of covariances: the independent pathway and the common pathway model. The estimated path coefficients of these models are depicted in Figure 2. The path coefficients were standardized and squared to calculate the proportion of variance accounted for by the latent predictor variables A and E, shown in percentages in Table 4. For example, the total variance in AI in the independent pathway model is 0.63 (0.402 + 0.422 + 0.142 + 0.532). The variance in AI accounted for by the common genetic factor divided by the total variance gives the proportion of variance in AI accounted for by the common genetic factor (0.422/0.63 = 0.28). The independent pathway model shows that around 20% to 28% of the variance in AI, IP, and NR can be explained by a common genetic factor while only 6% of the variance in SH can be explained by the common genetic factor. However, SH did load significantly on the common genetic factor since this path could not be left out of the model without a significant deterioration of the fit of the model. To calculate the percentage of variance accounted for by the latent predictor variables A and E in the common pathway model, a similar procedure is followed. For example, the total variance in IP is 0.50 (0.482 + 0.222 + 0.472). The variance in IP accounted for by genetic variation in the common factor can by calculated by dividing the product of the additive genetic variance of the latent predictor variable and the variance in IP accounted for by the latent predictor variable by the total variance in IP (0.712 * 0.482/0.50 = 0.23). In the common pathway model all scales load significantly on the latent BPD factor, but SH the least strongly (6% for SH and 27%, 23%, and 24% for AI, IP, and NR, respectively). Genetic model fitting results are summarized in Table 5. Both the independent pathway and the common pathway model did not fit the data significantly worse than the Cholesky decomposition. Based on the principle of model parsimony (the least complex model which gives an adequate account of the data), the common pathway model explained the data best. The four main features of BPD thus co-occur as a result of genetic and environmental factors that influence the four component in similar ways, through a latent predictor variable (the BPD construct).
FIGURE 2.
Graphical representation and path coefficients (95% confidence intervals) of the independent pathway model and the common pathway model. AI = affective instability; IP = identity problems; NR = negative relationships; SH = self harm; BPD = borderline personality disorder; Ac = genetic factor common to multiple traits; Ec = unique environmental factor common to multiple traits; As = specific genetic factors; Es = specific unique environmental factors. All latent A and E factors have unit variance.
TABLE 4.
Percentage of Variance Accounted for by the Genetic and Environmental Factors Common and Specific to Each Variable in the Independent and Common Pathway Model
| Independent Pathway Model |
Common Pathway Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ac | Ec | As | Es | Ac | Ec | As | Es | |
| AI | 28% | 25% | 3% | 44% | 27% | 26% | 4% | 43% |
| IP | 20% | 27% | 1% | 42% | 23% | 23% | 10% | 44% |
| NR | 24% | 26% | 9% | 41% | 24% | 23% | 10% | 43% |
| SH | 6% | 6% | 20% | 68% | 6% | 6% | 20% | 68% |
| BPD | — | — | — | — | 51% | 49% | — | — |
Note. AI = affective instability; IP = identity problems; NR = negative relationships; SH = self-harm; BPD = borderline personality disorder; Ac = common genetic factor; Ec = common unique environmental factor; As = specific genetic factor; Es = specific unique environmental factor.
TABLE 5.
Genetic Model Fitting Results Including Data from the Three Countries
| vs | −2 LL | df | χ 2 | Δ df | p | AIC | BIC | |
|---|---|---|---|---|---|---|---|---|
| 1. Cholesky | 55,284.2 | 26,589 | ||||||
| 2. Independent pathway model | 1. | 55,287.5 | 26,593 | 3.3 | 4 | 0.51 | −4.7 | −82,315.092 |
| 3. Common pathway model | 1. | 55,291.8 | 26,596 | 7.7 | 7 | 0.36 | −6.3 | −82,333.577 |
Note. Vs = versus; −2 LL = −2 log likelihood; df = degrees of freedom; AIC = Akaike’s information criterion; BIC = Bayesian information criterion.
DISCUSSION
The aim of the present study was to examine the relationship between the four scales of the PAI-BOR, reflecting four main features of BPD, in terms of genetic and environmental risk factors. Because BPD is a complex disorder with various manifestations, exploring the main features of BPD may lead to a better understanding of the etiology of BPD. We first established that there is a substantial association among the four scales. We then applied a series of multivariate genetic factor models, including the independent pathway and the common pathway models, to investigate the etiology of this association between the scales at the level of genetic and environmental influences. The common pathway model was the most parsimonious. This model tests the assumption that the covariation among the four scales is determined by a single latent factor. Genetic and environmental factors thus influence AI, IP, NR, and SH through the same mechanism. Additive genetic factors explained 51% of the variance in the latent BPD factor and unique environmental factors explained the remaining 49%. This heritability estimate is somewhat higher than the estimate we obtained for the total PAI-BOR score (h2 = .42; Distel, Hottenga, et al., 2008), which was based on a sum score of all items and on data of twins only. In the present study, the four scales AI, IP, NR, and SH were moderately heritable with estimates ranging from 26% (SH) to 35% (NR). These estimates were equal for men and women and the same genes influenced variation in men and women. Thus, although BPD is more often diagnosed in women than in men, there is no evidence for gender differences in genetic and environmental effects on BPD. In addition, there was no support for a different factor structure for men and women. All scales load substantially on the latent BPD factor except for SH of which only 12% of the variance is explained by the common factor. Each scale was also influenced by specific genetic factors, which do not overlap with each other. These genetic factors specific to each scale explained a much smaller amount of variance than the common genetic factor, except for SH (4% versus 27% for AI, 10% versus 23% for IP, 10% versus 24% for NR, and 20% versus 6% for SH). Thus there is support for a genetic factor which makes individuals vulnerable to all four main features of BPD. In addition, genetic effects specific to each scale contribute modestly to individual differences in each of the four scales.
Though the twin correlations suggested a contribution of nonadditive genetic influence, nonadditive genetic effects were not significant. This may be partly due to the low statistical power of the classical twin study to resolve the effects of genetic nonadditivity (Martin & Eaves, 1977; Neale, Eaves, & Kendler, 1994; Visscher, 2004). The heritability estimate in this study is thus likely to include some nonadditive effects.
An interesting finding of our study is the strong unique environmental covariance between the four scales of the PAI-BOR. This means there are environmental factors which simultaneously increase the risk for AI, IP, NR, and SH. Many studies into the aetiology of BPD focused on the environmental determinants of BPD and demonstrated that traumatic life events such as sexual or physical abuse and parental divorce, loss or illness are generally more common in patients with BPD than in nonpatients or patients with other personality disorders (Westen, Ludolph, Misle, Ruffins, & Block, 1990; Parker et al., 1999; Bandelow et al., 2005; Paris, Zwergfrank, & Guzder, 1994a, 1994b; Zanarini et al., 1997; Ogata et al., 1990; Helgeland & Torgersen, 2004; Horesh, Ratner, Zaor, & Toren, 2008). Also, the total number of negative life events to which BPD patients have been exposed is higher than for control subjects (Horesh et al., 2008; Jovev & Jackson, 2006). Based on this study it is likely that these life events influence all four main features of BPD. However, not all individuals who have experienced a traumatic event develop BPD, thus a genetic vulnerability in addition to the influence of environment is a likely requirement. Also, gene-environment interaction in which the effect of exposure to environmental factors depends on a person’s genotype may play a role. In the presence of gene-environment interaction, individuals with a sensitive genotype will be at greater risk if the predisposing environment is present, than individuals with an insensitive genotype (Boomsma & Martin, 2002; Rutter, 2007). If gene by environment interaction is present for BPD, this will have increased the estimates for E. In addition, certain life events may be a consequence, rather than a cause, of BPD features.
Several limitations should be kept in mind when interpreting the results of this study. First, some selection bias may have been present in the sample. The Dutch sample, which constituted the largest in the present study, was shown to be representative of the general population with regard to a number of variables such as socioeconomic status, smoking behavior, and religion (Boomsma, Vink, et al., 2002). However, individuals from less cooperative families (i.e., families in which only some individuals participate) show slightly more borderline personality features than individuals from highly cooperative families (i.e., families in which most individuals participate; Distel et al., 2007). Second, while the four scales of the PAI-BOR are all important clinical characteristics of the disorder, one (Fossati et al., 1999), two (Rosenberger & Miller, 1989) and three (Clarkin et al., 1993; Sanislow et al., 2000; Sanislow et al., 2002) factor structures have also been reported when different measures are used.
In conclusion, the results of this study suggest that genetic and environmental effects influence affective instability, identity problems, negative relationships, and self-harm through an intermediate phenotype, the BPD construct. A single genetic factor underlies most of the genetic variance in this latent variable and thus in most symptoms, although genetic effects specific to each components are also present, particularly for SH. This is important for future studies trying to find the causative genes for BPD features.
Acknowledgments
The Borderline Personality Disorder Research Foundation; Spinozapremie (NWO/SPI 56-464-14192); Center for Neurogenomics and Cognitive Research, VU; Center for Medical Systems Biology (NWO Genomics); Twin-family database for behavior genetics and genomics studies (NWO 480-04-004); Genome-wide analyses of European twin and population cohorts (EU/QLRT-2001-01254); Psychometric and genetic assessments of substance use (PI Neale; NIH DA-18673, DA-026119).
Contributor Information
Marijn A. Distel, VU University Amsterdam, Amsterdam, The Netherlands.
Gonneke Willemsen, VU University Amsterdam, Amsterdam, The Netherlands.
Lannie Ligthart, VU University Amsterdam, Amsterdam, The Netherlands.
Catherine A. Derom, University Hospital Gasthuisberg, Katholieke Universiteit Leuven, Belgium.
Nicholas G. Martin, Queensland Institute of Medical Research, Brisbane, Australia.
Michael C. Neale, Virginia Commonwealth University, USA.
Timothy J. Trull, University of Missouri-Columbia, Columbia, MO, USA.
Dorret I. Boomsma, VU University Amsterdam, Amsterdam, The Netherlands.
REFERENCES
- Akaike H. Factor-analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Press; Washington, DC: 2000. text revision. [Google Scholar]
- Bandelow B, Krause J, Wedekind D, Broocks A, Hajak G, Ruther E. Early traumatic life events, parental attitudes, family history, and birth risk factors in patients with borderline personality disorder and healthy controls. Psychiatry Research. 2005;134:169–179. doi: 10.1016/j.psychres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Becker DF, McGlashan TH, Grilo CM. Exploratory factor analysis of borderline personality disorder criteria in hospitalized adolescents. Comprehensive Psychiatry. 2006;47:99–105. doi: 10.1016/j.comppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- BellPringle VJ, Pate JL, Brown RC. Assessment of borderline personality disorder using the MMPI-2 and the personality assessment inventory. Assessment. 1997;4:131–139. [Google Scholar]
- Benazzi F. Borderline personality—bipolar spectrum relationship. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30:68–74. doi: 10.1016/j.pnpbp.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Blais MA, Hilsenroth MJ, Castlebury FD. Content validity of the DSM-IV borderline and narcissistic personality disorder criteria sets. Comprehensive Psychiatry. 1997;38:31–37. doi: 10.1016/s0010-440x(97)90050-x. [DOI] [PubMed] [Google Scholar]
- Blashfield RK, Intoccia V. Growth of the literature on the topic of personality disorders. American Journal of Psychiatry. 2000;157:472–473. doi: 10.1176/appi.ajp.157.3.472. [DOI] [PubMed] [Google Scholar]
- Blashfield RK, Mcelroy RA. The 1985 journal literature on the personality-disorders. Comprehensive Psychiatry. 1987;28:536–546. doi: 10.1016/0010-440x(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, de Geus EJC, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, et al. Netherlands twin register: From twins to twin families. Twin Research and Human Genetics. 2006;9:849–857. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Martin NG. Gene-environment interaction. In J. A. D’Haenen, J. A. den Boer, & P. Willner (Eds.), Biological Psychiatry. John Wiley & Sons, Ltd; Chichester: 2002. pp. 181–187. [Google Scholar]
- Boomsma DI, Molenaar PCM. Using lisrel to analyze genetic and environmental covariance structure. Behavior Genetics. 1986;16:237–250. doi: 10.1007/BF01070799. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Molenaar PCM, Orlebeke JF. Estimation of individual genetic and environmental factor scores. Genetic Epidemiology. 1990;7:83–91. doi: 10.1002/gepi.1370070115. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Vink JM, van Beijsterveldt TC, Geus EJC, Beem AL, Mulder EJ, et al. Netherlands twin register: A focus on longitudinal research. Twin Research. 2002;5:401–406. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- Clarkin JF, Hull JW, Hurt SW. Factor structure of borderline personality-disorder criteria. Journal of Personality Disorders. 1993;7:137–143. [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychological Bulletin. 2009;135:495–510. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor MHM, Distel MA, Trull TJ, Boomsma DI. Assessment of borderline personality disorder features in population samples: Is the personality assessment inventory-borderline scale measurement invariant across sex and age? Psychological Assessment. 2009;21:125–130. doi: 10.1037/a0014502. [DOI] [PubMed] [Google Scholar]
- Derom C, Derom R. The East flanders prospective twin survey. In: Blickstein I, Keith LG, editors. Multiple pregnancy: Epidemiology, gestation and perinatal outcome. 2 ed. Taylor and Francis; Oxford: 2005. pp. 39–47. [Google Scholar]
- Derom CA, Vlietinck RF, Thiery EW, Leroy FOG, Fryns JP, Derom RM. The East flanders prospective twin survey (EFPTS) Twin Research and Human Genetics. 2006;9:733–738. doi: 10.1375/183242706779462723. [DOI] [PubMed] [Google Scholar]
- Distel MA, Hottenga JJ, Trull TJ, Boomsma DI. Chromosome 9: Linkage for borderline personality disorder features. Psychiatric Genetics. 2008;18:302–307. doi: 10.1097/YPG.0b013e3283118468. [DOI] [PubMed] [Google Scholar]
- Distel MA, Ligthart L, Willemsen G, Nyholt DR, Trull TJ, Boomsma DI. Personality, health and lifestyle in a questionnaire family study: A comparison between highly cooperative and less cooperative families. Twin Research and Human Genetics. 2007;10:348–353. doi: 10.1375/twin.10.2.348. [DOI] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, et al. Heritability of borderline personality disorder features is similar across three countries. Psychological Medicine. 2008;38:1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th ed. Longman Group Ltd; Essex, England: 1996. [Google Scholar]
- Fossati A, Maffei C, Bagnato M, Donati D, Namia C, Novella L. Latent structure analysis of DSM-IV borderline personality disorder criteria. Comprehensive Psychiatry. 1999;40:72–79. doi: 10.1016/s0010-440x(99)90080-9. [DOI] [PubMed] [Google Scholar]
- Helgeland MI, Torgersen S. Developmental antecedents of borderline personality disorder. Comprehensive Psychiatry. 2004;45:138–147. doi: 10.1016/j.comppsych.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Horesh N, Ratner S, Laor N, Toren P. A comparison of life events in adolescents with major depression, borderline personality disorder and matched controls: A pilot study. Psychopathology. 2008;41:300–306. doi: 10.1159/000141925. [DOI] [PubMed] [Google Scholar]
- Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genetic Epidemiology. 1984;1 doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- Johansen M, Karterud S, Pedersen G, Gude T, Falkum E. An investigation of the prototype validity of the borderline DSM-IV construct. Acta Psychiatrica Scandinavica. 2004;109:289–298. doi: 10.1046/j.1600-0447.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- Jovev M, Jackson HJ. The relationship of borderline personality disorder, life events and functioning in an Australian psychiatric sample. Journal of Personality Disorders. 2006;20:205–217. doi: 10.1521/pedi.2006.20.3.205. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and symptoms of depression—Same genes, different environments. Archives of General Psychiatry. 1987;44 doi: 10.1001/archpsyc.1987.01800170073010. [DOI] [PubMed] [Google Scholar]
- Kurtz JE, Morey LC, Tomarken AJ. The concurrent validity of three self-report measures of borderline personality. Journal of Psychopathology and Behavioral Assessment. 1993;15:255–266. [Google Scholar]
- Lubke G, Neale MC. Distinguishing between latent classes and continuous factors: Resolution by maximum likelihood? Multivariate Behavioral Research. 2006;41:499–532. doi: 10.1207/s15327906mbr4104_4. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behavior Genetics. 2004;34:593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ. Genetic analysis of covariance structure. Heredity. 1977;38:79–95. doi: 10.1038/hdy.1977.9. [DOI] [PubMed] [Google Scholar]
- Morey LC. The personality assessment inventory: Professional manual. Psychological Assessment Resources; Odessa, FL: [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. MX: Statistical modelling. 6th edn Virginia Commonwealth University; Richmond, VA: 2003. [Google Scholar]
- Neale MC, Cardon L. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht: 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G × E interaction. Twin Research and Human Genetics. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR. On the probability of dizygotic twins being concordant for two alleles at multiple polymorphic loci. Twin Research and Human Genetics. 2006;9:194–197. doi: 10.1375/183242706776382383. [DOI] [PubMed] [Google Scholar]
- Ogata SN, Silk KR, Goodrich S, Lohr NE, Westen D, Hill EM. Childhood sexual and physical abuse in adult patients with borderline personality-disorder. American Journal of Psychiatry. 1990;147:1008–1013. doi: 10.1176/ajp.147.8.1008. [DOI] [PubMed] [Google Scholar]
- Paris J, Zweigfrank H, Guzder J. Psychological risk-factors for borderline personality-disorder in female-patients. Comprehensive Psychiatry. 1994;35:301–305. doi: 10.1016/0010-440x(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Paris J, Zweigfrank H, Guzder J. Risk-factors for borderline personality in male outpatients. Journal of Nervous and Mental Disease. 1994b;182:375–380. doi: 10.1097/00005053-199407000-00002. [DOI] [PubMed] [Google Scholar]
- Parker G, Roy K, Wilhelm K, Mitchell P, Austin MP, Hadzi-Pavlovic D. An exploration of links between early parenting experiences and personality disorder type and disordered personality functioning. Journal of Personality Disorders. 1999;13:361–374. doi: 10.1521/pedi.1999.13.4.361. [DOI] [PubMed] [Google Scholar]
- Polderman TJC, Posthuma D, De Sonneville LMJ, Stins JF, Verhulst FC, Boomsma DI. Genetic analyses of the stability of executive functioning during childhood. Biological Psychology. 2007;76:11–20. doi: 10.1016/j.biopsycho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Rosenberger PH, Miller GA. Comparing borderline definitions—DSM-III borderline and schizotypal personality-disorders. Journal of Abnormal Psychology. 1989;98:161–169. doi: 10.1037//0021-843x.98.2.161. [DOI] [PubMed] [Google Scholar]
- Rutter M. Gene-environment interdependence. Developmental Science. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Grilo CM, McGlashan TH. Factor analysis of the DSM-III-R borderline personality disorder criteria in psychiatric inpatients. American Journal of Psychiatry. 2000;157:1629–1633. doi: 10.1176/appi.ajp.157.10.1629. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Grilo CM, Morey LC, Bender DS, Skodol AE, Gunderson JG, et al. Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: Findings from the collaborative longitudinal personality disorders study. American Journal of Psychiatry. 2002;159:284–290. doi: 10.1176/appi.ajp.159.2.284. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Stein MB, Pinkster-Aspen JH, Hilsenroth MJ. Borderline pathology and the personality assessment inventory (PAI): An evaluation of criterion and concurrent validity. Journal of Personality Assessment. 2007;88:81–89. doi: 10.1080/00223890709336838. [DOI] [PubMed] [Google Scholar]
- Taylor J, Reeves M. Structure of borderline personality disorder symptoms in a nonclinical sample. Journal of Clinical Psychology. 2007;63:805–816. doi: 10.1002/jclp.20398. [DOI] [PubMed] [Google Scholar]
- Trull TJ. Borderline personality disorder features in nonclinical young adults: 1. Identification and validation. Psychological Assessment. 1995;7:33–41. [Google Scholar]
- Visscher PM. Power of the classical twin design revisited. Twin Research. 2004;7:505–512. doi: 10.1375/1369052042335250. [DOI] [PubMed] [Google Scholar]
- Westen D, Ludolph P, Misle B, Ruffins S, Block J. Physical and sexual abuse in adolescent girls with borderline personality-disorder. American Journal of Orthopsychiatry. 1990;60:55–66. doi: 10.1037/h0079175. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF, et al. Reported pathological childhood experiences associated with the development of borderline personality disorder. American Journal of Psychiatry. 1997;154:1101–1106. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]