Abstract
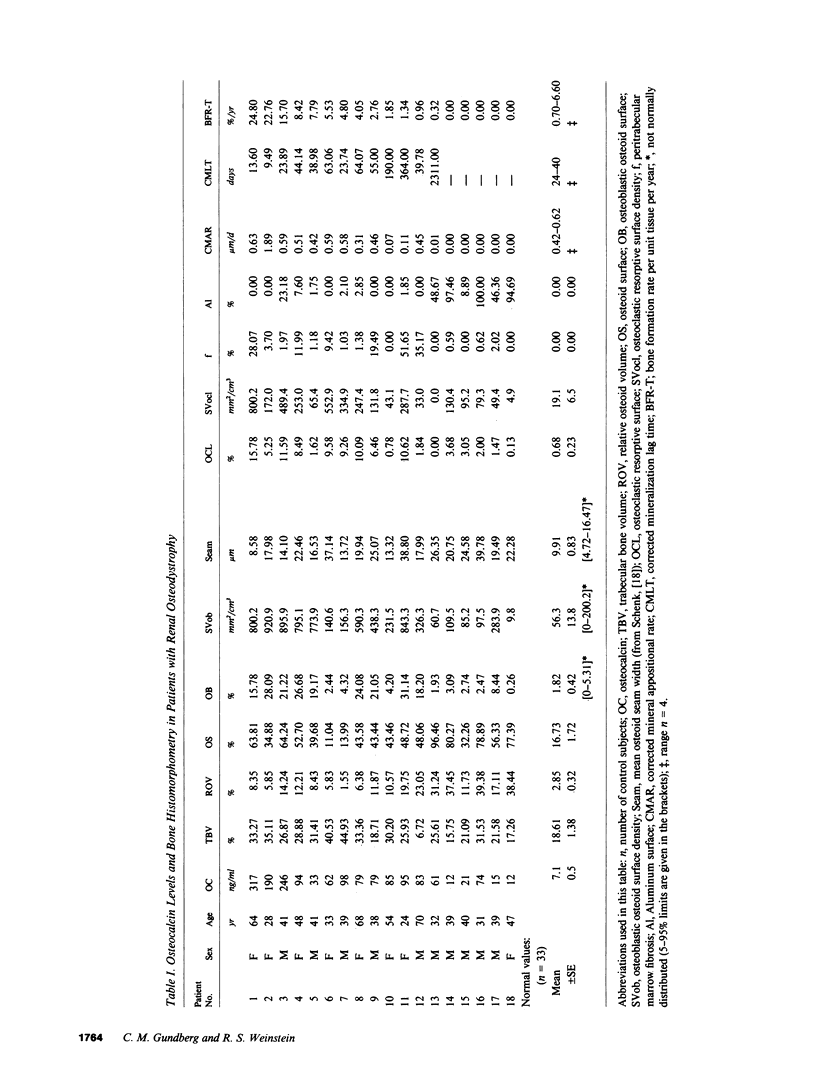
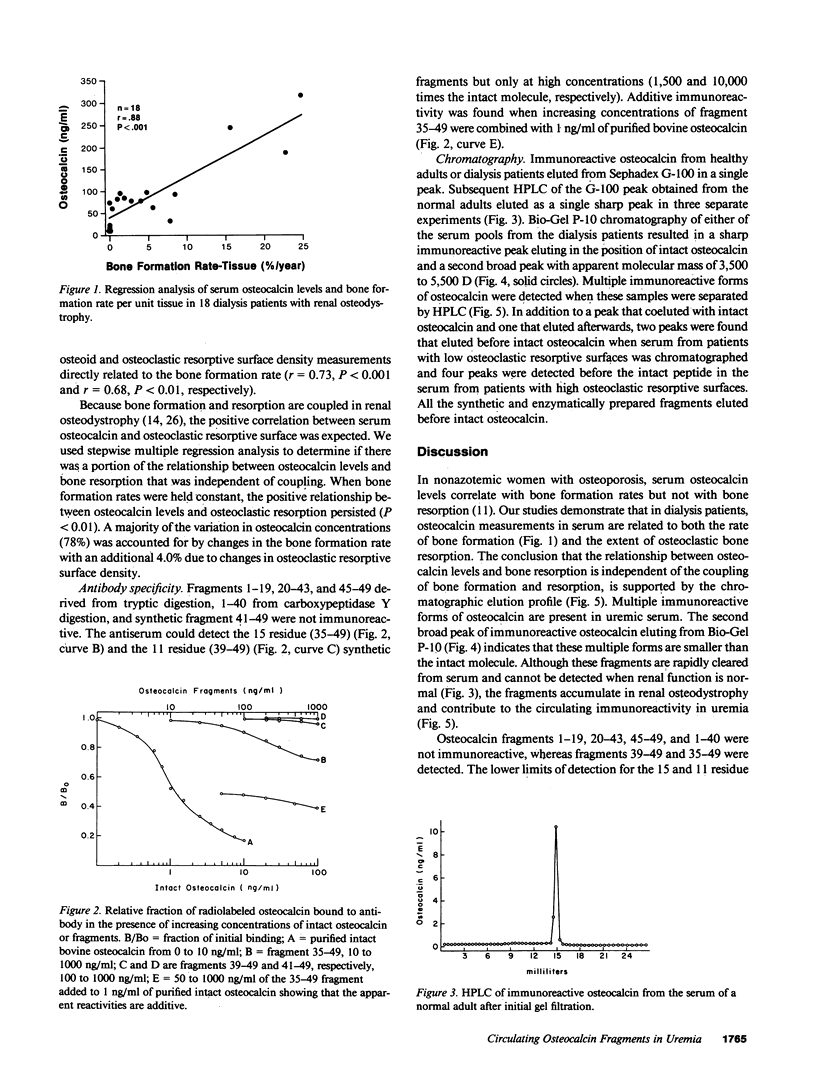
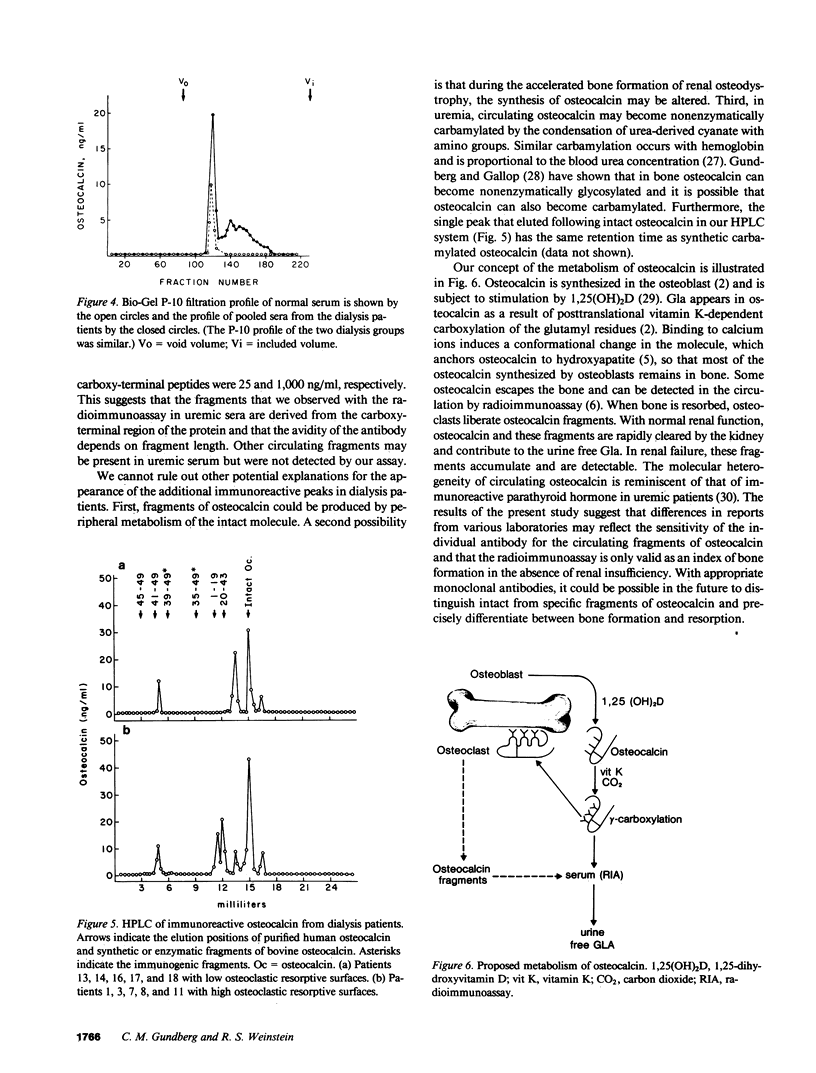
Circulating osteocalcin, which normally reflects the rate of bone formation, is elevated in uremia. In 18 patients receiving maintenance hemodialysis, serum osteocalcin levels were directly related to the bone formation rate (r = 0.88, P less than 0.001), osteoblastic osteoid surface density (r = 0.65, P less than 0.01), and osteoclastic resorptive surface density (r = 0.75, P less than 0.001). Multiple regression analysis showed that osteocalcin levels remained positively correlated with osteoclastic resorption when the bone formation rate was held constant (P less than 0.01). The intimation that the coupling of bone formation and resorption could not explain the relationship between osteocalcin and resorption led us to determine whether fragments of this abundant matrix protein are released by bone resorption and retained in uremia. Sera from dialysis patients with renal osteodystrophy were fractionated by sequential gel filtration and HPLC, and assayed for immunoreactive osteocalcin. When normal serum was analyzed, a single sharp peak was found. In pooled sera from patients with high osteoclastic resorptive surfaces identified by histomorphometry, we found five additional immunoreactive peaks, while three additional peaks were detected in sera from patients with lower osteoclastic surfaces. Bio-Gel P-10 chromatography showed that these multiple peaks were of lower molecular weight than intact osteocalcin. We suggest that the liberation of bone matrix by osteoclasts contributes to the circulating osteocalcin immunoreactivity in uremia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. P., Delmas P. D., Malaval L., Edouard C., Chapuy M. C., Meunier P. J. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984 May 19;1(8386):1091–1093. doi: 10.1016/s0140-6736(84)92506-6. [DOI] [PubMed] [Google Scholar]
- Butler W. T. Matrix macromolecules of bone and dentin. Coll Relat Res. 1984 Aug;4(4):297–307. doi: 10.1016/s0174-173x(84)80037-0. [DOI] [PubMed] [Google Scholar]
- Canterbury J. M., Reiss E. Multiple immunoreactive molecular forms of parathyroid hormone in human serum. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1393–1398. doi: 10.3181/00379727-140-36681. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Parthemore J. G., Price P. A. Changes in plasma bone GLA protein during treatment of bone disease. Calcif Tissue Int. 1982 Mar;34(2):121–124. doi: 10.1007/BF02411221. [DOI] [PubMed] [Google Scholar]
- Delmas P. D., Wilson D. M., Mann K. G., Riggs B. L. Effect of renal function on plasma levels of bone Gla-protein. J Clin Endocrinol Metab. 1983 Nov;57(5):1028–1030. doi: 10.1210/jcem-57-5-1028. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Harmon W., Meier W., Loo S., Gabbay K. H. Hemoglobin carbamylation in uremia. N Engl J Med. 1981 Apr 2;304(14):823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Lian J. B., Hauschka P. V. Carboxylated calcium-binding proteins and vitamin K. N Engl J Med. 1980 Jun 26;302(26):1460–1466. doi: 10.1056/NEJM198006263022608. [DOI] [PubMed] [Google Scholar]
- Gundberg C. M., Lian J. B., Gallop P. M., Steinberg J. J. Urinary gamma-carboxyglutamic acid and serum osteocalcin as bone markers: studies in osteoporosis and Paget's disease. J Clin Endocrinol Metab. 1983 Dec;57(6):1221–1225. doi: 10.1210/jcem-57-6-1221. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Frenkel J., DeMuth R., Gundberg C. M. Presence of osteocalcin and related higher molecular weight 4-carboxyglutamic acid-containing proteins in developing bone. J Biol Chem. 1983 Jan 10;258(1):176–182. [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. B., Friedman P. A. The vitamin K-dependent synthesis of gamma-carboxyglutamic acid by bone microsomes. J Biol Chem. 1978 Oct 10;253(19):6623–6626. [PubMed] [Google Scholar]
- Malluche H. H., Faugere M. C., Fanti P., Price P. A. Plasma levels of bone Gla-protein reflect bone formation in patients on chronic maintenance dialysis. Kidney Int. 1984 Dec;26(6):869–874. doi: 10.1038/ki.1984.230. [DOI] [PubMed] [Google Scholar]
- Maloney N. A., Ott S. M., Alfrey A. C., Miller N. L., Coburn J. W., Sherrard D. J. Histological quantitation of aluminum in iliac bone from patients with renal failure. J Lab Clin Med. 1982 Feb;99(2):206–216. [PubMed] [Google Scholar]
- Merz W. A., Schenk R. K. A quantitative histological study on bone formation in human cancellous bone. Acta Anat (Basel) 1970;76(1):1–15. doi: 10.1159/000143476. [DOI] [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem. 1980 Dec 25;255(24):11660–11663. [PubMed] [Google Scholar]
- Price P. A., Nishimoto S. K. Radioimmunoassay for the vitamin K-dependent protein of bone and its discovery in plasma. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2234–2238. doi: 10.1073/pnas.77.4.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Parthemore J. G., Deftos L. J. New biochemical marker for bone metabolism. Measurement by radioimmunoassay of bone GLA protein in the plasma of normal subjects and patients with bone disease. J Clin Invest. 1980 Nov;66(5):878–883. doi: 10.1172/JCI109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Williamson M. K., Lothringer J. W. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981 Dec 25;256(24):12760–12766. [PubMed] [Google Scholar]
- Schwartz M. P., Recker R. R. Comparison of surface density and volume of human iliac trabecular bone measured directly and by applied stereology. Calcif Tissue Int. 1981;33(6):561–565. doi: 10.1007/BF02409492. [DOI] [PubMed] [Google Scholar]
- Slovik D. M., Gundberg C. M., Neer R. M., Lian J. B. Clinical evaluation of bone turnover by serum osteocalcin measurements in a hospital setting. J Clin Endocrinol Metab. 1984 Aug;59(2):228–230. doi: 10.1210/jcem-59-2-228. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S. L. Renal osteodystrophy. Hum Pathol. 1984 Apr;15(4):306–323. doi: 10.1016/s0046-8177(84)80028-3. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Bryce G. F., Sappington L. J., King D. W., Gallagher B. B. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrinol Metab. 1984 Jun;58(6):1003–1009. doi: 10.1210/jcem-58-6-1003. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Underwood J. L., Hutson M. S., DeLuca H. F. Bone histomorphometry in vitamin D-deficient rats infused with calcium and phosphorus. Am J Physiol. 1984 Jun;246(6 Pt 1):E499–E505. doi: 10.1152/ajpendo.1984.246.6.E499. [DOI] [PubMed] [Google Scholar]


