Abstract
Purpose: To retrospectively determine whether inapparent tumor at endorectal magnetic resonance (MR) imaging and MR spectroscopic imaging is a favorable prognostic finding in prostate cancer patients who select active surveillance for management.
Materials and Methods: Committee on Human Research approval was obtained and compliance with HIPAA regulations was observed, with waiver of requirement for written consent. Ninety-two men (mean age, 64 years; range, 43–85 years) were retrospectively identified who had biopsy-proved prostate cancer, who had undergone baseline endorectal MR imaging and MR spectroscopic imaging, and who had selected active surveillance for management. Their mean baseline serum prostate-specific antigen (PSA) level was 5.5 ng/mL, and the median Gleason score was 6. Two readers with 10 and 3 years of experience independently reviewed all MR images and determined whether tumor was apparent on the basis of evaluation of established morphologic and metabolic findings. Another investigator compiled data about baseline clinical stage, biopsy findings, and serum PSA measurements. Multiple logistic regression analysis was used to investigate the relationship between the clinical parameters and tumor apparency at MR imaging and the biochemical outcome.
Results: At baseline MR imaging, readers 1 and 2 considered 54 and 26 patients, respectively, to have inapparent tumor (fair interobserver agreement; κ = 0.30). During a mean follow-up of 4.8 years, 52 patients had a stable PSA level and 40 had an increasing PSA level. In multivariate analysis, no significant association was found between the baseline clinical stage, Gleason score, serum PSA level, or the presence of apparent tumor at endorectal MR imaging and MR spectroscopic imaging for either reader and the biochemical outcome (P > .05 for all).
Conclusion: Endorectal MR imaging and MR spectroscopic imaging findings of tumor apparency or inapparency in prostate cancer patients who select active surveillance for management do not appear to be of prognostic value.
© RSNA, 2008
Keywords: PSA = prostate-specific antigen
The lifetime risk of receiving a diagnosis of prostate cancer is 16%, but the lifetime risk of dying of prostate cancer is only 3% (1). The primary dilemma in the management of this malignancy is distinguishing patients with progressive disease that will become life-threatening from patients with indolent disease that may not require treatment. Determining this distinction remains challenging, even with the emergence of prognostic models that incorporate baseline predictors such as serum prostate-specific antigen (PSA) level, Gleason score, and clinical stage as determined with digital rectal examination (2). Although the prognostic utility of the pretreatment PSA level and Gleason score is generally accepted, the value of clinical stage in low-risk patients remains controversial.
Results of a multivariate analysis of patients who were managed with watchful waiting indicated that a clinical stage of T2 or greater was independently predictive of the need for later treatment (3), but findings in other studies indicated no association between clinical staging and pathologic staging, biopsy results, or 5-year freedom from disease (4,5). The inadequacy of such predictive models has driven the investigation of endorectal magnetic resonance (MR) imaging and MR spectroscopic imaging as potential incrementally useful methods for evaluation of tumor extent, stage, and aggressiveness (6,7). One study (8) showed that the finding of extracapsular extension or seminal vesicle invasion (stage T3) at endorectal MR imaging prior to radical prostatectomy was predictive of a significantly worse biochemical outcome in patients with intermediate- and high-risk prostate cancer and that such a radiologic distinction (T3 vs T2 tumor) was incrementally useful for standard clinical risk stratification.
On the basis of the foregoing considerations and our institutional experience that many patients with lower-risk biopsy-proved prostate cancer (ie, the population that most frequently selects active surveillance for management) do not have visible tumor at MR examination, we hypothesized that inapparent tumor at baseline endorectal MR imaging and MR spectroscopic imaging might be a favorable prognostic finding in prostate cancer patients who select active surveillance.
To our knowledge, the predictive value of MR imaging and MR spectroscopic imaging findings in the active-surveillance population has not been explored, even though candidates for active surveillance could benefit from additional prognostic information at baseline to guide the management of their cancer. More specifically, research is lacking on the predictive value of tumor inapparency at imaging, despite that tumor inapparency at imaging is a part of the definition of stage T1 disease. We therefore undertook this study to retrospectively determine whether inapparent tumor at endorectal MR imaging and MR spectroscopic imaging is a favorable prognostic finding in prostate cancer patients who select active surveillance for management.
MATERIALS AND METHODS
Patient Cohort
The study was approved by our institutional Committee on Human Research, with a waiver of the requirement for written consent, and was compliant with the Health Insurance Portability and Accountability Act. We retrospectively identified 134 men with biopsy-proved prostate cancer who underwent combined endorectal MR imaging and MR spectroscopic imaging at our institution between 2000 and 2001 and who selected active surveillance for management. This time was chosen to generate a cohort with a substantial duration of follow-up. Patients were identified by means of a computerized search of our prostate cancer database, a secure registry maintained by the Magnetic Resonance Science Center at our institution that includes all patients who have undergone MR imaging and MR spectroscopic imaging at our facility and that contains details about patient management. One of the authors (A.R.C.) collected baseline and follow-up data about all the patients by reviewing all available medical records. Data collected included demographic details, baseline biopsy findings, baseline clinical stage determined with digital rectal examination as performed by an attending urologist, baseline and subsequent serum PSA levels, and, if applicable, progression to treatment or development of clinically overt metastatic disease. Patients in whom we could not establish the baseline PSA level or Gleason score or those in whom follow-up PSA levels were monitored during less than 3 months were excluded (n = 42) so that 92 patients remained in the final study population. These 92 men had a mean age of 64 years ± 9 (standard deviation) (range, 43–85 years), a mean baseline serum PSA level of 5.5 ng/mL ± 3.0 (range, 0.7–22 ng/mL), and a median Gleason score of 6 (range, 4–7). Clinical stage at baseline digital rectal examination was T1 in 61 patients, T2 in 22 patients, and unavailable in nine patients. The mean interval between transrectal biopsy and MR imaging was 158 days (median, 254 days; range, 30–2437 days).
Imaging Technique
Imaging studies were performed with a 1.5-T whole-body MR imager (Signa; GE Medical Systems, Milwaukee, Wis). Patients were imaged in the supine position by using a body coil for excitation and a pelvic phased-array coil (GE Medical Systems) in combination with a commercially available balloon-covered expandable endorectal coil (Medrad, Pittsburgh, Pa) for signal reception. Sequences included thin-section high-spatial-resolution transverse and coronal T2-weighted fast spin-echo MR imaging of the prostate and seminal vesicles with the following parameters: repetition time msec/echo time msec, 5000/96 (effective); echo train length, 16; section thickness, 3 mm; intersection gap, 0 mm; field of view, 14 cm; matrix, 256 × 192; frequency-encoding direction, anteroposterior (to prevent obscuration of the prostate from endorectal coil motion artifact); and number of signals acquired, three.
After review of the transverse T2-weighted MR images, an MR spectroscopic imaging volume was selected to maximize coverage of the prostate and minimize the inclusion of periprostatic fat and rectal air. Three-dimensional MR spectroscopic imaging data were acquired by using a water-and-lipid–suppressed double spin-echo point-resolved spatially localized spectroscopic sequence that utilized spectral spatial pulses for the two 180° excitation pulses (9–11). The spectral-spatial pulses allowed precise volume selection, as well as frequency selection, to reduce the water resonance and suppress lipid resonances. Data sets were acquired as 16 × 8 × 8 phase-encoded spectral arrays (1024 voxels with a spatial resolution of 0.24–0.34 cm3), with 1000/130 and a 17-minute acquisition time. MR spectroscopic imaging data were overlaid on the corresponding transverse T2-weighted images, including the raw spectra and the choline-creatine ratio and the choline-plus-creatine–citrate ratio. The total examination time was 1 hour, and the time included coil placement and patient positioning.
Image Interpretation
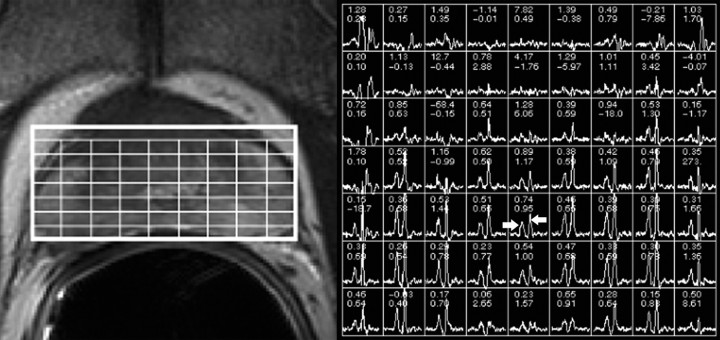
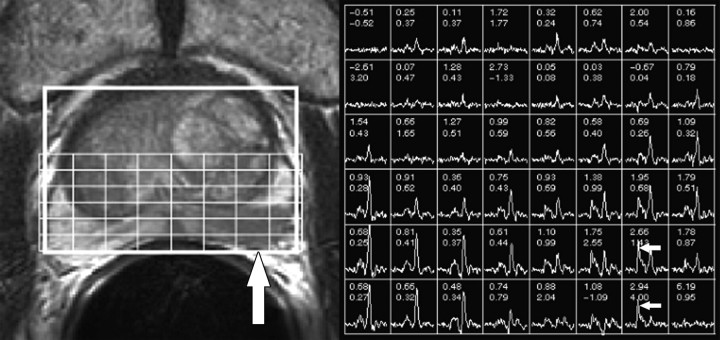
Two attending radiologists (F.V.C. and A.C.W., with 10 and 3 years of experience, respectively, in the interpretation of endorectal MR images and MR spectroscopic images of the prostate) independently reviewed the MR images at a picture archiving and communication system workstation (Impax; Agfa, Mortsel, Belgium). Readers were aware that the patients had biopsy-proved prostate cancer and had chosen active surveillance for management, but they were unaware of other clinical and histopathologic findings, including patient outcome. By using established morphologic and metabolic criteria for the endorectal MR imaging and MR spectroscopic imaging evaluation of prostate cancer (Figs 1, 2) (12–14), readers assigned a radiologically determined tumor stage that was based on the American Joint Committee on Cancer staging system. T1 tumors were not apparent on MR images and MR spectroscopic images, T2 tumors were visible on MR images and MR spectroscopic images but were organ confined, T3 tumors extended outside the capsule or into the seminal vesicles, and T4 tumors invaded adjacent structures such as the bladder or rectum.
Figure 1:
Photomontage shows representative example of biopsy-proved prostate cancer that is not apparent at endorectal MR imaging and MR spectroscopic imaging (radiologic stage T1 disease). Left: Transverse T2-weighted fast spin-echo MR image (5000/96 [effective]) shows no masslike foci of low signal intensity suggestive of tumor. Overlying grid corresponds to adjacent MR spectral array shown at right. Right: Metabolic patterns without abnormalities. In representative peripheral zone voxel, arrow pointing right shows nonelevated choline peak and arrow pointing left shows appropriately high citrate peak.
Figure 2:
Photomontage shows representative example of prostate cancer that is apparent at endorectal MR imaging and MR spectroscopic imaging. Left: Transverse T2-weighted fast spin-echo MR image (5000/96 [effective]) shows ill-defined masslike focus (vertical arrow) of low signal intensity in left peripheral zone interpreted as organ-confined tumor (radiologic stage T2 disease) by both readers. Overlying grid corresponds to adjacent MR spectral array shown at right. Right: Metabolic patterns consistent with malignancy. Voxels corresponding to low T2 signal intensity focus in left peripheral zone demonstrate markedly elevated choline peaks (horizontal arrows).
Extracapsular extension was considered present if tumor abutted the prostate capsule and demonstrated an irregular margin with the adjacent periprostatic tissue or if frank extension of tumor outside the confines of the prostatic capsule was present. Seminal vesicle, bladder, or rectal invasion was considered present if tumor was seen to extend into any part of the respective structure. Of note, fixed quantitative criteria for prostate cancer identification were not used and have not been reported, but, in general, tumor was characterized morphologically as an ovoid masslike or crescentic subcapsular focus of reduced T2 signal intensity and metabolically by the presence of one or more voxels of suspicious metabolism (elevation of the choline peak or reduction of the citrate peak such that the two peaks were of similar height or the choline peak was higher than the citrate peak). To investigate the potential confounding effect of postbiopsy hemorrhage, each reader also recorded the degree of postbiopsy hemorrhage on a five-point scale (score 0, no hemorrhage visible; score 1, faint diffuse increased T1 signal intensity; score 2, hemorrhage in one to two sextants; score 3, hemorrhage in three to four sextants; and score 4, hemorrhage in five to six sextants). Hemorrhage was identified as increased T1 signal intensity in the prostate.
Statistical Analysis
Tumors that were classified as stage T1 by the MR image readers were considered inapparent, and all other tumors (stage T2 or higher) were considered apparent. Interobserver agreement between the two readers was assessed by using the κ statistic. Level of agreement was defined as follows: poor agreement, κ = 0–0.20; fair agreement, κ = 0.21–0.40; moderate agreement, κ = 0.41–0.60; good agreement, κ = 0.61–0.80; and very good agreement, κ = 0.81–1.00 (15). Biochemical outcomes that were based on serial PSA measurements were dichotomized as stable or progressive. This dichotomization was performed by excluding any PSA measurements obtained after progression to treatment and then constructing regression lines for log-transformed PSA values over time to facilitate best fit. Patients with a significantly increased slope at a 5% level were considered biochemically progressive. That is, the lower boundary of the 95% confidence interval of the log-linear regression slope was more than zero. Otherwise, patients were considered biochemically stable.
Multiple logistic regression analysis was used to investigate the relationship between the baseline PSA level, clinical stage, Gleason score, and tumor apparency at MR imaging with the biochemical outcome. The full logistic regression model, as well as models selected by using backward, forward, or stepwise approaches, were tried to investigate whether tumor apparency at MR imaging had any prognostic significance. Because the two readers did not always agree whether the tumors were apparent, we performed separate analyses for reader 1 only, reader 2 only, apparency to both readers versus inapparency to one or both readers, and apparency to at least one reader versus inapparency to both readers. All statistical analyses were performed by using software (SAS, version 9.1; SAS Institute, Cary, NC). A 5% level was used to define a significant difference.
RESULTS
PSA Level and Distant Metastases
After a mean follow-up of 4.8 years ± 1.2 (range, 0.4–5.3 years), with a mean number of serial PSA measurements of 10 per patient ± 7 (range, 3–44), regression line analysis of the serial PSA data showed that 52 patients had biochemically stable disease, whereas 40 had biochemically progressive disease. The mean PSA level increase in those with stable disease was 0.3 ng/mL per year compared with 2.0 ng/mL per year in those with progressive disease (P < .001). No patient received a diagnosis of distant metastases.
Staging and Associations
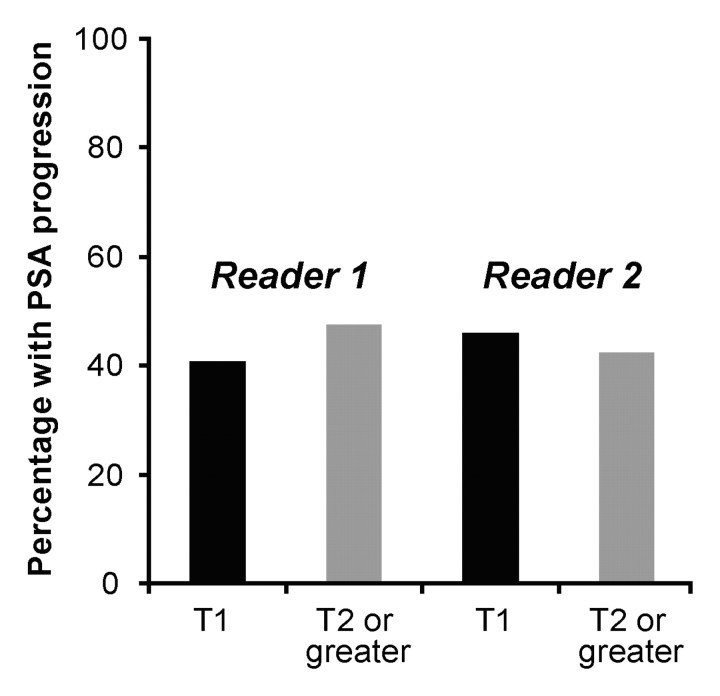
At baseline MR imaging and MR spectroscopic imaging, reader 1 considered 54, 33, five, and zero patients to have stage T1, T2, T3, and T4 disease, respectively. The corresponding numbers for reader 2 were 26, 50, 16, and zero for stage T1, T2, T3, and T4 disease, respectively. Readers demonstrated fair interobserver agreement, with a κ value of 0.30 (95% confidence interval: 0.16, 0.45). In multivariate analysis, no significant association was found between the biochemical outcome and the baseline clinical stage, Gleason score, serum PSA level, presence of apparent tumor at endorectal MR imaging and MR spectroscopic imaging for either reader, or combinations of the aforementioned variables (Fig 3, Table). The Table presents the results of the multivariate logistic regression, and the results incorporate interpretation of the imaging data for reader 1. Results of the analysis of the interpretation for reader 2 also indicated no association between imaging findings and outcome. The results of multivariate analysis were unchanged after adjustment for the degree of postbiopsy hemorrhage.
Figure 3:
Percentage of patients with biochemical progression, as a function of baseline stage at MR imaging for both readers.
Results of Multivariate Logistic Regression Analysis of Relationship between Baseline Clinical Characteristics and Biochemical Outcome
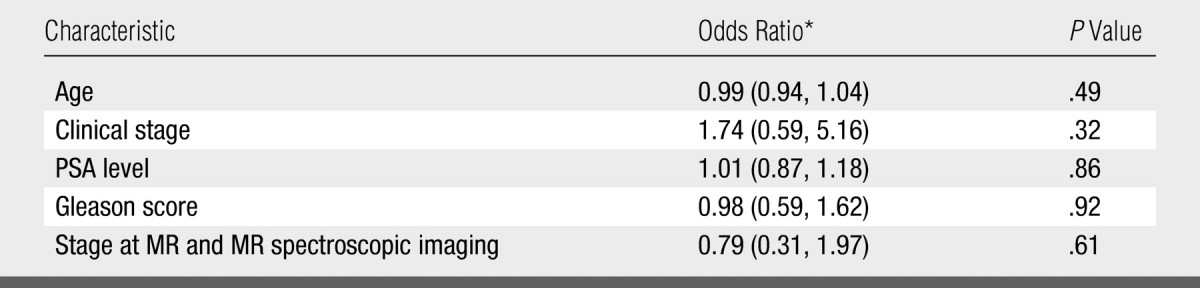
Numbers in parentheses are the 95% confidence intervals.
DISCUSSION
Our results suggest that, in men with low-risk prostate cancer who select active surveillance, findings at baseline endorectal MR imaging and MR spectroscopic imaging are not helpful in predicting disease progression. In particular, we had hypothesized that inapparent tumor at baseline MR imaging and MR spectroscopic imaging, which suggests low tumor volume (6), would imply a more favorable prognosis, but patients with radiologically inapparent tumors (T1 disease) were just as likely to develop an increasing PSA level as those with radiologically apparent tumors. These findings are counterintuitive, given prior research results that support the accuracy of endorectal MR imaging at staging, that show a correlation of MR spectroscopic imaging findings with Gleason score, and that show an association of stage and grade with clinical progression (6,7,16,17). However, previous evidence suggests that staging, whether through digital rectal examination or imaging, is less useful for prognostication in low-risk disease—the type of disease that affected our subjects.
Philip et al (5) found no association among digital rectal examination findings, biopsy findings, and pathologic staging in low-risk patients, and they questioned the utility of digital rectal examination–based staging in men with a PSA level of 2.5–10 ng/mL. In patients treated with radiation therapy, Liebross et al (4) found no difference in the 5-year freedom from failure rate between patients with stage T1 disease at digital rectal examination and those with stage T2 disease. In men treated with radical prostatectomy, D'Amico et al (8) found that pretreatment endorectal MR imaging imparted prognostic information incremental to standard clinical factors (ie, stage, Gleason score, PSA level) only in patients with intermediate- to high-risk disease. In patients with low-risk disease, staging distinguishes primarily between T1 and T2 tumors rather than between T2 and T3 tumors; the latter distinction, which differentiates organ-confined from extracapsular disease, may hold more clinical importance because extracapsular extension implies more invasive malignancy.
Given that the preponderance of patients who consider watchful waiting or active surveillance have low-risk cancer, endorectal MR imaging and MR spectroscopic imaging in these patients may offer limited prognostic utility no matter how accurately it delineates tumors. In fact, none of the parameters included in our study, including the established predictors of Gleason score and PSA level, were useful in the prediction of progression of disease. This finding probably reflects the limitations of all baseline predictors in a population of patients with predominantly low-risk disease, and our results should be placed in the context of this global inability to further substratify patients with favorable disease rather than considered as a negative reflection on the utility of imaging alone. Our study also calls into question the value of distinguishing patients with T1 versus T2 tumor, although the American Joint Committee on Cancer staging system for prostate cancer relies on digital rectal examination findings incorporated with all available information, including imaging findings, prior to a decision about management (12). Prior research has failed to show, however, that patients in whom tumors are upstaged by using transrectal ultrasonographic (US) or endorectal MR imaging findings have a different outcome following definitive treatment compared with those in whom tumors are not upstaged (4,18).
Our study had several limitations. First, we used an increasing PSA level as a surrogate marker for prostate cancer progression. An escalating PSA level is not always associated with tumor growth or increasing metabolic abnormality, and clinicians who are monitoring patients who select active surveillance generally consider some combination of PSA velocity, clinical progression (eg, stage progression on the basis of digital rectal examination or transrectal US findings, obstructive symptoms), and histologic progression when they are determining whether a patient's disease has progressed enough to justify treatment (19). Numerous studies have shown, however, that lower PSA level doubling times correlate strongly with disease progression (20,21). Digital rectal examination and transrectal US results are unreliable, and obstructive symptoms usually reflect benign prostatic hyperplasia rather than tumor. Histologic progression at repeat biopsy more likely represents inadequate sampling at the original biopsy than it does tumor dedifferentiation (22,23). Moreover, although clinicians at our institution follow serial PSA values in all patients who select active surveillance, their performance of serial digital rectal examination, transrectal US, or biopsy is far less regular or uniform. Employing the latter data, therefore, would have risked introducing detection bias. An alternative would have been to use “progression to treatment” as our end point, but patients who select active surveillance sometimes elect treatment in the absence of evidence for disease progression (19–21). In other words, progression to treatment does not imply progression of disease. Although increasing PSA values are an imperfect surrogate for disease progression, these increasing values represented our most reliable option; furthermore, this surrogate has recently been validated as a longitudinal tumor marker in prostate cancer patients who choose active surveillance (24).
Second, we examined a relatively small population of patients, and we cannot exclude the possibility that a study with a greater number of patients would have allowed detection of group differences. Our inability to find trends even approaching significance, however, makes this improbable.
Third, we performed the study retrospectively at a single academic institution. The extent to which our results can be extrapolated to other populations is unknown, and we cannot exclude the possibility of sample bias in the patients who chose to have their cancer managed at our institution. We cannot identify any reason, however, why patients treated at our institution would differ from other patients with respect to the association between their baseline imaging findings and subsequent clinical course.
Fourth, only two readers assessed the patients' baseline imaging results. Although they based their evaluations on criteria that researchers are endeavoring to standardize for MR imaging and MR spectroscopic imaging interpretation, subjectivity is generally inherent to radiologic evaluation, and the extent to which their abilities can be extrapolated to other radiologists is unknown. The presence of only fair concordance between our two MR image readers with a subspecialty interest in the prostate seems to corroborate this concern. Of note, however, although reader 1 had 7 more years of experience than did reader 2 in the interpretation of prostate MR images, neither observed findings predictive of clinical course.
Finally, it is curious that baseline PSA levels and Gleason scores also failed to have prognostic importance in our sample. Our sample may have been atypical in some way that we were unable to determine. Overall, we do not intend to undermine the role of endorectal MR imaging and MR spectroscopic imaging in the management of prostate cancer but rather highlight the limitations of currently available technology and existing tumor criteria as predictive variables in the particular subset of prostate cancer patients who select active surveillance for management. Future studies should investigate whether 3.0-T endorectal MR imaging and MR spectroscopic imaging provide findings of greater prognostic importance and whether findings on serial MR images and MR spectroscopic images in patients who select active surveillance can help determine whether to continue surveillance or progress to definitive treatment.
In conclusion, 1.5-T endorectal MR imaging and MR spectroscopic imaging findings of tumor apparency or inapparency in prostate cancer patients who select active surveillance for management do not appear to be of prognostic value, possibly because these patients generally have low-risk tumors.
ADVANCES IN KNOWLEDGE
-
•.
In multivariate analysis, no significant association was found between the baseline clinical stage, Gleason score, serum prostate-specific antigen level, or the presence of apparent tumor at endorectal MR imaging and MR spectroscopic imaging and the biochemical outcome in patients with prostate cancer who select active surveillance for management.
-
•.
The subset of patients with prostate cancer who select active surveillance for management and who have no MR imaging findings of tumor do not appear to have a better prognosis than those who have visible tumor at endorectal MR imaging and MR spectroscopic imaging.
IMPLICATIONS FOR PATIENT CARE
-
•.
By using current technology, endorectal MR imaging and MR spectroscopic imaging findings of tumor apparency or inapparency in prostate cancer patients who select active surveillance for management do not appear to be of prognostic value.
-
•.
Our data support the view that the distinction between stage T1 and stage T2 prostate cancer is of questionable prognostic value.
Endorectal MR imaging and MR spectroscopic imaging findings of tumor apparency or inapparency in prostate cancer patients who select active surveillance for management do not appear to be of prognostic value, possibly because these patients generally have low-risk tumors.
Footnotes
Author contributions: Guarantors of integrity of entire study, A.R.C., F.V.C.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, A.R.C.; clinical studies, A.R.C., A.C.W., S.Z., K.S., P.R.C., J.K.; statistical analysis, Y.L.; and manuscript editing, all authors
Authors stated no financial relationship to disclose.
References
- 1.Sakr WA, Grignon DJ. Prostate cancer: indicators of aggressiveness. Eur Urol 1997;32(suppl 3):15–23. [PubMed] [Google Scholar]
- 2.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol 2003;170:1792–1797. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Sun L, Moul JW, et al. Watchful waiting and factors predictive of secondary treatment of localized prostate cancer. J Urol 2004;171:1111–1116. [DOI] [PubMed] [Google Scholar]
- 4.Liebross RH, Pollack A, Lankford SP, von Eschenbach AC, Zagars GK. Relationship of ultrasound staging and bilateral biopsy positivity to outcome in stage T1c prostate cancer treated with radiotherapy. Urology 1998;52:647–652. [DOI] [PubMed] [Google Scholar]
- 5.Philip J, Dutta Roy S, Ballal M, Foster CS, Javlé P. Is a digital rectal examination necessary in the diagnosis and clinical staging of early prostate cancer? BJU Int 2005;95:969–971. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima J, Tanimoto A, Imai Y, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology 2004;64:101–105. [DOI] [PubMed] [Google Scholar]
- 7.Huch Boni RA, Boner JA, Debatin JF, et al. Optimization of prostate carcinoma staging: comparison of imaging and clinical methods. Clin Radiol 1995;50:593–600. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico AV, Whittington R, Malkowicz B, et al. Endorectal magnetic resonance imaging as a predictor of biochemical outcome after radical prostatectomy in men with clinically localized prostate cancer. J Urol 2000;164:759–763. [DOI] [PubMed] [Google Scholar]
- 9.Males RG, Vigneron DB, Star-Lack J, et al. Clinical application of BASING and spectral/spatial water and lipid suppression pulses for prostate cancer staging and localization by in vivo 3D 1H magnetic resonance spectroscopic imaging. Magn Reson Med 2000;43:17–22. [DOI] [PubMed] [Google Scholar]
- 10.Star-Lack J, Vigneron DB, Pauly J, Kurhanewicz J, Nelson SJ. Improved solvent suppression and increased spatial excitation bandwidths for three-dimensional PRESS CSI using phase-compensating spectral/spatial spin-echo pulses. J Magn Reson Imaging 1997;7:745–757. [DOI] [PubMed] [Google Scholar]
- 11.Schricker AA, Pauly JM, Kurhanewicz J, Swanson MG, Vigneron DB. Dualband spectral-spatial RF pulses for prostate MR spectroscopic imaging. Magn Reson Med 2001;46:1079–1087. [DOI] [PubMed] [Google Scholar]
- 12.Prostate. In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC cancer staging manual. 6th ed. New York, NY: Springer, 2002; 309–316.
- 13.Coakley FV, Teh HS, Qayyum A, et al. Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology 2004;233:441–448. [DOI] [PubMed] [Google Scholar]
- 14.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology 2004;233:701–708. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Zakian KL, Sircar K, Hricak H, et al. Correlation of proton MR spectroscopic imaging with Gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology 2005;234:804–814. [DOI] [PubMed] [Google Scholar]
- 17.Hardie C, Parker C, Norman A, et al. Early outcomes of active surveillance for localized prostate cancer. BJU Int 2005;95:956–960. [DOI] [PubMed] [Google Scholar]
- 18.Pinover WH, Hanlon A, Lee WR, Kaplan EJ, Hanks GE. Prostate carcinoma patients upstaged by imaging and treated with irradiation: an outcome-based analysis. Cancer 1996;77:1334–1341. [DOI] [PubMed] [Google Scholar]
- 19.Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol 2002;167:1664–1669. [PubMed] [Google Scholar]
- 20.McLaren DB, McKenzie M, Duncan G, Pickles T. Watchful waiting or watchful progression? prostate specific antigen doubling times and clinical behavior in patients with early untreated prostate carcinoma. Cancer 1998;82:342–348. [DOI] [PubMed] [Google Scholar]
- 21.de Vries SH, Raaijmakers R, Kranse R, Blijenberg BG, Schroder FH. Prostate cancer characteristics and prostate specific antigen changes in screening detected patients initially treated with a watchful waiting policy. J Urol 2004;172:2193–2196. [DOI] [PubMed] [Google Scholar]
- 22.Choo R, Do V, Sugar L, et al. Comparison of histologic grade between initial and follow-up biopsy in untreated, low to intermediate grade, localized prostate cancer. Can J Urol 2004;11:2118–2124. [PubMed] [Google Scholar]
- 23.Hruby G, Choo R, Klotz L, et al. The role of serial transrectal ultrasonography in a ‘watchful waiting’ protocol for men with localized prostate cancer. BJU Int 2001;87:643–647. [DOI] [PubMed] [Google Scholar]
- 24.Coakley FV, Chen I, Qayyum A, et al. Validity of prostate-specific antigen as a tumour marker in men with prostate cancer managed by watchful-waiting: correlation with findings at serial endorectal magnetic resonance imaging and spectroscopic imaging. BJU Int 2007;99:41–45. [DOI] [PubMed] [Google Scholar]