Abstract
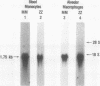
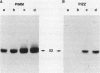
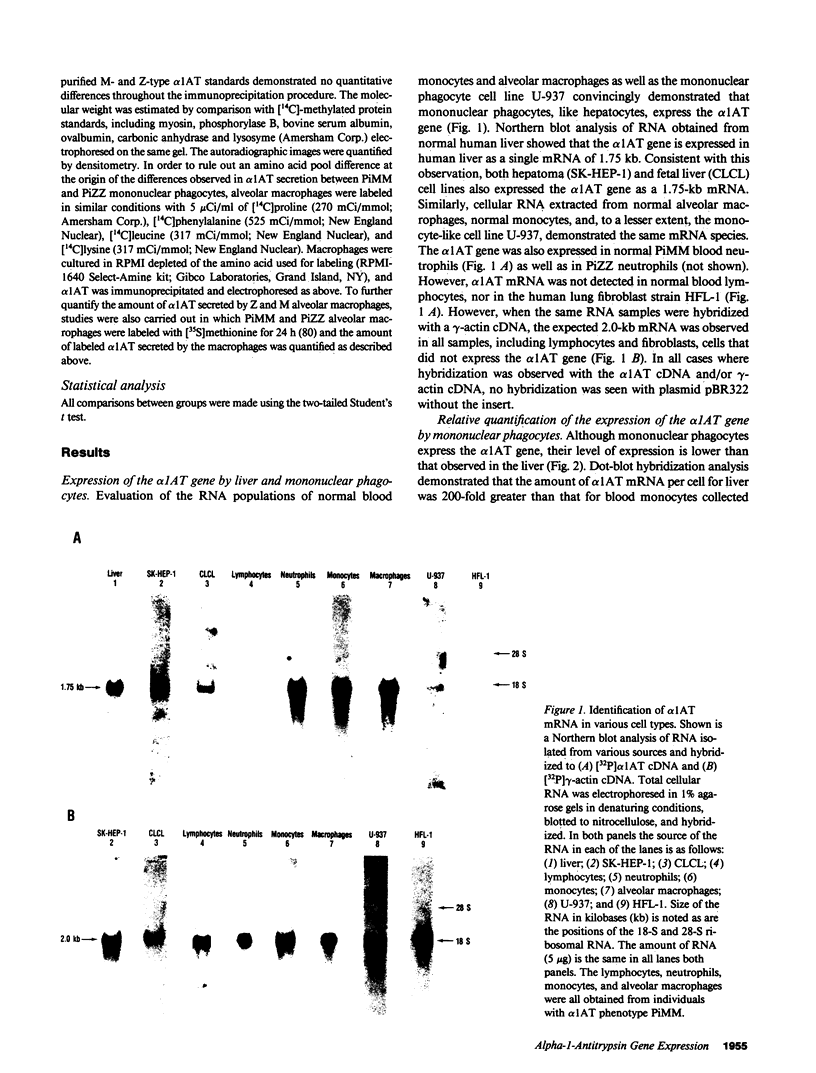
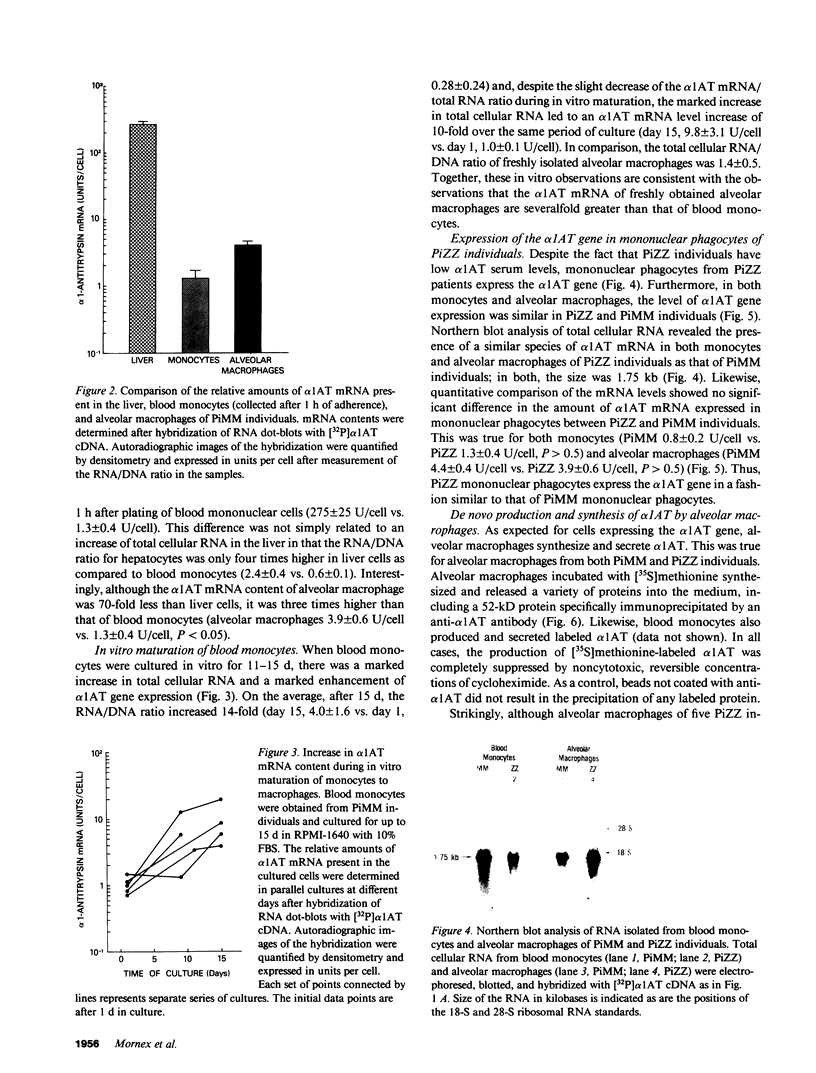
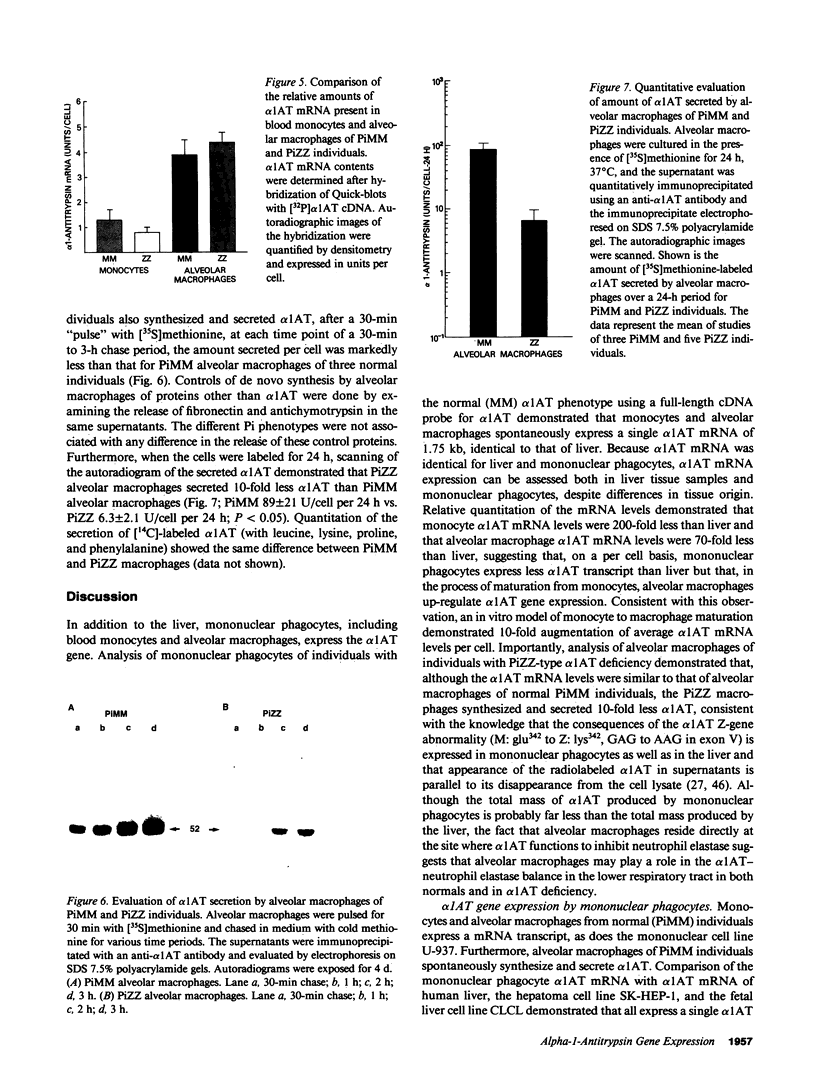
To evaluate the contribution of mononuclear phagocytes, and particularly alveolar macrophages, to alpha-1-antitrypsin (alpha 1AT) production in normal and alpha 1AT-deficient individuals, Northern analysis with a human alpha 1AT complementary DNA was used to demonstrate that alpha 1AT messenger RNA (mRNA) can be detected in liver, blood monocytes, and alveolar macrophages. Quantification of alpha 1AT mRNA expression demonstrated that: (a) type PiMM monocytes and alveolar macrophages expressed, respectively, 200-fold and 70-fold less alpha 1AT mRNA per cell than the liver; (b) the level of expression of the alpha 1AT gene was increased during the in vitro maturation of blood monocytes; and (c) blood monocyte and alveolar macrophage levels of expression of the alpha 1AT gene were the same in PiMM and PiZZ individuals. However, the amount of newly synthesized alpha 1AT secreted by ZZ alveolar macrophages was 10 times lower than that secreted by MM alveolar macrophages. Thus, mononuclear phagocytes of PiZZ individuals express a secretory defect in alpha 1AT in a fashion similar to hepatocytes. Not only do mononuclear phagocytes provide a readily accessible cell to evaluate the regulation of alpha 1AT gene expression, but these cells may contribute to the levels of alpha 1AT present in the lower respiratory tract in the normal and ZZ states.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler C. P., Ringlage W. P., Böhm N. DNS-Gehalt und Zellzahl in Herz und Leber von Kindern. Vergleichende biochemische, cytophotometrische und histologische Untersuchungen. Pathol Res Pract. 1981 Jul;172(1-2):25–41. [PubMed] [Google Scholar]
- Alpert S. E., Auerbach H. S., Cole F. S., Colten H. R. Macrophage maturation: differences in complement secretion by marrow, monocyte, and tissue macrophages detected with an improved hemolytic plaque assay. J Immunol. 1983 Jan;130(1):102–107. [PubMed] [Google Scholar]
- Andersen M. M. Leucocyte-associated plasma proteins in leucocytes during disease states, and in leukaemic cells. Scand J Clin Lab Invest. 1984 May;44(3):257–265. doi: 10.3109/00365518409083805. [DOI] [PubMed] [Google Scholar]
- Andersen M. M. Leucocyte-associated plasma proteins. Association of prealbumin, albumin, orosomucoid, alpha 1-antitrypsin, transferrin and haptoglobin with human lymphocytes, monocytes, granulocytes and a promyelocytic leukaemic cell line (HL-60). Scand J Clin Lab Invest. 1983 Feb;43(1):49–59. doi: 10.1080/00365518309168222. [DOI] [PubMed] [Google Scholar]
- Andreesen R., Osterholz J., Bodemann H., Bross K. J., Costabel U., Löhr G. W. Expression of transferrin receptors and intracellular ferritin during terminal differentiation of human monocytes. Blut. 1984 Sep;49(3):195–202. doi: 10.1007/BF00319822. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Stenflo J., Errington D. M., Carrell R. W. Translation and processing of normal (PiMM) and abnormal (PiZZ) human alpha 1-antitrypsin. FEBS Lett. 1983 Mar 21;153(2):270–274. doi: 10.1016/0014-5793(83)80622-x. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Baumann H. An evolutionary switch in tissue-specific gene expression. Abundant expression of alpha 1-antitrypsin in the kidney of a wild mouse species. J Biol Chem. 1985 Jan 25;260(2):1160–1165. [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Grand R. J., Colten H. R., Alper C. A. Liver in alpha1-antitrypsin deficiency: morphologic observations and in vitro synthesis of alpha1-antitrypsin. Pediatr Res. 1976 Jan;10(1):35–40. doi: 10.1203/00006450-197601000-00007. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Loriau R., Herzog A., Hérion P. Expression of human alpha 1-antitrypsin in Escherichia coli. FEBS Lett. 1984 Jan 23;166(1):67–70. doi: 10.1016/0014-5793(84)80046-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bresser J., Doering J., Gillespie D. Quick-blot: selective mRNA or DNA immobilization from whole cells. DNA. 1983;2(3):243–254. doi: 10.1089/dna.1983.2.243. [DOI] [PubMed] [Google Scholar]
- Breul S. D., Bradley K. H., Hance A. J., Schafer M. P., Berg R. A., Crystal R. G. Control of collagen production by human diploid lung fibroblasts. J Biol Chem. 1980 Jun 10;255(11):5250–5260. [PubMed] [Google Scholar]
- Budek W., Bünning P., Heinrich P. C. Rat lung tissue is a site of alpha 1-proteinase inhibitor synthesis: evidence by cell-free translation. Biochem Biophys Res Commun. 1984 Jul 18;122(1):394–400. doi: 10.1016/0006-291x(84)90488-1. [DOI] [PubMed] [Google Scholar]
- Carlson J., Stenflo J. Rat alpha 1-antitrypsin, preliminary characterisation of the in vitro mRNA translation product. FEBS Lett. 1981 Aug 3;130(2):297–300. doi: 10.1016/0014-5793(81)81143-x. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen A. B. Interrelationships between the human alveolar macrophage and alpha-1-antitrypsin. J Clin Invest. 1973 Nov;52(11):2793–2799. doi: 10.1172/JCI107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F. S., Auerbach H. S., Goldberger G., Colten H. R. Tissue-specific pretranslational regulation of complement production in human mononuclear phagocytes. J Immunol. 1985 Apr;134(4):2610–2616. [PubMed] [Google Scholar]
- Colten H. R. Expression of the MHC class III genes. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):355–366. doi: 10.1098/rstb.1984.0096. [DOI] [PubMed] [Google Scholar]
- Constans J., Viau M., Gouaillard C. Pi M4: an additional Pi M subtype. Hum Genet. 1980;55(1):119–121. doi: 10.1007/BF00329137. [DOI] [PubMed] [Google Scholar]
- Cookson S. L., Adams D. O. A simple, sensitive assay for determining DNA in mononuclear phagocytes and other leukocytes. J Immunol Methods. 1978;23(1-2):169–173. doi: 10.1016/0022-1759(78)90120-5. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Gehr P., Bachofen M., Weibel E. R. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982 Aug;126(2):332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- DAVIDSON J. N., LESLIE I., WHITE J. C. The nucleic-acid content of the cell. Lancet. 1951 Jun 16;1(6668):1287–1290. doi: 10.1016/s0140-6736(51)91768-0. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Astrin K. H., Muirhead S. P., Desnick R. J., Smith M. Assignment of human alpha 1-antitrypsin to chromosome 14 by somatic cell hybrid analysis. Proc Natl Acad Sci U S A. 1982 Feb;79(3):870–873. doi: 10.1073/pnas.79.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSSON S. PULMONARY EMPHYSEMA AND ALPHA1-ANTITRYPSIN DEFICIENCY. Acta Med Scand. 1964 Feb;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg L., Fedorcsak I., Solymosy F. Diethyl pyrocarbonate in nucleic acid research. Prog Nucleic Acid Res Mol Biol. 1976;16:189–262. doi: 10.1016/s0079-6603(08)60758-8. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Alm R., Astedt B. Organ cultures of human fetal hepatocytes in the study of extra-and intracellular alpha1-antitrypsin. Biochim Biophys Acta. 1978 Sep 6;542(3):496–505. doi: 10.1016/0304-4165(78)90379-3. [DOI] [PubMed] [Google Scholar]
- Eriksson S. The effect of tamoxifen in intermediate alpha 1-antitrypsin deficiency associated with the phenotype PiSZ. Ann Clin Res. 1983 Apr;15(2):95–98. [PubMed] [Google Scholar]
- Errington D. M., Bathurst I. C., Janus E. D., Carrell R. W. In vitro synthesis of M and Z forms of human alpha 1-antitrypsin. FEBS Lett. 1982 Nov 1;148(1):83–86. doi: 10.1016/0014-5793(82)81247-7. [DOI] [PubMed] [Google Scholar]
- FAARVANG H. J., LAURITSEN O. S. INCREASE OF TRYPSIN INHIBITOR IN SERUM DURING PREGNANCY. Nature. 1963 Jul 20;199:290–291. doi: 10.1038/199290b0. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Foreman R. C., Judah J. D., Colman A. Xenopus oocytes can synthesise but do not secrete the Z variant of human alpha 1-antitrypsin. FEBS Lett. 1984 Mar 12;168(1):84–88. doi: 10.1016/0014-5793(84)80211-2. [DOI] [PubMed] [Google Scholar]
- Frants R. R., Noordhoek G. T., Eriksson A. W. Separator isoelectric focusing for identification of alpha-1-antitrypsin (Pi M) subtypes. Scand J Clin Lab Invest. 1978 Sep;38(5):457–462. doi: 10.3109/00365517809108451. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Grystal R. G. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977 Sep;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganrot P. O., Bjerre B. Alpha-1-antitrypsin and alpha-2-macroglobulin concentration in serum during pregnancy. Acta Obstet Gynecol Scand. 1967;46(2):126–137. [PubMed] [Google Scholar]
- Gautier M., Martin J. P., Polini G. In vitro synthesis of alpha-1-antitrypsin in long term monolayer human liver cell cultures. Biomedicine. 1977 Apr;27(3):116–119. [PubMed] [Google Scholar]
- Geiger T., Northemann W., Schmelzer E., Gross V., Gauthier F., Heinrich P. C. Synthesis of alpha 1-antitrypsin in rat-liver hepatocytes and in a cell-free system. Eur J Biochem. 1982 Aug;126(1):189–195. doi: 10.1111/j.1432-1033.1982.tb06765.x. [DOI] [PubMed] [Google Scholar]
- Glasgow J. E., Bagdasarian A., Colman R. W. Functional alpha 1 protease inhibitor produced by a human hepatoma cell line. J Lab Clin Med. 1982 Jan;99(1):108–117. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K., Frost J. K., Geddes S., Aracil B., Davidovski F. Morphological identification of alpha-1-antitrypsin in pulmonary macrophages. Hum Pathol. 1979 May;10(3):345–347. doi: 10.1016/s0046-8177(79)80030-1. [DOI] [PubMed] [Google Scholar]
- Hercz A., Harpaz N. Characterization of the oligosaccharides of liver Z variant alpha 1-antitrypsin. Can J Biochem. 1980 Aug;58(8):644–648. doi: 10.1139/o80-089. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Isaacson P., Jones D. B., Millward-Sadler G. H., Judd M. A., Payne S. Alpha-1-antitrypsin in human macrophages. J Clin Pathol. 1981 Sep;34(9):982–990. doi: 10.1136/jcp.34.9.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Koj A., Regoeczi E., Toews C. J., Leveille R., Gauldie J. Synthesis of antithrombin III and alpha-1-antitrypsin by the perfused rat liver. Biochim Biophys Acta. 1978 Apr 3;539(4):496–504. doi: 10.1016/0304-4165(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Black L. F. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis. 1974 Aug;110(2):176–194. doi: 10.1164/arrd.1974.110.2.176. [DOI] [PubMed] [Google Scholar]
- Kueppers F. Genetically determined differences in the response of alpha-antitrypsin levels in human serum to typhoid vaccine. Humangenetik. 1968;6(3):207–214. doi: 10.1007/BF00291864. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Kullander S., Thorell J. Effect of administration of a combined estrogen-progestin contraceptive on the level of individual plasma proteins. Scand J Clin Lab Invest. 1968;21(4):337–343. doi: 10.3109/00365516809077003. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., Musson R. A., Henson P. M. Production of growth factor activity for fibroblasts by human monocyte-derived macrophages. J Leukoc Biol. 1984 Aug;36(2):143–159. doi: 10.1002/jlb.36.2.143. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Mittman C. Dynamic response of alpha1-antitrypsin variants to diethylstilbestrol. Am J Hum Genet. 1973 Nov;25(6):610–617. [PMC free article] [PubMed] [Google Scholar]
- Lieberman J., Mittman C., Kent J. R. Screening for heterozygous 1 -antitrypsin deficiency. 3. A provocative test with diethylstilbestrol and effect of oral contraceptives. JAMA. 1971 Aug 30;217(9):1198–1206. doi: 10.1001/jama.217.9.1198. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Olsen G. N., Harris J. O., Castle J. R., Waldman R. H., Karmgard H. J. Alpha-1-antitrypsin content in the serum, alveolar macrophages, and alveolar lavage fluid of smoking and nonsmoking normal subjects. J Clin Invest. 1975 Feb;55(2):427–430. doi: 10.1172/JCI107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Kay R. M., Cole F. S., Rossing T. H., Van Thiel D., Colten H. R. The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Kalsheker N., Wallis S., Speer A., Coutelle C. H., Woods D., Humphries S. E. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):375–382. doi: 10.1016/0006-291x(83)90532-6. [DOI] [PubMed] [Google Scholar]
- Saltini C., Hance A. J., Ferrans V. J., Basset F., Bitterman P. B., Crystal R. G. Accurate quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Oct;130(4):650–658. doi: 10.1164/arrd.1984.130.4.650. [DOI] [PubMed] [Google Scholar]
- Stone P. J. The elastase-antielastase hypothesis of the pathogenesis of emphysema. Clin Chest Med. 1983 Sep;4(3):405–412. [PubMed] [Google Scholar]
- Sugiura M., Hayakawa S., Adachi T., Ito Y., Hirano K., Sawaki S. A simple one-step purification of human alpha 1-proteinase inhibitor by immunoadsorbent column chromatography. J Biochem Biophys Methods. 1981 Dec;5(5):243–249. doi: 10.1016/0165-022x(81)90034-8. [DOI] [PubMed] [Google Scholar]
- Sztein M. B., Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of human peripheral blood monocyte DR antigen expression in vitro by lymphokines and recombinant interferons. J Clin Invest. 1984 Feb;73(2):556–565. doi: 10.1172/JCI111243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Turner V. S. Secretion of alpha 1-antitrypsin by an established human hepatoma cell line and by human/mouse hybrids. Somatic Cell Genet. 1980 Jan;6(1):1–14. doi: 10.1007/BF01538692. [DOI] [PubMed] [Google Scholar]
- Vercaigne D., Bourguignon J., Lefebvre F., Martin J. P. Identification of the translation products of alpha -1-antitrypsin mRNA from baboon liver polysomes. FEBS Lett. 1982 Jan 25;137(2):231–235. doi: 10.1016/0014-5793(82)80356-6. [DOI] [PubMed] [Google Scholar]
- Weiel J. E., Adams D. O., Hamilton T. A. Murine monocytes express transferrin receptors: evidence for similarity to inflammatory macrophages. Cell Immunol. 1984 Oct 15;88(2):343–349. doi: 10.1016/0008-8749(84)90167-9. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Rennard S. I., Hance A. J., Bitterman P. B., Crystal R. G. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest. 1984 Dec;74(6):2208–2218. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Lee D., Habicht G. S., Janoff A. Secretion of alpha 1-proteinase inhibitor by cultured rat alveolar macrophages. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):447–449. doi: 10.1164/arrd.1981.123.4.447. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Mega T. Carbohydrate composition of normal and variant human alpha 1-protease inhibitors. Arch Biochem Biophys. 1979 Jul;195(2):591–595. doi: 10.1016/0003-9861(79)90385-0. [DOI] [PubMed] [Google Scholar]
- Zuckerman A. J., Taylor P. E., Jacobs J. P., Jones C. A. Chromosome studies of virus-infected semi-continuous human embryonic liver cells. Br J Exp Pathol. 1970 Feb;51(1):92–96. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]