Abstract
Objective
The associations between oral diseases and increased risk of pancreatic cancer have been reported in several prospective cohort studies. In this study, we measured variations of salivary microbiota and evaluated their potential associations with pancreatic cancer and chronic pancreatitis.
Methods
This study was divided into three phases: (1) microbial profiling using the Human Oral Microbe Identification Microarray to investigate salivary microbiota variation between 10 resectable patients with pancreatic cancer and 10 matched healthy controls, (2) identification and verification of bacterial candidates by real-time quantitative PCR (qPCR) and (3) validation of bacterial candidates by qPCR on an independent cohort of 28 resectable pancreatic cancer, 28 matched healthy control and 27 chronic pancreatitis samples.
Results
Comprehensive comparison of the salivary microbiota between patients with pancreatic cancer and healthy control subjects revealed a significant variation of salivary microflora. Thirty-one bacterial species/clusters were increased in the saliva of patients with pancreatic cancer (n=10) in comparison to those of the healthy controls (n=10), whereas 25 bacterial species/clusters were decreased. Two out of six bacterial candidates (Neisseria elongata and Streptococcus mitis) were validated using the independent samples, showing significant variation (p<0.05, qPCR) between patients with pancreatic cancer and controls (n=56). Additionally, two bacteria (Granulicatella adiacens and S mitis) showed significant variation (p<0.05, qPCR) between chronic pancreatitis samples and controls (n=55). The combination of two bacterial biomarkers (N elongata and S mitis) yielded a receiver operating characteristic plot area under the curve value of 0.90 (95% CI 0.78 to 0.96, p<0.0001) with a 96.4% sensitivity and 82.1% specificity in distinguishing patients with pancreatic cancer from healthy subjects.
Conclusions
The authors observed associations between variations of patients’ salivary microbiota with pancreatic cancer and chronic pancreatitis. This report also provides proof of salivary microbiota as an informative source for discovering non-invasive biomarkers of systemic diseases.
INTRODUCTION
The poor outcome associated with pancreatic cancer stems from its propensity to rapidly disseminate to the lymphatic system and distant organs.1–3 This aggressive biology, resistance to conventional and targeted therapeutic agents, and lack of biomarkers for early detection result in a 5-year survival rate of only 5% among patients diagnosed as having pancreatic cancer.4,5 Around 15%–20% of patients have surgically resectable disease at the time of presentation, but only around 20% of these survive to 5 years.3 Cigarette smoking is considered to be the only established modifiable risk factor for cancer of the pancreas, although some data also suggest an association of diabetes, obesity and insulin resistance with increased risk of developing pancreatic cancer. Additionally, the association of chronic pancreatitis with an extremely high risk of pancreatic cancer suggests that inflammation may be involved in the initiation and/or promotion of pancreatic cancer. Inflammation may enhance cellular proliferation and mutagenesis, reduce adaptation to oxidative stress, promote angiogenesis, inhibit apoptosis and increase secretion of inflammatory mediators.
The oral cavity is a large reservoir of bacteria composed of more than 700 species or phylotypes, of which approximately 35% have not been cultured.6 Periodontitis is an inflammatory disease of the oral cavity due to bacteria. Several prospective studies have shown positive associations between oral inflammation (periodontitis) and an increased risk of pancreatic cancer.7–9 Additional studies have also illustrated the potential role of periodontal disease as a risk factor for cardiovascular and cerebrovascular diseases,10–12 preterm birth13 and certain cancers.14 In addition, bacteria have been implicated in the pathogenesis of pancreatic diseases including autoimmune pancreatitis and pancreatic ductal adenocarcinoma.15–28
Assessing bacterial flora composition appears to be of increasing importance in order to unravel bacterial role or to better understand flora changes upon disease onset or between different disease stages. The role of oral microbiota composition on chronic disease development and progression is important to evaluate, especially in the context of developing non-invasive diagnostic tests. A recently developed 16S rRNA-based oligonucleotide microarray, the Human Oral Microbe Identification Microarray (HOMIM) (http://mim.forsyth.org/index.html), made it possible to profile and monitor the oral microbial changes. HOMIM allows the simultaneous detection of about 300 of the most prevalent oral bacterial species, including those that cannot yet be grown in vitro.29
In this study, we performed a comprehensive comparison of the oral microbiota in human saliva from healthy control subjects and patients with either pancreatic cancer or chronic pancreatitis using HOMIM array and quantitative real-time PCR (qPCR). Furthermore, we evaluated the performance and potential translational utilities of salivary microbial signatures as an additional biomarker source for non-invasive detection of pancreatic cancer.
PATIENTS AND METHODS
Study design, populations and samples
This study was approved by the UCLA Institutional Review Board. The study design followed the principle of PRoBE design (prospective specimen collection before outcome ascertainment and retrospective blinded evaluation).30 All subjects were recruited from the UCLA Medical Center prospectively. The saliva bank of pancreatic diseases at the UCLA Dental Research Institute had collected 283 saliva samples. Of these, 103 saliva pellet samples, including 38 pancreatic cancer, 38 matched healthy control and 27 chronic pancreatitis samples, were selected for the discovery and validation phase of this study. Inclusion criteria of disease patients consisted of confirmed diagnosis of pancreatic cancer confined to the pancreas, either resectable or borderline resectable (due to superior mesenteric vein or portal vein involvement), and chronic pancreatitis. Exclusion criteria included evidence of locally advanced pancreatic cancer due to arterial involvement or direct extension into adjacent organs, metastatic pancreatic cancer, chemotherapy or radiation therapy prior to saliva collection and a diagnosis of other malignancies within 5 years from the time of saliva collection. Written informed consents and questionnaire data sheets were obtained from all patients who agreed to serve as saliva donors. The information on individual characteristics, such as age, gender, ethnicity, smoking and drinking history (current or past), is presented in table 1. Healthy control individuals were matched for age, gender and ethnicity to the cancer group. Unstimulated saliva samples were consistently collected, stabilised and preserved as previously described.31 The sample pellets were preserved at −80°C prior to assay.
Table 1.
Demographic information of subjects in the discovery and validation phases
| Discovery phase |
Validation phase |
|||||||
|---|---|---|---|---|---|---|---|---|
| Demographic variable |
Characteristics | Pancreatic cancer (n=10) |
Healthy control (n=10) |
p Value | Pancreatic cancer (n=28) |
Healthy control (n=28) |
Chronic pancreatitis (n=27) |
p Value* |
| Age (years) | Mean±SD | 66.5±8.9 | 66.4±10.5 | 0.98 | 69.9±11.6 | 65.1±10.1 | 57.8±11.0 | 0.10 |
| Sex | Male | 8 | 8 | 1 | 17 | 18 | 15 | 1 |
| Female | 2 | 2 | 11 | 10 | 12 | |||
| Ethnicity | Caucasian | 10 | 10 | 1 | 19 | 19 | 18 | 1 |
| African American |
0 | 0 | 2 | 2 | 2 | |||
| Asian | 0 | 0 | 4 | 4 | 3 | |||
| Hispanic | 0 | 0 | 3 | 3 | 4 | |||
| Smoking | 0 | 0 | 1 | 5 | 2 | 11 | 0.23 | |
| Drinking | 0 | 0 | 1 | 2 | 3 | 2 | 0.65 | |
For the validation samples, p value was calculated between pancreatic cancer and healthy control
This study consisted of a discovery phase and a verification phase, followed by an independent validation phase. The salivary microflora in the pellet samples from 10 patients with pancreatic cancer and 10 healthy control subjects were profiled using the HOMIM array.32 Biomarkers identified from the microarray study were first verified using qPCR on the discovery sample set (10 cancers and 10 healthy controls). An independent sample set, including 28 patients with pancreatic cancer, 28 matched healthy controls and 27 patients with chronic pancreatitis, was used for the biomarker validation phase (figure 1). The validated biomarkers were evaluated within three levels of clinical discrimination categories: pancreatic cancer versus healthy control, pancreatic cancer versus chronic pancreatitis and pancreatic cancer versus combined non-cancer (healthy control + chronic pancreatitis). The purpose of including the patients with chronic pancreatitis in the validation is to evaluate whether the discovered biomarkers can also differentiate patients with cancer from patients with chronic pancreatitis, which has phenotypic overlap with early pancreatic cancer.
Figure 1.
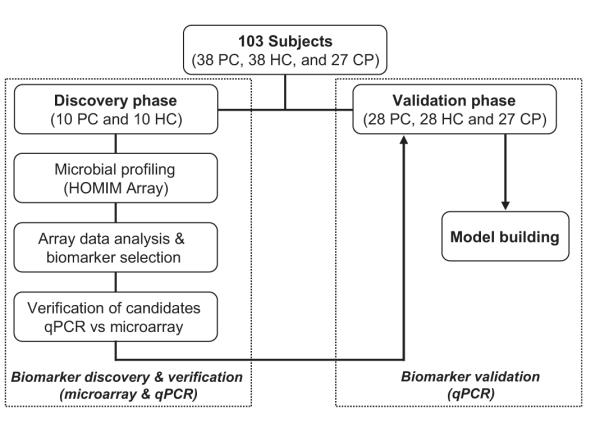
chematic of the strategy used for the discovery (including verification) and validation of salivary bacterial biomarkers. PC, pancreatic cancer; HC, healthy control; CP, chronic pancreatitis.
Salivary microflora profiling and microbial biomarker validation
Bacterial DNA was extracted using the UltraClean Microbial DNA Isolation Kit (MO BIO Laboratories Inc, Carlsbad, California, USA). PCR amplification was performed using 16S universal primers (forward primer, 5′-GAG AGT TTG ATY MTG GCT CAG-3′; reverse primer, 5′-GAA GGA GGT GWT CCA RCC GCA-3′),33 followed by hybridisation to HOMIM array.32 Selection of bacterial candidates was based on Present detection call and p value by Mann–Whitney U test (P call ≥20%, p<0.05). Quantities of bacterial species in the original DNA samples were determined by qPCR. Specific primers were designed for the 16S rRNA genes of the bacterial biomarker candidates (table 2). qPCR was carried out in duplicate in reaction volumes of 10 μl using power SYBR-Green Master Mix (Applied Biosystems, Foster City, California, USA) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 s and 60°C for 30 s in the ABI 7900HT Fast Real Time PCR system (Applied Biosystems). Verified microbial biomarkers were then subjected to independent validation by qPCR using the validation samples.
Table 2.
16S rRNA primers for the six verified bacterial biomarkers
| Strains | 16S rRNA primer sequences (59′–39′) |
|---|---|
| Atopobium parvulum | F: CGAATACTTCGAGACTTCCGCA |
| R: CAATCTGGCTGGTCGGTCTC | |
| Granulicatella adiacens | F: CAAGCTTCTGCTGATGGATGGA |
| R: CTCAGGTCGGCTATGCATCAC | |
| Neisseria elongata | F: CATGCCGCGTGTCTGAAGAA |
| R: CCGTCAGCAGAAACGGGTATT | |
| Prevotella nigrescens | F: GACGGCATCCGATATGAAACA |
| R: TGCACGCTACTTGGCTGGT | |
| Streptococcus australis | F: AGAACGCTGAAGGAAGGAGCTT |
| R: CAATAGTTATCCCCCGCTACCA | |
| Streptococcus mitis | F: CCGCATAATAGCAGTTRTTGCA |
| R: ACAACGCAGGTCCATCTGGTA |
Statistical analysis
Fisher’s exact test and the Wilcoxon rank sum test were used to compare the distributions of the clinical characteristics across groups. The Wilcoxon test was also used to compare the biomarkers between groups. For each biomarker, we constructed the receiver operating characteristic (ROC) curve and computed the area under the curve (AUC) value by numerical integration. Next, the validated salivary biomarkers were fit into logistic regression models (separately for each group comparisons). The sensitivity and specificity for the biomarker combinations were estimated by identifying the cut-off point of the predicted probability that yielded the highest sum of sensitivity and specificity.34,35
RESULTS
Significant variation of microflora profiles in the saliva of patients with pancreatic cancer versus matched healthy controls
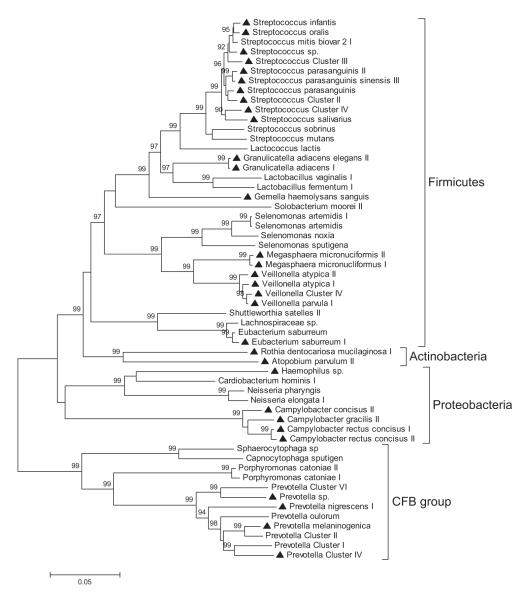
Out of 410 oligonucleotide probes on HOMIM, 149 probes targeting different species or higher taxa showed detectable signals after hybridisation. In all, 56 predominant species or clusters were defined as showing a mean signal intensity >10% of the positive control signal (16S rRNA universal probe on the HOMIM array), of which 31 species/clusters were increased in the saliva pellets of patients with pancreatic cancer (n=10) in comparison to those of the healthy controls (n=10), whereas 25 species/clusters were decreased. Predominant species/clusters detected in the saliva pellets belonged to five different bacterial phyla, namely, the Firmicutes (eg, Streptococcus and Granulicatella), Proteobacteria (eg, Campylobacter and Neisseria), CFB group bacteria (eg, Prevotella and Porphyromonas) and Actinobacteria (eg, Atopobium and Rothia). Firmicutes was the most diverse phylum, comprising 34 different genus/clusters, and Streptococcus was the most diverse genus, comprising 13 different species/groups (figure 2).
Figure 2.
16S rRNA gene-based phylogenetic tree of 56 varied clusters/genera between patients with pancreatic cancer and healthy controls. Thirty-one clusters/species increased in the saliva of pancreatic cancer patients were marked with triangles. The phylogenetic tree was inferred by a minimum evolution analysis of 16S rRNA sequences.
Identification and independent validation of bacterial biomarkers
Based on the HOMIM data, 16 species/clusters showing significant difference between pancreatic cancer and matched healthy controls (p<0.05, n=20; mean signal intensity >20% of the positive control signal) were selected as biomarker candidates. These 16 species/clusters represented six different genera, including Streptococcus (3 species/groups), Prevotella (4 species/groups), Campylobacter (4 species/groups), Granulicatella (2 species), Atopobium (1 species) and Neisseria (2 species). qPCR was performed to verify the HOMIM array results. Using the original sample set of 10 pancreatic cancer samples and 10 matched healthy controls, 6 out of 16 species were confirmed by qPCR. All six microbial biomarker candidates showed significant differences between patients with pancreatic cancer and healthy controls (p<0.05, n=20). These candidates were then subjected to independent validation by qPCR (28 pancreatic cancer, 28 matched healthy controls and 27 chronic pancreatitis). Two microbial biomarkers (N elongata and S mitis) showed significant difference between patients with pancreatic cancer and healthy controls (p<0.05, n=56), yielding ROC-plot AUC values of 0.657 and 0.680, respectively (table 3). The levels of both bacterial markers were decreased in pancreatic cancer as shown by the results of qPCR, which were consistent with the results obtained by HOMIM array. Interestingly, the levels of one increased species (G adiacens) and one decreased species (S mitis) were significantly different between pancreatic cancer and chronic pancreatitis (p<0.05, n=55). The levels of G adiacens and S mitis were also significantly different between pancreatic cancer (n=28) and non-cancer subjects (chronic pancreatitis and healthy controls, n=55) (p<0.05) (table 3).
Table 3.
Quantitative PCR results of six bacterial biomarkers using the validation samples (n=83)
| Pancreatic cancer versus healthy control |
Pancreatic cancer versus chronic pancreatitis |
Pancreatic cancer versus non-cancer |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | p Value | AUC | Fold change | p Value | AUC | Fold change | p Value | AUC | Fold change |
| Atopobium parvulum | 0.84 | 0.55 | 0.11 | 0.59 | 0.31 | 0.59 | |||
| Granulicatella adiacens | 0.17 | 0.58 | 0.04 | 0.61 | 3.50 (+) | 0.02 | 0.64 | 2.30 (+) | |
| Neisseria elongata | 0.02 | 0.66 | 2.84 (−) | 0.77 | 0.52 | 0.10 | 0.59 | ||
| Prevotella nigrescens | 0.09 | 0.60 | 0.15 | 0.63 | 0.82 | 0.52 | |||
| Streptococcus australis | 0.29 | 0.55 | 0.12 | 0.61 | 0.65 | 0.53 | |||
| Streptococcus mitis | 0.02 | 0.68 | 2.45 (−) | 0.01 | 0.69 | 2.06 (−) | 0.002 | 0.68 | 2.25 (−) |
qPCR was performed to validate the HOMIM microarray findings of an independent clinical cohort, including saliva from 28 patients with pancreatic cancer, 28 healthy control subjects and 27 patients with chronic pancreatitis.
Wilcoxon test: validated if p<0.05. (+): increased risk in pancreatic cancer; (−): decreased risk in pancreatic cancer.
Fold change is only shown for the validated biomarkers.
AUC, area under the curve.
Biomarker combination analysis
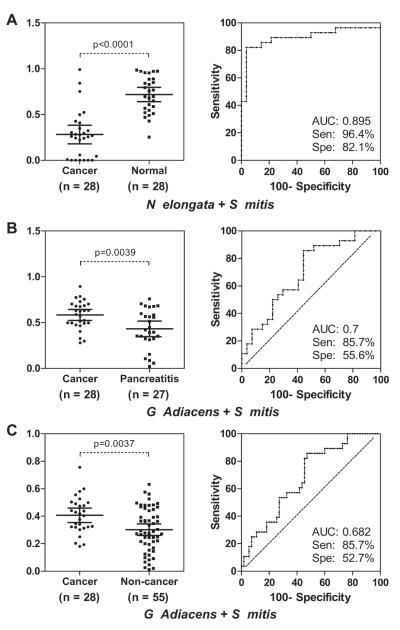
Logistic regression was used to evaluate different combinations of two biomarkers for three levels of clinical discrimination: pancreatic cancer versus healthy control, pancreatic cancer versus chronic pancreatitis and pancreatic cancer versus non-cancer (healthy control + chronic pancreatitis). For pancreatic cancer versus healthy control, the combination of two microbial biomarkers (N elongata and S mitis) yielded an ROC-plot AUC value of 0.90 (95% CI 0.78 to 0.96, p<0.0001) with 96.4% sensitivity and 82.1% specificity in distinguishing patients with pancreatic cancer from healthy subjects (figure 3A). For pancreatic cancer versus chronic pancreatitis, the combination of two microbial biomarkers (G adiacens and S mitis) yielded an ROC-plot AUC value of 0.70 (95% CI 0.56 to 0.81, p=0.0047) with 85.7% sensitivity and 55.6% specificity in distinguishing patients with pancreatic cancer from healthy subjects (figure 3B). For the discrimination of pancreatic cancer versus non-cancer, the combination of the same two microbial biomarkers as pancreatic cancer versus chronic pancreatitis (G adiacens and S mitis) yielded an ROC-plot AUC value of 0.68 (95% CI 0.57 to 0.78, p=0.0063) with 85.7% sensitivity and 52.7% specificity (figure 3C).
Figure 3.
nteractive dot diagram analysis and receiver operating characteristic (ROC) curve analysis for the predictive power of combined salivary bacterial biomarkers. The validated biomarkers were evaluated by logistic regression within three levels of clinical discrimination categories: pancreatic cancer versus healthy control (A), pancreatic cancer versus chronic pancreatitis (B) and pancreatic cancer versus non-cancer (healthy control + chronic pancreatitis) (C). The sensitivity and specificity for each model were obtained by identifying the cut-off point in the predicted probabilities from the logistic regression that maximised the sum of the sensitivity plus specificity. In general, these cut-off points correspond well with the proportion of patients with cancer evaluated in each model.
DISCUSSION
Our study is among the first systematic surveys profiling the microbiome in saliva samples of patients with pancreatic cancer or chronic pancreatitis. We applied the HOMIM array profiling technology to assess salivary microflora alterations in pancreatic cancer and chronic pancreatitis, and possible discriminatory salivary microbial biomarkers that can be validated for these systemic diseases. By addressing both questions, our profiling results and further prevalidation of detection biomarkers open new research directions supporting the idea of systemic inflammation contributing to pancreatic diseases and that saliva is a scientifically feasible and credible biomarker source for non-oral diseases. The early detection of cancer can significantly improve survival rates, especially for pancreatic cancer which, unlike some cancers such as colon cancer, has no clear symptoms or screening methods. Cancer detection tools need to be sufficiently non-invasive and inexpensive to allow widespread applicability. The harnessing of valuable disease-specific biomarkers using less invasive methods such as salivary microflora alterations supports this concept.
The HOMIM profiling of microflora in saliva revealed that microbial composition shifts significantly between patients with pancreatic cancer and healthy controls. The validated bacterial signatures discovered in our study can be linked to pancreatic cancer in multiple aspects. Recent prospective studies showed associations between periodontal disease/tooth loss and an increased risk of pancreatic cancer.7–9 The oral cavity is a large reservoir of bacteria composed of more than 700 species or phylotypes, of which approximately 35% have not been cultured.6 The study of oral bacteria extends beyond the focus of oral disease to systemic diseases. Several studies have illustrated the potential role of periodontal disease as a risk factor for cardiovascular and cerebrovascular diseases,10–12 preterm birth13 and certain cancers.14 Additionally, researchers have found that certain bacteria or variation of the microbiota diversity is associated with atheromas,36 preterm birth, low birth weight37 and human cancers.38–44 P gingivalis is associated with periodontal disease and has been shown to accelerate atheroma deposition in animal models45 by activating host innate immune responses associated with atherosclerosis. P gingivalis, Actinobacillus actinomycetemcomitans and Treponema denticola were detected in atheromatous plaques of humans with atherosclerosis.46–48 Serum antibodies to P gingivalis have also been associated with elevated risk of coronary heart disease.49,50
In our study, the levels of N elongata and S mitis were significantly decreased in patients with pancreatic cancer relative to healthy controls. The level of G adiacens was significantly elevated in patients with pancreatic cancer relative to all non-cancer subjects. These results validate an association between N elongata and G adiacens with periodontal disease.51–53 In addition, G adiacens isolates have been detected in bacteraemia/septicaemia in patients with infective endocarditis/atheroma and in primary bacteraemia.54,55 Together, these observations indicate that G adiacens, often considered opportunistic pathogens, may be associated with systemic inflammations. An elevation of G adiacens may be related to a decrease in S mitis levels. It has been indicated that S mitis plays a protective role against the adhesion of cariogenic bacteria56 and the loss of colonisation by Streptococcus spp. may contribute to aggressive periodontitis.57
Bacteria have been implicated in the pathogenesis of pancreatic diseases including autoimmune pancreatitis and pancreatic ductal adenocarcinoma. A role of Helicobacter pylori infection in the pathogenesis of autoimmune pancreatitis has been suggested.15–19 In a recent study of patients with autoimmune pancreatitis, the peptide AIP1–7, which is homologous to amino acid sequence of PBP of H pylori, was identified from the majority of patients with autoimmune pancreatitis.22 However, this peptide was also identified in a small number of patients with pancreatic adenocarcinoma. H pylori was recently isolated from a human cirrhotic liver,58 suggesting that microorganisms may infect the pancreas and associated tissues by ascending gastric infections or retrograde transfer from the small bowel.20,21 Other data support an association between H pylori colonisation and pancreatic cancer.23–28 Whether a variation in bacterial abundance is a causative factor for cancer carcinogenesis or a derivational reflection of cancer onset due to the change of oral niches needs to be further explored in longitudinal studies. Meanwhile, the link between chronic inflammation and the development of pancreatic ductal adenocarcinoma is becoming clearer. Chronic pancreatitis is now considered a risk factor for the development of pancreatic cancer.59
Taken together, these data suggest that the association between variations in oral microbiota and pancreatic disease may likely be causative rather than reactive. However, this study does not explore changes in oral flora after the surgical resection of pancreatic cancer to address this question. Whether and how local oral infection without bacteria entering the blood stream could potentially result in systemic diseases such as chronic inflammation or neoplasia are currently under active investigation. For example, the immune system recognises the presence of bacterial pathogens through the expression of a family of membrane receptors known as Toll-like receptors (TLRs). Lipopolysaccharide (LPS) on bacteria is specifically recognised by TLR4. Recognition of microbial components by TLRs initiates signal transduction pathways, which upregulate genes involved in innate immune responses and further instruct development of antigen-specific acquired immunity. These pathways are further regulated by TLR domain-containing adaptors such as TIRAP/Mal, TRIF, TRAM and MyD88.
In addition to its effects on immune cells, LPS can also act on certain epithelial cells including cancer cells and promote their phenotypic transformation. For example, nuclear factor-κB is a transcriptional factor that controls the expression of numerous genes involved in inflammation and genes encoding growth factors and cellular invasion-related molecules.60,61 It is constitutively activated in several types of cancers, including pancreatic cancer, and can be induced by several types of inflammatory cytokines including interleukin-1b in pancreatic cancer.62–65 In addition, it has also been shown that LPS, released from the surface of the cell membrane of gram-negative bacteria, promotes nuclear factor-κB activation in pancreatic cancer, providing a possible link between inflammation and cancer development and progression.66 Given the limited understanding of pancreatic cancer aetiology, further investigation into the role of bacterial associated systemic inflammation in pancreatic carcinogenesis is warranted. Finally, additional risk factors for pancreatic cancer should be further researched, including obesity and type 2 diabetes that are associated with inflammation, gastric acidity and high nitrosamines which are caused by nitrate-reducing bacteria.67
Screening for pancreatic cancer carries two major challenges. First is the need to detect early small pancreatic cancers confined to the pancreas or even precancerous stages, also known as PanIN stages. The second is in the ability to differentiate pancreatic cancer from the phenotypically similar chronic pancreatitis, a benign pancreatic disease. The determination of specific profiles of microflora changes in specific cancer types is important because it is possible that the different cancers may have overlapping signatures. We have evaluated the specificity of the validated microbial biomarkers against another HOMIM profiling study that had been performed in our laboratory using lung cancer. None of the bacterial biomarkers validated in this study was significantly altered in the microflora profile of lung cancer. This cross-disease comparison indicated that the validated microbial biomarkers in saliva are likely to be specific for pancreatic cancer detection. This is a discovery study with an initial validation of the statistically significant markers. Hence, in the absence of developing and testing of a prediction panel, this is a prevalidation study, and the biomarker model will need to be tested in an independent clinically relevant cohort in order to be ‘validated’.
This study has some limitations. Primarily, the cross-sectional nature of the study does not enable us to understand the mechanisms and time sequence of the associations. Additional large cohort studies are needed to establish the time sequence and evaluate changes in the oral microbiome from early to later stages of pancreatic cancer. Furthermore, the small sample size does not allow for subgroup analysis to assess whether the associations are consistent across different populations defined by factors such as race, ethnicity and smoking status. For example, none of the patients in our discovery group and very few of the patients in our validation group had a history of smoking. However, smoking is clearly a risk factor for pancreatic cancer, and cigarettes themselves may represent a source for exposure to a wide range of potentially pathogenic microbes.68 However, this does not detract from the potential value of these markers for diagnostic testing, which is currently being evaluated in a nested, case-controlled study using a population-based cohort.
Significance of this study What is already known about this subject?
What is already known about this subject?
▶ Previous studies suggest a link between oral disease, especially periodontitis, and systemic disease, including pancreatic cancer.
▶ Chronic inflammation of the pancreas is associated with an increased risk of developing pancreatic cancer.
▶ Bacteria have been implicated in the pathogenesis of autoimmune pancreatitis and pancreatic ductal adenocarcinoma.
What are the new findings?
▶ First study showing how variation of oral microbiota diversity is associated with pancreatic cancer.
▶ Oral microbiota may function as non-invasive diagnostic biomarkers of pancreatic disease.
How it might impact on clinical practice in the foreseeable future?
▶ Although unclear if the association is causative or reactive, this research may allow for intervention in altering the natural history of pancreatic cancer pathogenesis, especially in high-risk populations, through manipulation of the oral flora.
Acknowledgements
We thank Ali Ammar for collecting and processing saliva samples. We also thank Susan Boches for technical assistance.
Funding Funding support was provided by the National Institute of Health (RO1DE017170 and R21CA126733).
Footnotes
Competing interests DTWW disclose ownership of intellectual property related to the saliva diagnostics field. The other authors disclosed no potential conflicts of interests.
Ethics approval UCLA IRB Committee. This study was approved by the UCLA Institutional Review Board.
Contributors LZ, JF and DW supervised all aspects of this study including study design, execution and data interpretation. LZ, JF and BP conducted the experiments and analysed experimental data. LZ, HZ, DE and BP contributed to data acquisition and data interpretation. JF provided human saliva samples. LZ, JF and KJ wrote the final manuscript. All authors reviewed the manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb DC. Inflammation and cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–19. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–e96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2005, National Cancer Institute. SEER data submission, posted to the SEER website; Bethesda, MD: 2008. based on November 2007. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 6.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaud DS, Joshipura K, Giovannucci E, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171–5. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 8.Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13:312–16. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 9.Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr. 2003;78:176–81. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]
- 10.Joshipura KJ, Douglass CW, Willett WC. Possible explanations for the tooth loss and cardiovascular disease relationship. Ann Periodontol. 1998;3:175–83. doi: 10.1902/annals.1998.3.1.175. [DOI] [PubMed] [Google Scholar]
- 11.Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15:403–13. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- 12.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg RL, Culhane JF. Preterm birth and periodontal disease. N Engl J Med. 2006;355:1925–7. doi: 10.1056/NEJMe068210. [DOI] [PubMed] [Google Scholar]
- 14.Meyer MS, Joshipura K, Giovannucci E, et al. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieder G, Karnholz A, Stoeckelhuber M, et al. H pylori infection causes chronic pancreatitis in Mongolian gerbils. World J Gastroenterol. 2007;13:3939–47. doi: 10.3748/wjg.v13.i29.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kountouras J, Zavos C, Gavalas E, et al. Challenge in the pathogenesis of autoimmune pancreatitis: potential role of Helicobacter pylori infection via molecular mimicry. Gastroenterology. 2007;133:368–9. doi: 10.1053/j.gastro.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Kountouras J, Zavos C, Chatzopoulos D. Autoimmune pancreatitis, Helicobacter pylori infection, and apoptosis: a proposed relationship. Pancreas. 2005;30:192–3. doi: 10.1097/01.mpa.0000151576.91790.f8. [DOI] [PubMed] [Google Scholar]
- 18.Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005;9:196–207. doi: 10.1111/j.1582-4934.2005.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005;9:741–4. doi: 10.1111/j.1582-4934.2005.tb00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson HO, Stenram U, Ihse I, et al. Re: Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2002;94:632–3. doi: 10.1093/jnci/94.8.632. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson HO, Stenram U, Ihse I, et al. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J Gastroenterol. 2006;12:3038–43. doi: 10.3748/wjg.v12.i19.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frulloni L, Lunardi C, Simone R, et al. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med. 2009;361:2135–42. doi: 10.1056/NEJMoa0903068. [DOI] [PubMed] [Google Scholar]
- 23.Risch HA, Yu H, Lu L, et al. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case–control study. J Natl Cancer Inst. 2010;102:502–5. doi: 10.1093/jnci/djq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93:937–41. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 25.de Martel C, Llosa AE, Friedman GD, et al. Helicobacter pylori infection and development of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1188–94. doi: 10.1158/1055-9965.EPI-08-0185. [DOI] [PubMed] [Google Scholar]
- 26.Lindkvist B, Johansen D, Borgstrom A, et al. A prospective study of Helicobacter pylori in relation to the risk for pancreatic cancer. BMC Cancer. 2008;8:321. doi: 10.1186/1471-2407-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosunen TU, Pukkala E, Seppälä K, et al. The effect of eradication therapy for Helicobacter infection on the incidence of gastric and other cancers. Helicobacter. 2004;9:534. [Google Scholar]
- 28.Wadstrom T, Fryzek JP, Demirjian S, et al. Antibodies to Helicobacter bilis in patients with pancreatic carcinoma. Helicobacter. 2004;9:538–9. [Google Scholar]
- 29.Huyghe A, Francois P, Charbonnier Y, et al. Novel microarray design strategy to study complex bacterial communities. Appl Environ Microbiol. 2008;74:1876–85. doi: 10.1128/AEM.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–8. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Farrell JJ, Zhou H, et al. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010;138:949–57.e1-7. doi: 10.1053/j.gastro.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preza D, Olsen I, Willumsen T, et al. Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2009;28:509–17. doi: 10.1007/s10096-008-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 35.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 36.Herzberg MC, Weyer MW. Dental plaque, platelets, and cardiovascular diseases. Ann Periodontol. 1998;3:151–60. doi: 10.1902/annals.1998.3.1.151. [DOI] [PubMed] [Google Scholar]
- 37.Dasanayake AP, Li Y, Wiener H, et al. Salivary Actinomyces naeslundii genospecies 2 and Lactobacillus casei levels predict pregnancy outcomes. J Periodontol. 2005;76:171–7. doi: 10.1902/jop.2005.76.2.171. [DOI] [PubMed] [Google Scholar]
- 38.Anttila T, Koskela P, Leinonen M, et al. Chlamydia pneumoniae infection and the risk of female early-onset lung cancer. Int J Cancer. 2003;107:681–2. doi: 10.1002/ijc.11353. [DOI] [PubMed] [Google Scholar]
- 39.Biarc J, Nguyen IS, Pini A, et al. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S bovis) Carcinogenesis. 2004;25:1477–84. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- 40.Gold JS, Bayar S, Salem RR. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch Surg. 2004;139:760–5. doi: 10.1001/archsurg.139.7.760. [DOI] [PubMed] [Google Scholar]
- 41.Koyi H, Branden E, Gnarpe J, et al. An association between chronic infection with Chlamydia pneumoniae and lung cancer. A prospective 2-year study. APMIS. 2001;109:572–80. doi: 10.1034/j.1600-0463.2001.d01-177.x. [DOI] [PubMed] [Google Scholar]
- 42.Littman AJ, White E, Jackson LA, et al. Chlamydia pneumoniae infection and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1624–30. [PubMed] [Google Scholar]
- 43.Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mager DL, Haffajee AD, Devlin PM, et al. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson FC, 3rd, Yumoto H, Takahashi Y, et al. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–21. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 46.Padilla C, Lobos O, Hubert E, et al. Periodontal pathogens in atheromatous plaques isolated from patients with chronic periodontitis. J Periodontal Res. 2006;41:350–3. doi: 10.1111/j.1600-0765.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 47.Cavrini F, Sambri V, Moter A, et al. Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by FISH: report of two cases. J Med Microbiol. 2005;54:93–6. doi: 10.1099/jmm.0.45845-0. [DOI] [PubMed] [Google Scholar]
- 48.Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–60. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 49.Pussinen PJ, Alfthan G, Tuomilehto J, et al. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2004;11:408–11. doi: 10.1097/01.hjr.0000129745.38217.39. [DOI] [PubMed] [Google Scholar]
- 50.Pussinen PJ, Jousilahti P, Alfthan G, et al. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:1250–4. doi: 10.1161/01.ATV.0000072969.71452.87. [DOI] [PubMed] [Google Scholar]
- 51.Kumar PS, Griffen AL, Barton JA, et al. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 52.Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 53.Siqueira JF, Jr, Rocas IN. Catonella morbi and Granulicatella adiacens: new species in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:259–64. doi: 10.1016/j.tripleo.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Wong JD, Janda JM. Association of an important Neisseria species, Neisseria elongata subsp. nitroreducens, with bacteremia, endocarditis, and osteomyelitis. J Clin Microbiol. 1992;30:719–20. doi: 10.1128/jcm.30.3.719-720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woo PC, Fung AM, Lau SK, et al. Granulicatella adiacens and Abiotrophia defectiva bacteraemia characterized by 16S rRNA gene sequencing. J Med Microbiol. 2003;52:137–40. doi: 10.1099/jmm.0.04950-0. [DOI] [PubMed] [Google Scholar]
- 56.van Hoogmoed CG, van der Mei HC, Busscher HJ. The influence of biosurfactants released by S. mitis BMS on the adhesion of pioneer strains and cariogenic bacteria. Biofouling. 2004;20:261–7. doi: 10.1080/08927010400027050. [DOI] [PubMed] [Google Scholar]
- 57.Stingu CS, Eschrich K, Rodloff AC, et al. Periodontitis is associated with a loss of colonization by Streptococcus sanguinis. J Med Microbiol. 2008;57:495–9. doi: 10.1099/jmm.0.47649-0. [DOI] [PubMed] [Google Scholar]
- 58.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 59.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 60.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 61.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115:2625–32. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Abbruzzese JL, Evans DB, et al. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 63.Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041–9. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 64.Yamanaka N, Morisaki T, Nakashima H, et al. Interleukin 1beta enhances invasive ability of gastric carcinoma through nuclear factor-kappaB activation. Clin Cancer Res. 2004;10:1853–9. doi: 10.1158/1078-0432.ccr-03-0300. [DOI] [PubMed] [Google Scholar]
- 65.Kiefel H, Bondong S, Erbe-Hoffmann N, et al. L1CAM-integrin interaction induces constitutive NF-kappaB activation in pancreatic adenocarcinoma cells by enhancing IL-1beta expression. Oncogene. 2010;29:4766–78. doi: 10.1038/onc.2010.230. [DOI] [PubMed] [Google Scholar]
- 66.Kojima M, Morisaki T, Izuhara K, et al. Lipopolysaccharide increases cyclo-oxygenase-2 expression in a colon carcinoma cell line through nuclear factor-kappa B activation. Oncogene. 2000;19:1225–31. doi: 10.1038/sj.onc.1203427. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro KB, Hotchkiss JH, Roe DA. Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem Toxicol. 1991;29:751–5. doi: 10.1016/0278-6915(91)90183-8. [DOI] [PubMed] [Google Scholar]
- 68.Sapkota AR, Berger S, Vogel TM. Human pathogens abundant in the bacterial metagenome of cigarettes. Environ Health Perspect. 2010;118:351–6. doi: 10.1289/ehp.0901201. [DOI] [PMC free article] [PubMed] [Google Scholar]