Abstract
Alkylglycerols (AKGs) from shark liver oil (SLO) were demonstrated to have strong potency to stimulate immune response. However, no study has been conducted on the effects of AKGs on diet-induced obesity and metabolic inflammatory disorder. The purpose of the present study was to investigate the effect of two AKGs isoforms on obesity and insulin resistance in mice fed high-fat (HF) diet. Forty-eight C57BL/6 mice were divided into normal, HF, HF + 20 mg/kg selachyl alcohol (SA), HF + 200 mg/kg SA, HF + 20 mg/kg batyl alcohol (BA), and HF + 200 mg/kg BA groups. Body weight, fasting glucose, lipids, insulin and leptin levels, serum IL-1β, and TNF-α levels were compared among different groups. Our results showed that high-dose SA decreased body weight, serum triglyceride, cholesterol, fasting glucose level, insulin level, and serum leptin level of the HF fed mice, while high-dose BA increased fasting insulin level of the HF fed mice. Pretreatment of primary adipocytes with 10 μM SA or BA differentially modulates LPS-mediated MAPK and NF-κB signaling. Our study demonstrated that oral administration of AKGs has differential effects on HF-induced obesity and metabolic inflammatory disorder in mice.
1. Introduction
Obesity has become a common public health issue with a cluster of metabolic abnormalities. The incidence of obesity-related chronic diseases is increasing rapidly worldwide [1]. Evidence has accumulated indicating that obesity is closely associated with a state of systematic, low-grade inflammation characterized by activation of inflammatory signaling pathways and abnormal cytokine production in adipose tissue [2, 3]. The cytokines produced by adipocytes include several inflammatory markers such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and monocyte chemoattractant protein (MCP)-1 [4]. These cytokines are elevated in patients with obesity and insulin resistance and are highly associated with the development of cardiovascular diseases and type 2 diabetes mellitus.
Recently, dietary supplements have been used for prevention of obesity and diabetes mellitus due to their high compliance and low toxicity. Shark liver oil (SLO), a well-known dietary supplement, contains alkylglycerols (AKGs), squalene, and essential fatty acids [5]. It has recently been shown that SLO has various pharmacological benefits such as chemoprotective properties against reactive oxygen species as well as anti-inflammatory, antibacterial, antifungal, and anticancer potency [6]. AKGs, the major component of SLO, are glycerol ether lipids that have structural characteristics of an ether linkage between fatty acid and α-position of the glycerol backbone. According to the fatty acid chain length and the number of double bonds, several derivatives of AKGs have been identified. They include such substances as batyl alcohol (BA), chimyl alcohol (CA), and selachyl alcohol (SA) [7]. SA, the predominant component of bioactive AKGs in the SLO (accounting for 59.4%), contains an unsaturated bond in the long hydrocarbon chain (18C:1). CA and BA, which are saturated in their hydrocarbon chains (16C:0 CA, 18C:0 BA), account for a minor proportion of SLO (9.1% CA, 2.8% BA) [6]. AKGs are also found in immune organs such as bone marrow and spleen, indicating their important role in human immune activity [8]. AKGs mainly function by stimulating immune response to enhance the human defense against inflammation [9]. AKGs can also be applied to treat leukemia and solid tumor as well [10]. It was demonstrated that AKGs can inhibit the growth, vascularization, and dissemination of lung carcinoma tumors in mice [11, 12].
The antidiabetic effects of various bioactive food components have gained widespread attention. However, it was also demonstrated that some nutrients such as selenium have side-effect on energy metabolism if they are supplemented inappropriately [13]. AKGs have been shown to have capability of activating cytotoxic macrophages leading to an enhanced phagocytosis and elevating Th-1 cytokines such as TNF-α which are required for macrophage activation [14]. Adipose tissue macrophages play a key role in obesity-induced inflammation and insulin resistance [15]. However, no study has been conducted on the effects of AKGs on diet-induced obesity and metabolic inflammatory disorder. It is interesting to explore how AKGs affect energy metabolism if consumed daily as a nutrition supplement. Therefore, we examined the effect of AKGs on lipopolysaccharide- (LPS-) mediated insulin resistance and induction of inflammatory genes in high-fat (HF) fed mice.
2. Materials and Methods
2.1. Chemicals
SA was purchased from NIKKO Chemicals (Tokyo, Japan). BA was purchased from Bachem (Bubendorf, Switzerland). Escherichia coli LPS 0111:B6 was purchased from Sigma-Aldrich (St. Louis, MO). Glucose, cholesterol, and triglyceride kits were obtained from Kinghawk Pharmaceutical (Beijing, China). Insulin, leptin, IL-1β, and TNF-α ELISA kits were purchased from R&D systems (Minneapolis, MN). Antiphospho- (Thr183/Tyr185) and total JNK, antiphospho- (Thr202/Tyr204) and total ERK, and anti-IκBα were purchased from Santa Cruz (Santa Cruz, CA). All other chemical reagents used in the present study were of analytical grade.
2.2. Animals and Facilities
The study was approved by the Animal Ethics Committee of Xinhua Hospital. Forty-eight, 4-week old male C57BL/6 mice were purchased from SLAC Laboratories (Shanghai, China). All mice were housed in stainless steel cages with bedding (6 mice/cage). Sufficient bedding was used to keep mice dry and clean. All the mice were exposed to a 12-hour light and dark cycle. Frequent bedding changes and cage cleaning were performed as often as necessary.
2.3. Animal Study Design
After arrival, mice were acclimatized for 4 days. After acclimatation, forty-eight mice were randomly divided into six groups of 8 mice each. Both normal chow and high-fat diets were purchased from Shanghai Slac Laboratory Animal Co., Ltd. Normal chow diets contained 20.5% crude protein, 4.62% crude fat, 52.5% nitrogen-free extract, and 4.35% crude fibers (total calories 3.45 Kcal/g, 12% calories in fat). High-fat diets contained 18.8% crude protein, 16.2% crude fat, 45.2% nitrogen-free extract, and 3.98% crude fibers (total calories 3.79 Kcal/g, 38% calories in fat) [16]. For 8 weeks, groups 1 and 2 received the normal diets (ND) and high-fat diets (HF), respectively; groups 3 and 4 were fed the HF supplemented with 20 and 200 mg/kg SA, respectively; groups 5 and 6 were fed the HF supplemented with 20 and 200 mg/kg BA, respectively. Body weight was monitored weekly. At the end of the experiment, blood samples were collected after overnight fasting. Following 4 days recovery, all groups were fasted for 5 hours and then challenged with 100 ng LPS intraperitoneally. After 2 hours, animals were then euthanized and blood samples, liver, and epididymal fat were collected. Liver tissues and visceral adipose were immediately weighted after removal [17]. Serum was isolated by centrifugation at 1500 g at 4°C for 10 min and stored at −80°C until it was used for blood biochemical assays.
2.4. Culturing of Primary Adipocytes
Abdominal white adipose tissue was obtained from 4- to 5-week-old, wild-type mice. After blood washing, the adipose tissues were minced and digested with 1 mg/mL collagenase type I (Sigma-Aldrich, St. Louis, MO) for 30 min at 37°C. Cells were filtered through 200-μm pore size nylon meshes. The stromal vascular cells (SVCs) were separated from adipocytes by centrifugation and washed with DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). SVCs were plated and propagated to confluence in DMEM supplemented with 10% FBS, 50 μg/mL streptomycin, and 50 U/mL penicillin [18]. After attachment, the medium was replaced by induction medium containing 10 μg/mL insulin (INS), 1 μm dexamethasone (DEX), and 0.5 mm 3-isobutyl-1-methylxanthine (MIX) with 10% FBS and continued differentiation for 12 days. On day 12, cultures were pretreated with DMSO vehicle, or different concentrations of SA or BA for 24 hrs, and then treated with 10 μg/L LPS for 6 hrs.
2.5. Immunoblotting Analysis
Following treatment, cultures were harvested and protein was extracted with RIPA buffer. Immunoblotting analysis was performed as described previously [19].
2.6. Biochemical Analysis
Concentrations of insulin, leptin, IL-1β, and TNF-α were measured using ELISA kit. Glucose, total cholesterol, and triglyceride were tested using enzymatic methods. Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) was calculated from glucose and insulin concentrations (fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5) [20].
2.7. Statistical Analysis
Data are shown as means with their standard errors. Statistical significance was evaluated using one-way ANOVA followed by Duncan's multiple range test. P value < 0.05 was considered statistically significant.
3. Results
3.1. Effects of AKGs Diets on Body and Organ Weights
Daily food intake during the experimental period was not significantly different among groups. No changes of end-point body weight and net weight gain were observed between HF diet group and low-dose (20 mg/kg) AKGs (SA or BA) supplemented groups during the 60-day period. High-dose SA (200 mg/kg) supplementation significantly decreased the end-point body weight and net weight gain of the HF fed mice during the 60-day dietary intervention. Weights of epididymal white adipose tissue and liver were compared among the different dietary treatments. Epididymal fat was significantly decreased as percent body weight in mice that received 200 mg/kg SA supplementation (Table 1).
Table 1.
The effect of selachyl alcohol (SA) or batyl alcohol (BA) supplementation on body weights, tissue weights, and food consumption.
| Treatment1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P value6 | P value | P value | |||||||
| Item | ND | HF | HF + 20 mg/kg SA | HF + 200 mg/kg SA | HF + 20 mg/kg BA | HF + 200 mg/kg BA | (HF) | (SA) | (BA) |
| Initial body wt2 (g) | 14.05 ± 0.185 | 14.27 ± 0.21 | 14.38 ± 0.23 | 14.36 ± 0.27 | 14.22 ± 0.29 | 14.33 ± 0.23 | 0.299 | 0. 943 | 0.950 |
| End-point body wt3 (g) | 25.92 ± 0.56 | 35.21 ± 0.57 | 36.48 ± 0.59 | 32.80 ± 0.54* | 36.35 ± 0.44 | 36.17 ± 0.39 | <0.001 | <0.001 | 0.087 |
| Net weight gain4 (g) | 11.87 ± 0.63 | 20.93 ± 0.51 | 22.10 ± 0.67 | 18.43 ± 0.57* | 22.12 ± 0.55 | 21.83 ± 0.54 | <0.001 | <0.001 | 0.062 |
| Epididymal fat 3(%) | 1.76 ± 0.29 | 2.39 ± 0.09 | 2.20 ± 0.05 | 2.02 ± 0.10* | 2.48 ± 0.08 | 2.64 ± 0.07 | <0.001 | 0.019 | 0.130 |
| Liver weight3 (%) | 3.86 ± 0.04 | 3.29 ± 0.05 | 3.42 ± 0.07 | 3.49 ± 0.08 | 3.37 ± 0.02 | 3.18 ± 0.03† | <0.001 | 0.150 | 0.011 |
| Food intake (g/mouse/day) | 2.80 ± 0.05 | 2.64 ± 0.04 | 2.57 ± 0.04 | 2.58 ± 0.05 | 2.64 ± 0.06 | 2.69 ± 0.05 | 0.005 | 0.563 | 0.729 |
1ND: normal diet; HF: high-fat diet; SA: selachyl alcohol; BA: batyl alcohol.2Measured before dietary intervention. 3Measured after 8-week feeding period. 4Difference between end-point body weight and initial body weight. 5Values are mean ± SEM. Means with a mark (*) in HF + 20 mg/kg SA or HF + 200 mg/kg SA group differ significantly from the HF diet group. Means with a mark (†) in HF + 20 mg/kg BA or HF + 200 mg/kg BA group differ significantly from the HF diet group. 6 P value (HF) indicates the effect of HF treatment. P value (SA) indicates the effect of different doses of SA supplements on HF fed mice. P value (BA) indicates the effect of different doses of BA supplements on HF fed mice.
3.2. Effects of AKGs Diets on Serum Triglyceride and Cholesterol
There was a significant (110%) increase in the serum triglyceride level of the HF group compared with the ND group, whereas a 25% decrease of serum triglyceride was observed in the 200 mg/kg SA group relative to the HF group (P < 0.01) (Figure 1(a)). HF feeding caused a significant (50%) increase in the total cholesterol level. 200 mg/kg SA treatment; however, had reduced the serum cholesterol level by 30% as compared to the HF group (P < 0.001) (Figure 1(b)). There was no significant difference in triglyceride or cholesterol level between the HF group and the HF plus low-dose SA group. No significant change in triglyceride or cholesterol level was observed in HF plus BA diet group as compared with HF group.
Figure 1.
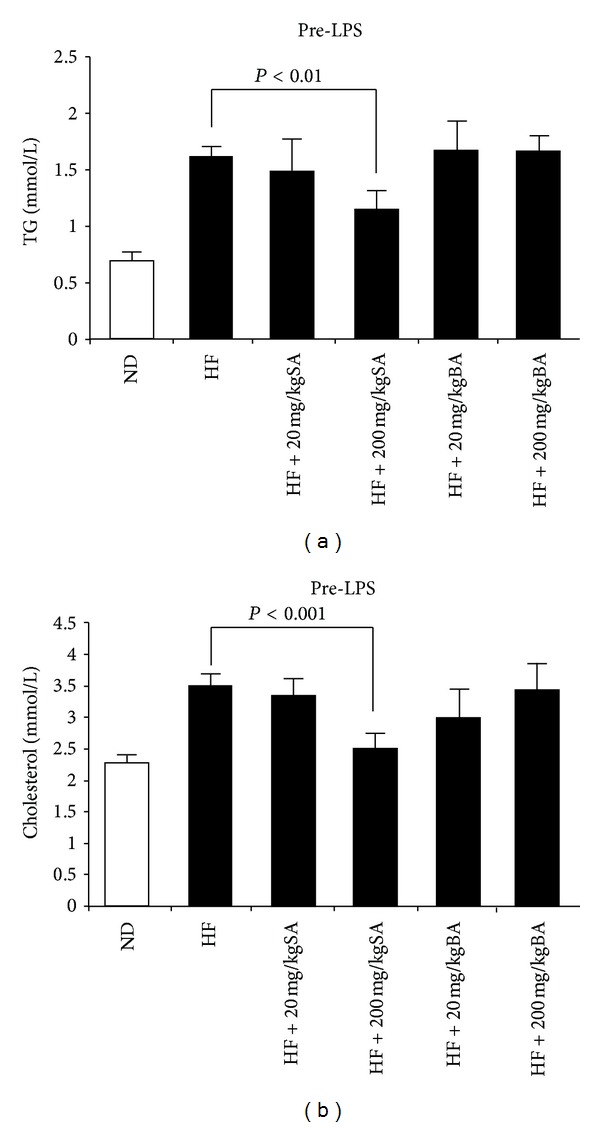
The effect of selachyl alcohol (SA) or batyl alcohol (BA) supplementation on serum triglycerides (a) and cholesterol (b). Data were presented as mean ± SE. Significant differences were tested with two-way ANOVA. Only significant comparisons are presented.
3.3. Effects of AKGs Diets on Glucose, Insulin, and HOMA-IR
To investigate the impact of different AKGs supplemented HF diets in comparison with HF diet on glucose metabolism, we examined blood glucose concentrations before and after LPS challenge in different dietary groups. HF diet group had significantly higher fasting blood glucose concentration as compared to ND diet group (9.84 mmol/L versus 7.62 mmol/L; P < 0.001) (Table 2). Fasting blood glucose was significantly lower in mice fed HF diet plus 20 or 200 mg/kg SA than that in mice fed HF diet (P < 0.001). After intraperitoneal injection of LPS, a drop of blood glucose concentration was observed in all groups. HF diet plus 200 mg/kg SA caused lower blood glucose concentration after LPS challenge as compared to HF diet group (5.05 mmol/L versus 5.71 mmol/L; P < 0.05) (Table 2).
Table 2.
The effect of selachyl alcohol (SA) or batyl alcohol (BA) supplementation on serum glucose, insulin, and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) before and after 100 ng lipopolysaccharide (LPS) challenge.
| Pre-LPS1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P value4 | P value | P value | |||||||
| Item | ND2 | HF | HF + 20 mg/kg SA | HF + 200 mg/kg SA | HF + 20 mg/kg BA | HF + 200 mg/kg BA | (HF) | (SA) | (BA) |
| Glucose (mmol/L) | 7.62 ± 0.303 | 9.84 ± 0.34 | 8.69 ± 0.20* | 7.47 ± 0.29* | 10.03 ± 0.37 | 10.06 ± 0.54 | <0.001 | <0.001 | 0.761 |
| Insulin (μIU/mL) | 13.49 ± 1.14 | 27.81 ± 1.85 | 25.05 ± 2.03 | 20.92 ± 1.5* | 25.33 ± 2.73 | 34.41 ± 2.03† | <0.001 | 0.043 | 0.025 |
| HOMA-IR | 4.63 ± 0.54 | 12.11 ± 0.78 | 9.71 ± 0.86* | 7.00 ± 0.69* | 11.12 ± 1.02 | 15.39 ± 1.17† | <0.001 | <0.001 | 0.017 |
|
| |||||||||
| Post-LPS | |||||||||
| Glucose (mmol/L) | 6.19 ± 0.30 | 5.71 ± 0.16 | 5.73 ± 0.21 | 5.05 ± 0.22* | 5.16 ± 0.15 | 5.88 ± 0.30 | 0.182 | 0.038 | 0.062 |
| Insulin (μIU/mL) | 21.20 ± 3.35 | 37.13 ± 2.86 | 33.03 ± 3.65 | 22.99 ± 3.08* | 33.86 ± 2.42 | 39.92 ± 2.33 | <0.005 | 0.018 | 0.246 |
| HOMA-IR | 5.71 ± 0.79 | 9.48 ± 0.91 | 8.35 ± 0.91 | 5.11 ± 0.70* | 7.80 ± 0.62 | 10.62 ± 1.08 | 0.008 | 0.004 | 0.096 |
1Serum glucose and insulin were measured before LPS challenge (Pre-LPS) and 2 hours following 100 ng LPS challenge (Post-LPS). 2ND: normal diet; HF: high-fat diet; SA: selachyl alcohol; BA: batyl alcohol. 3Values are mean ± SEM. Means with a mark (*) in HF + 20 mg/kg SA or HF + 200 mg/kg SA group differ significantly from the HF diet group. Means with a mark (†) in HF + 20 mg/kg BA or HF + 200 mg/kg BA group differ significantly from the HF diet group. 4 P value (HF) indicates the effect of HF treatment. P value (SA) indicates the effect of different doses of SA supplements on HF fed mice. P value (BA) indicates the effect of different doses of BA supplements on HF fed mice.
The impact of different forms of AKGs on insulin resistance induced by HF diet was also assessed. The plasma insulin concentrations were examined before and after LPS challenge in different dietary groups. As expected, HF diet group had significantly higher fasting insulin concentrations as compared to ND diet group (27.81 μIU/mL versus 13.49 μIU/mL; P < 0.001) (Table 2). It was noted that fasting insulin concentration was decreased in mice fed HF diet plus 200 mg/kg SA relative to mice fed HF diet (20.92 μIU/mL versus 27.81 μIU/mL; P < 0.05), whereas insulin concentration was increased in mice fed HF diet plus 200 mg/kg BA (P < 0.05). After intraperitoneal injection of LPS, mice fed HF plus 200 mg/kg SA showed lower insulin increase compared to mice fed HF diet (22.92 μIU/mL versus 37.13 μIU/mL; P < 0.05). No effects of low-dose AKGs (SA or BA) supplementation were observed on fasting insulin concentrations at pre- and post-LPS challenge (Table 2). The HOMA-IR score was calculated from fasting blood glucose and insulin concentration to assess whether AKGs diet protected mice from insulin resistance. In fact, mice that received 20 or 200 mg/kg SA supplementation had significantly lower HOMA-IR scores (P < 0.001), however, mice received 200 mg/kg BA supplementation had significantly higher HOMA-IR scores as compared to mice that received HF diet at pre-LPS challenge (P < 0.05). After intraperitoneal injection of LPS, only 200 mg/kg SA supplementation showed significant protective effect on insulin resistance which was indicated by HOMA-IR scores (P < 0.005) (Table 2).
3.4. Effect of AKGs on Serum Cytokines and Leptin
The proinflammatory cytokines such as IL-1β and TNF-α were elevated after LPS challenge. Interestingly, high-dose SA or BA showed differential effects on serum IL-1β and TNF-α production after 100 ng LPS challenge (Table 3). 200 mg/kg SA supplementation suppressed serum IL-1β and TNF-α level induced by LPS challenge (P < 0.05), whereas 200 mg/kg BA supplementation significantly enhanced serum IL-1β and TNF-α level induced by LPS challenge (P < 0.05). However, there was no significant effect on IL-1β and TNF-α response to LPS challenge by low-dose AKGs (SA or BA) supplementation.
Table 3.
The effect of selachyl alcohol (SA) or batyl alcohol (BA) supplementation on serum cytokines and leptin before and after 100 ng lipopolysaccharide (LPS) challenge.
| Pre-LPS1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P value4 | P value | P value | |||||||
| Item | ND2 | HF | HF + 20 mg/kg SA | HF + 200 mg/kg SA | HF + 20 mg/kg BA | HF + 200 mg/kg BA | (HF) | (SA) | (BA) |
| IL-1β (pg/mL) | 2.3 ± 0.193 | 8.8 ± 0.70 | 8.25 ± 1.09 | 7.1 ± 0.82 | 8.18 ± 0.59 | 10.47 ± 1.37 | <0.001 | 0.404 | 0.074 |
| TNF-α (pg/mL) | 4.83 ± 0.56 | 5.83 ± 0.29 | 5.5 ± 0.44 | 5.95 ± 0.25 | 5.07 ± 0.39 | 5.68 ± 0.36 | 0.138 | 0.625 | 0.289 |
| Leptin (ng/mL) | 7.26 ± 0.50 | 16.55 ± 0.60 | 16.18 ± 0.59 | 13.57 ± 0.49* | 16.52 ± 0.42 | 16.13 ± 0.55 | <0.001 | 0.005 | 0.837 |
|
| |||||||||
| Post-LPS | |||||||||
| IL-1β (pg/mL) | 100.85 ± 9.2 | 196.2 ± 15.4 | 187.1 ± 10.1 | 149.6 ± 6.7* | 204.5 ± 10.8 | 253.1 ± 8.7† | <0.001 | 0.016 | 0.005 |
| TNF-α (pg/mL) | 231 ± 8.78 | 347 ± 10.23 | 320.75 ± 13.06 | 288.87 ± 9.49* | 370.25 ± 14.44 | 476.79 ± 19.28† | <0.001 | 0.004 | <0.001 |
| Leptin (ng/mL) | 9.18 ± 0.32 | 18.16 ± 0.83 | 16.74 ± 0.76 | 16.27 ± 0.78 | 16.37 ± 0.8 | 16.81 ± 0.68 | <0.001 | 0.063 | 0.238 |
1Serum cytokines and leptin were measured before LPS challenge (Pre-LPS) and 2 hours following 100 ng LPS challenge (Post-LPS). 2ND: normal diet; HF: high-fat diet; SA: selachyl alcohol; BA: batyl alcohol. 3Values are mean ± SEM. Means with a mark (*) in HF + 20 mg/kg SA or HF + 200 mg/kg SA group differ significantly from the HF diet group. Means with a mark (†) in HF + 20 mg/kg BA or HF + 200 mg/kg BA group differ significantly from the HF diet group. 4 P value (HF) indicates the effect of HF treatment. P value (SA) indicates the effect of different doses of SA supplements on HF fed mice. P value (BA) indicates the effect of different doses of BA supplements on HF fed mice.
As for serum leptin, mice that received HF diet had significantly higher serum leptin concentration compared to those received ND diet (16.55 ng/mL versus 7.26 ng/mL; P < 0.05). We found that serum leptin concentration was significantly decreased in mice that received 200 mg/kg SA supplementation as compared to mice that received HF diet at pre-LPS challenge (13.57 ng/mL versus 16.55 ng/mL; P < 0.05) (Table 3).
3.5. Effect of AKGs on LPS-Mediated MAPK and NF-κB Activation
Given the role of MAPK pathway in inducing inflammatory gene expression via Toll-like-receptor- (TLR-) 4 activation, we examined the effects of AKGs on MAPK phosphorylation. Pretreatment of mice adipocyte cultures with 10 μM SA modestly decreased LPS-mediated phosphorylation of JNK and ERK (Figure 2). However, pretreatment of mice adipocyte cultures with 10 μM BA increased LPS-mediated phosphorylation of JNK and ERK. In the absence of LPS, AKGs treatment did not enhance the MAPK activation. Because the activation of NF-κB also plays a crucial role in the transcriptional activation of inflammation-responsive genes, the effects of AKGs on NF-κB activation were examined by detecting IκBα degradation via immunoblot. We found that the pretreatment with SA attenuated IκBα degradation by LPS (Figure 2). However, BA did not present effect on LPS-induced NF-κB activation in our experiments.
Figure 2.
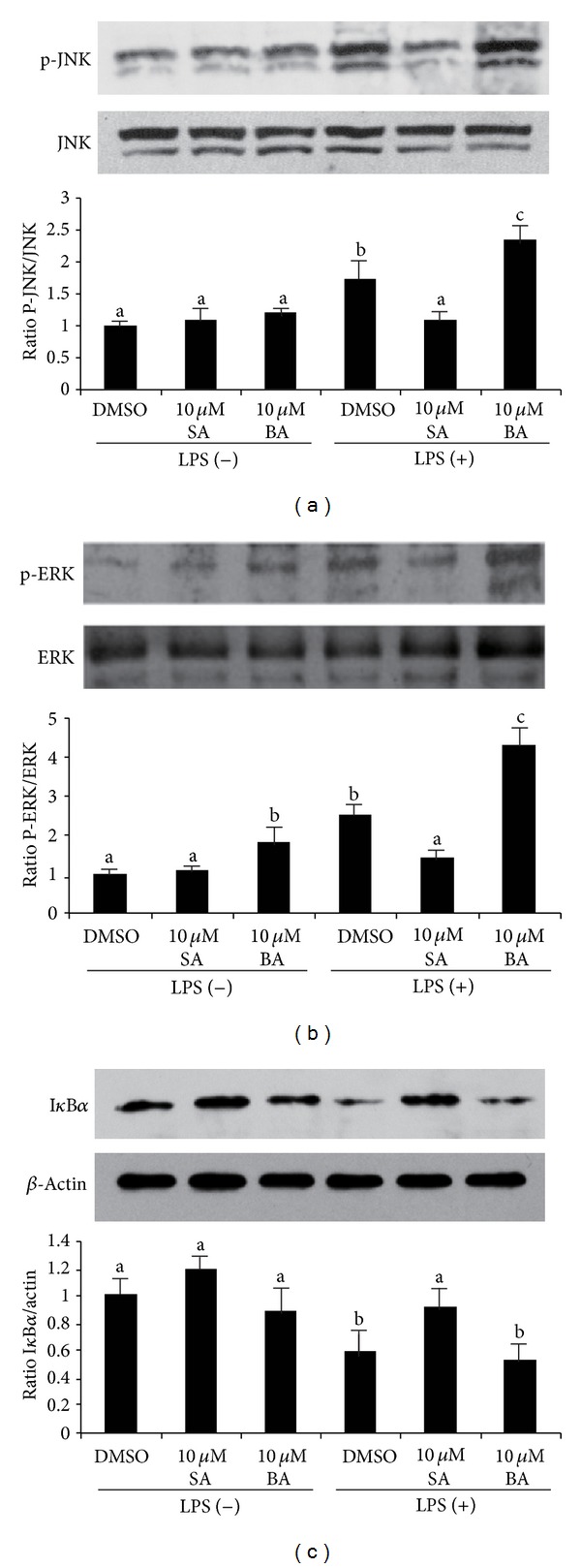
The effect of selachyl alcohol (SA) or batyl alcohol (BA) on TLR-4 signaling activated by LPS in mice primary adipocytes. Newly differentiated cells were pretreated with DMSO vehicle (−) or 10 μM SA or 10 μM BA for 24 h and then treated with 10 μg/L LPS for 6 h. (a) Phosphorylation of JNK (P-JNK-to-JNK ratio). (b) Phosphorylation of ERK (P-ERK-to-ERK ratio). (c) Expression of IκBα (IκBα-to-actin ratio). Levels of protein were measured by western blotting. Data are a mean of ± SE and corrected for loading. Significant differences between each group are indicated by different letters.
4. Discussion
SLO has been widely used in the past years in the Scandinavian medicine because of its properties as immunity boosters and a remedy against radiation therapy and cancer [21]. AKGs are the major components in SLO which could stimulate immunity both in vitro and in vivo [22, 23]. Daily consumption of AKGs-rich SLO showed benefits to the immune system. Despite widespread intake of AKGs, safety studies on AKGs extract were poor. The acute and repeated (28 days) oral toxicity has been evaluated for oral AKGs administration in rats at doses of 200 and 1000 times the maximum recommended dose in humans [24]. In that study, AKGs administration showed no adverse effects on mortality at either acute or subchronic dose. However, the correlation between long-term AKGs supplement and HF-induced obesity has not been shown. In previous studies, AKGs were demonstrated to have potency to activate cytotoxic macrophages and increase humoral immune response [22]. AKGs could also stimulate the IL-12 and IFN-gamma production and elicit Th1 response [25, 26]. As we know, adipose infiltrated macrophage and secreted cytokines play important roles in obesity and insulin resistance [27–29]. Thus, the potency of AKGs to stimulate immunity spurred our interest to examine the AKGs effect on HF-induced obesity and insulin resistance.
Studies have shown that HF diets induce adipose tissue inflammation and stimulate TLR-4 expression [30]. TLR-4, a subclass of the TLR family, plays a critical role in activating innate immune and inflammation response in mammals by recognizing bacterial LPS [31, 32]. Activation of TLR-4 in adipocytes leads to the activation of MAPK and NF-κB signaling pathways, and induction of many inflammatory cytokines [33, 34]. These cytokines are involved in inducing glucose intolerance, insulin resistance, and infiltration of macrophages into adipose tissue. Recent study showed that TLR-4 responds to nonbacterial ligands such as fatty acids [35–37]. It was demonstrated that saturated fatty acids such as lauric acid and palmitic acid are able to induce cyclooxygenase-2 (COX-2) expression; however, unsaturated fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are able to inhibit saturated fatty acid-induced COX-2 expression [38]. In present study, we demonstrated that AKGs with saturated chain increased LPS-mediated activation of the MAPK signaling, which could cause the expression of inflammatory genes and insulin resistance in adipocytes. AKGs with unsaturated chain decreased LPS-mediated activation of the MAPK and NF-κB signaling, thus ameliorated insulin resistance. However, both AKGs with saturated and unsaturated chain did not activate MAPK or NF-κB signaling in the absence of LPS. Accordingly, it was indicated that both AKGs with saturated chain and unsaturated chain modulated TLR-4 signaling and insulin response in the hyperinflammatory environment such as high-fat diet feeding or LPS treatment but did not show direct effect in the hypoinflammatory environment. This result was also noticed by Ocaña et al., who showed that BA activated the expression of IL-1β and IL-6 genes only in TNF-α-induced adipocytes but not in the nonstimulated cells [39].
Several studies have demonstrated that different forms of AKGs have differential biological effects. The unsaturated AKGs with 16 or 18 carbon alkyl chains showed strong anti-tumor and antimetastasis activities in mice model [40]. By contrast, the saturated AKGs with 16 or 18 carbon alkyl chains showed less antitumor effect or even tumor-promoting activity. Previous studies also demonstrated that proinflammatory responses were enhanced by most saturated fatty acids but reduced by most unsaturated fatty acids. In metabolic experiments, rats fed saturated fatty acids had higher triacylglycerol, cholesterol, and low-density lipoprotein cholesterol, whereas rats fed unsaturated fatty acids had lower triacylglycerol, cholesterol, and low-density lipoprotein cholesterol as compared with control rats [41, 42]. Moreover, the increased ratio of P/S (polyunsaturated/saturated) fatty acids was beneficial in depleting white adipose tissue accumulation and improved the metabolic status in diet-induced obese hamster [43]. The controlled clinical trials have also indicated that replacing saturated fat with unsaturated fat was more effective in lowering risk of metabolic disorder than simply reducing total fat consumption [44, 45]. Our studies showed that AKGs had the similar effects on metabolic and inflammatory status as free fatty acids in spite of their ether bond with glycerol. As we know, SLO and human breast milk are both good sources of AKGs, with unsaturated forms as the predominant components. In clinical studies, SLO showed beneficial effect on lipid metabolism in patients with hypertension [46]. Breast milk has also been demonstrated to be protective against the development of childhood overweight and obesity [47]. Thus, we postulated that the predominant unsaturated AKGs in SLO and breast milk would neutralize the adverse effects of saturated AKGs on metabolism and play a prominent role of preventing metabolic syndrome.
Increasing studies have indicated that dietary supplementation of oils extracted from marine species can alter the metabolic and immunologic status which is highly associated with the incidence of obesity and type 2 diabetes. Different marine oils have distinct lipid profiles, which result in potential differences in their effects on inflammation and metabolism. Fish oil, the typical marine oil product, was demonstrated to have protective effect on LPS-induced inflammation and insulin resistance [17]. It was also shown that dietary fish oil supplementation could reduce body weight gain in HF-induced obese mice [48]. The anti-inflammation and antiobesity effects of fish oil were attributed to its richness of (n − 3) PUFA [49]. SLO, which was also rich of (n − 3) PUFA, was known to contain high proportion of squalene and AKGs. Both squalene and AKGs have potent immunological activity, thus eliciting people's interest of investigating their impact on metabolic balance. Squalene has already been demonstrated to be able to elevate the body weight and serum cholesterol in high-fat fed hamsters due to its involvement in hepatic cholesterol metabolism [50]. However, little is known about the association between AKGs intake and metabolic alteration. Our results showed that AKGs differentially modulated HF-induced obesity and insulin resistance in mice, which gave us indication that more attention should be paid to the impact of these immune-stimulating chemicals on metabolism.
Actually, it has already been noticed that oral SLO intake has adverse effect on liver function if supplemented at high dosage [51]. Therefore, it is important to use AKGs in animal experiment at the dosage comparable to that of human supplementation for nutritional purpose. The Biomare Immuno which is manufactured by Hankintatukku (Finland) contains 60 mg AKGs per capsule. On the label, it recommends six capsules daily, which equals to 7.2 mg AKGs/kgweight (60 mg × 6/50 kgweight) provided that a male adult weighs 50 kg on average. In the present study, AKGs at 20 mg/kgdiet and 200 mg/kgdiet dietary supplementation corresponded to 4 mg/kgweight (20 mg/kgdiet × 0.005 kgdiet/0.025 kgweight) and 40 mg/kgweight (200 mg/kgdiet×0.005 kgdiet/0.025 kgweight), respectively, assuming that a male adult mouse weighs 25 g and expends 5 g diet on average. Thus, the amount of 20 mg/kgdiet AKGs dietary supplement was comparable to that commonly consumed by a SLO-supplement consumer. The results obtained from rodents showed that the 20 mg/kgdiet dietary AKGs supplementation did not have significant effect on body weight but only showed minor effect on glucose metabolism. Nevertheless, 200 mg/kgdiet dietary AKGs supplementation showed obvious effects not only on body weight but also on lipid, glucose metabolism, and pro-inflammatory cytokine production. Although these results were not directly applicable to humans, they provided some implications for individuals who routinely consume AKGs supplement or AKGs-rich SLO. Taken together, our results provided novel insights into differential effects of saturated- and unsaturated-chain AKGs on adipose tissue inflammation, which suggested that routine consumption of SLO consisting of different isoforms of AKGs is safe at normal dosage and shows balanced effect on obesity and insulin resistance.
5. Conclusion
Collectively, these data demonstrate that the unsaturated AKGs (SA) have potency to decrease the HF-induced obesity at high dosage and ameliorate insulin resistance at normal or high dosage. The saturated AKGs (BA) can increase insulin resistance at high dosage. Our data also suggest that AKGs show differential effects on LPS-induced inflammation in adipocytes. The effect of SLO rich for AKGs on adipose metabolism and insulin response should be evaluated in the future, to warrant supplementing SLO products at a safe dosage and prevent its potential hazardous effects on metabolism.
Acknowledgments
This research was supported by Grants 81000242, 30901472, 30772270, and 30972427 from the National Natural Science Foundation of China (to Linxi Qian, Wei Cai, and Shuna Sun), Program for Innovative Research Team of Shanghai Municipal Education Commission (to Wei Cai), 11QA1405400 Hesper Foundation of Shanghai Scientific Bureau (to Linxi Qian), 2010Y157 Junior Scientist Foundation of Shanghai Health Bureau (to Linxi Qian), and 11DZ2260500 Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition (to Wei Cai).
Abbreviations
- AKGs:
alkylglycerols
- SA:
Selachyl alcohol
- BA:
Batyl alcohol
- HF:
High fat
- ND:
Normal diets
- LPS:
Lipopolysaccharides
- IL:
Interleukin
- TNF:
Tumor necrosis factor
- SLO:
Shark liver oil
- SVCs:
Stromal vascular cells
- HOMA-IR:
Model assessment-insulin resistance
- TLR:
Toll-like-receptor
- PUFAs:
Polyunsaturated fatty acids.
References
- 1.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Reviews Immunology. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 5.Bordier CG, Sellier N, Foucault AP, le Goffic F. Purification and characterization of deep sea shark Centrophorus squamosus liver oil 1-O-alkylglycerol ether lipids. Lipids. 1996;31(5):521–528. doi: 10.1007/BF02522646. [DOI] [PubMed] [Google Scholar]
- 6.Iannitti T, Palmieri B. An update on the therapeutic role of alkylglycerols. Marine Drugs. 2010;8(8):2267–2300. doi: 10.3390/md8082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brohult A, Brohult J, Brohult S. Biochemical effects of alkoxyglycerols and their use in cancer therapy. Acta chemica Scandinavica. 1970;24(2):p. 730. doi: 10.3891/acta.chem.scand.24-0730. [DOI] [PubMed] [Google Scholar]
- 8.Hallgren B, Niklasson A, Ställberg G, Thorin H. On the occurrence of 1-O-alkylglycerols and 1-O-(2-methoxyalkyl)glycerols in human colostrum, human milk, cow’s milk, sheep’s milk, human red bone marrow, red cells, blood plasma and a uterine carcinoma. Acta chemica Scandinavica B. 1974;28(9):1029–1034. doi: 10.3891/acta.chem.scand.28b-1029. [DOI] [PubMed] [Google Scholar]
- 9.Osmond DG, Roylance PJ, Webb AJ, Yoffey JM. The action of batyl alcohol and selachyl alcohol on the bone marrow of the guinea pig. Acta haematologica. 1963;29:180–186. doi: 10.1159/000207341. [DOI] [PubMed] [Google Scholar]
- 10.Langen P, Brachwitz H, Schildt J. Inhibition of proliferation of Ehrlich ascites carcinoma cells in vitro and in vivo by halogeno analogues of long chain acyl- and alkylglycerols. Acta Biologica et Medica Germanica. 1979;38(7):965–974. [PubMed] [Google Scholar]
- 11.Pédrono F, Martin B, Leduc C, et al. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutrition and Cancer. 2004;48(1):64–69. doi: 10.1207/s15327914nc4801_9. [DOI] [PubMed] [Google Scholar]
- 12.Skopińska-Rózewska E, Chorostowska-Wynimko J, Krotkiewski M, et al. Inhibitory effect of Greenland shark liver oil combined with squalen and arctic birch ashes on angiogenesis and L-1 sarcoma growth in Balb/c mice. Polish Journal of Veterinary Sciences. 2003;6(supplement 3):54–56. [PubMed] [Google Scholar]
- 13.Zeng M-S, Li X, Liu Y, et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radical Biology and Medicine. 2012;52(8):1335–1342. doi: 10.1016/j.freeradbiomed.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berdel WE, Bausert WR, Weltzien HU. The influence of alkyl-lysophospholipids and lysophospholipid-activated macrophages on the development of metastasis of 3-Lewis lung carcinoma. European Journal of Cancer and Clinical Oncology. 1980;16(9):1199–1204. doi: 10.1016/0014-2964(80)90179-6. [DOI] [PubMed] [Google Scholar]
- 15.Ortega Martinez de Victoria E, Xu X, Koska J, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy pima Indians. Diabetes. 2009;58(2):385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024520.e24520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijay-Kumar M, Vanegas SM, Patel N, Aitken JD, Ziegler TR, Ganji V. Fish oil rich diet in comparison to saturated fat rich diet offered protection against lipopolysaccharide-induced inflammation and insulin resistance in mice. Nutrition and Metabolism. 2011;8(1, article 16) doi: 10.1186/1743-7075-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masaki M, Kurisaki T, Shirakawa K, Sehara-Fujisawa A. Role of meltrin α (ADAM12) in obesity induced by high-fat diet. Endocrinology. 2005;146(4):1752–1763. doi: 10.1210/en.2004-1082. [DOI] [PubMed] [Google Scholar]
- 19.Hwang D, Jang BC, Yu G, Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-κB signaling pathways in macrophages. Biochemical Pharmacology. 1997;54(1):87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 20.Holness MJ, Greenwood GK, Smith ND, Sugden MC. PPARα activation and increased dietary lipid oppose thyroid hormone signaling and rescue impaired glucose-stimulated insulin secretion in hyperthyroidism. American Journal of Physiology—Endocrinology and Metabolism. 2008;295(6):E1380–E1389. doi: 10.1152/ajpendo.90700.2008. [DOI] [PubMed] [Google Scholar]
- 21.Brohult A, Brohult J, Brohult S, Joelsson I. Effect of alkoxyglycerols on the frequency of fistulas following radiation therapy for carcinoma of the uterine cervix. Acta Obstetricia et Gynecologica Scandinavica. 1979;58(2):203–207. doi: 10.3109/00016347909154583. [DOI] [PubMed] [Google Scholar]
- 22.Homma S, Millman I, Yamamoto N. A serum factor for macrophage activation after in vitro dodecylglycerol treatment of mouse lymphocytes. Immunology and Cell Biology. 1990;68, part 2:137–142. doi: 10.1038/icb.1990.19. [DOI] [PubMed] [Google Scholar]
- 23.Oh SY, Jadhav LS. Effects of dietary alkylglycerols in lactating rats on immune responses in pups. Pediatric Research. 1994;36(3):300–305. doi: 10.1203/00006450-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Anadón A, Martínez MA, Ares I, et al. Acute and repeated dose (28 days) oral safety studies of an alkoxyglycerol extract from shark liver oil in rats. Journal of Agricultural and Food Chemistry. 2010;58(3):2040–2046. doi: 10.1021/jf903384c. [DOI] [PubMed] [Google Scholar]
- 25.Lewkowicz P, Banasik M, Głowacka E, Lewkowicz N, Tchórzewski H. Effect of high doses of shark liver oil supplementation on T cell polarization and peripheral blood polymorphonuclear cell function. Polski Merkuriusz Lekarski. 2005;18(108):686–692. [PubMed] [Google Scholar]
- 26.Ziegler R, Jobst W, Minne H, Faulhaber JD. Calciotropic hormones and lipolysis of human adipose tissue: role of extracellular calcium as conditioning but not regulating factor. Endokrinologie. 1980;75(1):77–88. [PubMed] [Google Scholar]
- 27.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. International Journal of Obesity. 2008;32(3):451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 28.Jager J, Grémeaux T, Cormont M, le Marchand-Brustel Y, Tanti J-F. Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148(1):241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor α inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(11):4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gäbele E, Dostert K, Dorn C, Patsenker E, Stickel F, Hellerbrand C. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcoholism. 2011;35(7):1361–1367. doi: 10.1111/j.1530-0277.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 31.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430(6996):257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 32.Akira S. Toll-like receptors and innate immunity. Advances in Immunology. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 33.Faure E, Thomas L, Xu H, Medvedev AE, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-γ induce toll-like receptor 2 and toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. Journal of Immunology. 2001;166(3):2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 34.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochemical and Biophysical Research Communications. 2006;346(3):739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 35.Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through Toll-like receptor 4-derived signaling pathways. The FASEB Journal. 2001;15(14):2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. Journal of Biological Chemistry. 2001;276(20):16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 37.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. Journal of Lipid Research. 2003;44(3):479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Lee SA, Kim HJ, Chang KC, et al. DHA and EPA down-regulate COX-2 expression through suppression of NF-κB activity in LPS-treated human umbilical vein endothelial cells. Korean Journal of Physiology and Pharmacology. 2009;13(4):301–307. doi: 10.4196/kjpp.2009.13.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ocaña A, Gómez-Asensio C, Arranz-Gutiérrez E, Torres C, Señoráns FJ, Reglero G. In vitro study of the effect of diesterified alkoxyglycerols with conjugated linoleic acid on adipocyte inflammatory mediators. Lipids in Health and Disease. 2010;9, article 36 doi: 10.1186/1476-511X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deniau A-L, Mosset P, Pédrono F, Mitre R, le Bot D, Legrand AB. Multiple beneficial health effects of natural alkylglycerols from shark liver oil. Marine Drugs. 2010;8(7):2175–2184. doi: 10.3390/md8072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang NW, Huang PC. Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids. 1998;33(5):481–487. doi: 10.1007/s11745-998-0231-9. [DOI] [PubMed] [Google Scholar]
- 42.Diniz YS, Cicogna AC, Padovani CR, Santana LS, Faine LA, Novelli ELB. Diets rich in saturated and polyunsaturated fatty acids: metabolic shifting and cardiac health. Nutrition. 2004;20(2):230–234. doi: 10.1016/j.nut.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Liao F-H, Liou T-H, Chiu W-C, Shieh M-J, Chien Y-W. Differential effects of high MUFA with high or low P/S ratio (polyunsaturated to saturated fatty acids) on improving hepatic lipolytic enzymes and mediating PPARγ related with lipoprotein lipase and hormone-sensitive lipase of white adipose tissue in diet-induced obese hamster. International Journal of Obesity. 2010;34(11):1608–1617. doi: 10.1038/ijo.2010.88. [DOI] [PubMed] [Google Scholar]
- 44.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. Journal of the American College of Nutrition. 2001;20(1):5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 45.Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. American Journal of Clinical Nutrition. 2001;73(6):1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 46.Pogozheva AV, Derbeneva SA, Anykina NV, et al. The clinical and experimental research of metabolic effects of shark liver oil. Voprosy Pitaniia. 2007;76(6):28–32. [PubMed] [Google Scholar]
- 47.McCrory C, Layte R. Breastfeeding and risk of overweight and obesity at nine-years of age. Social Science and Medicine. 2012;75(2):323–330. doi: 10.1016/j.socscimed.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 48.Mori T, Kondo H, Hase T, Tokimitsu I, Murase T. Dietary fish oil upregulates intestinal lipid metabolism and reduces body weight gain in C57BL/6J mice. Journal of Nutrition. 2007;137(12):2629–2634. doi: 10.1093/jn/137.12.2629. [DOI] [PubMed] [Google Scholar]
- 49.Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 1993;264(6, part 2):R1111–R1118. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Yeung WK, Huang Y, Chen Z-Y. Effect of squalene and shark liver oil on serum cholesterol level in hamsters. International Journal of Food Sciences and Nutrition. 2002;53(5):411–418. doi: 10.1080/0963748021000044750. [DOI] [PubMed] [Google Scholar]
- 51.Kilincalp S, Deveci M, Basar O, Ekiz F, Coban S, Yuksel O. Shark liver oil: hidden dangers. Annals of Hepatology. 11(5):728–730. [PubMed] [Google Scholar]


