Abstract
Using a panel of monoclonal antibodies and rabbit heteroantisera, we have studied the cell surface markers of peripheral blood (PB) Sezary cells from six patients with mycosis fungoides or Sezary syndrome, disease grouped within the spectrum of cutaneous T cell lymphomas (CTCL). Furthermore, we have studied two cell lines (Hut 78 and Hut 102) derived from malignant Sezary T cells from CTCL patients. The monoclonal antibody 3A1 defines a major human PB T cell subset (85% of PB T cells) while the antigen defined by the monoclonal antibody 4F2 is present on a subset (70%) of activated PB T cells and on circulating PB monocytes.
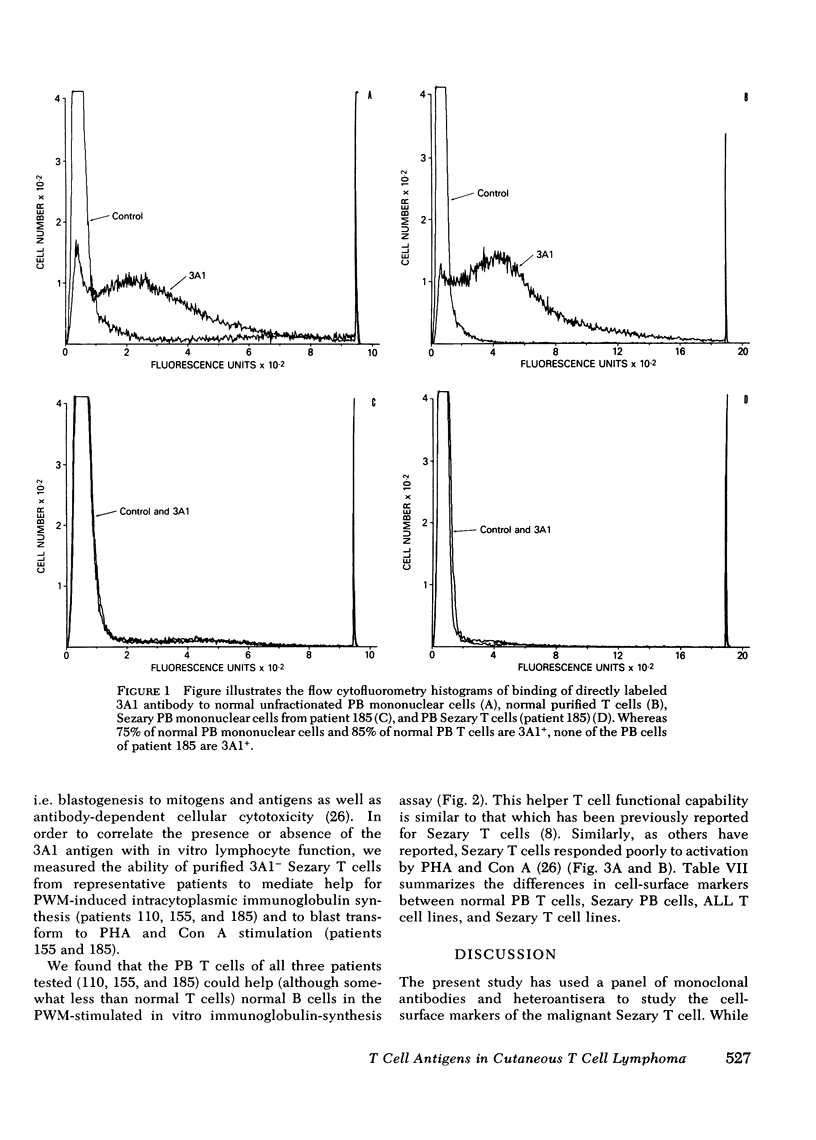
In contrast to normal subjects in whom 60-70% of circulating PB mononuclear cells were 3A1+ T cells, PB mononuclear cells from six CTCL patients studied had an average of only 10.6±3.2% 3A1+ T cells. Whereas 85% of E-rosette positive cells from normal individuals were 3A1+, virtually all E-rosette positive T cells from the Sezary patients were 3A1-. Two patients with high numbers of circulating Sezary T cells had both aneuploid and diploid PB T cell populations present; after separation of PB T cells into 3A1+ and 3A1- cell suspensions, all 3A1- cells were found to be aneuploid. In contrast to normal resting PB T cells which were 4F2-, all PB Sezary cells were 4F2+, suggesting a state of activation. The 3A1 antigen was on a variety of acute lymphoblastic leukemia T cell lines (HSB-2, RPMI-8402, MOLT4, CEM) but was absent on the Hut 78 and Hut 102 Sezary T cell lines.
Using rabbit anti-human T and anti-human Ia (p23, 30) antisera, we found that all malignant Sezary PB cells tested were killed by anti-T cell antiserum plus complement but not by anti-Ia plus complement. In contrast, Sezary cell lines Hut 78 and 102, were killed by both anti-T cell antiserum and anti-Ia plus complement.
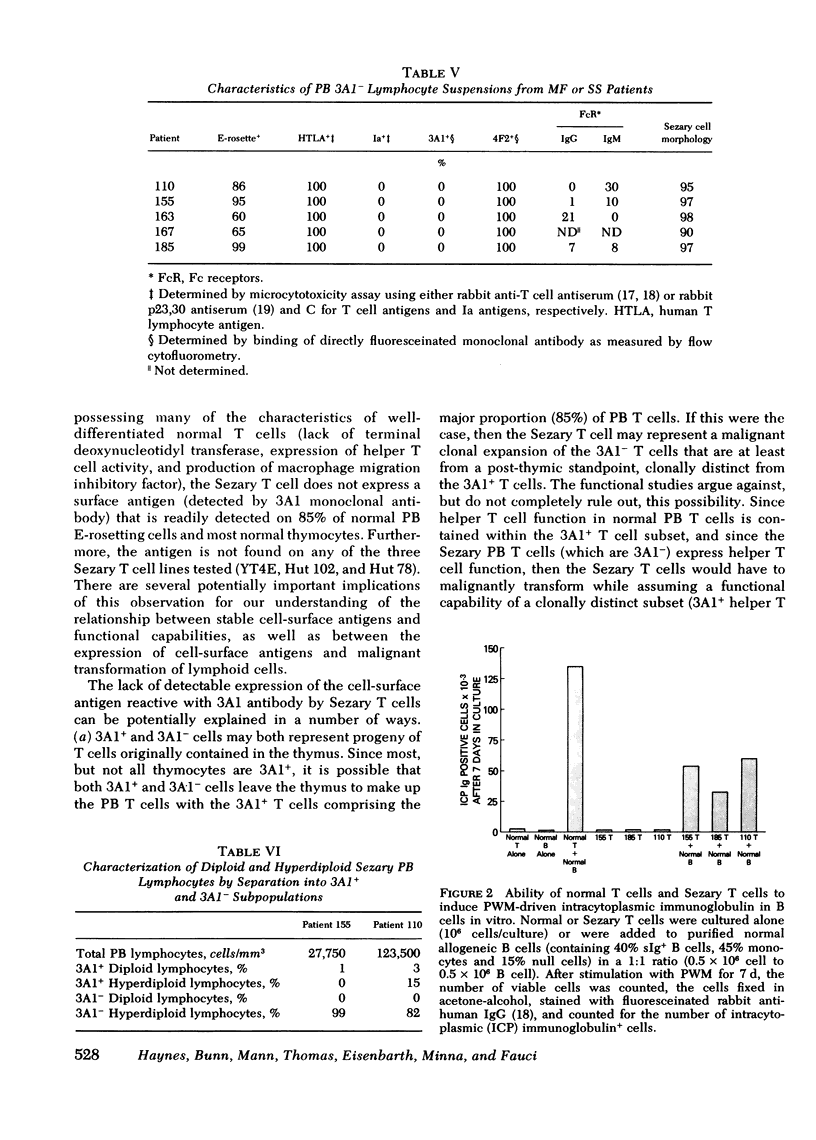
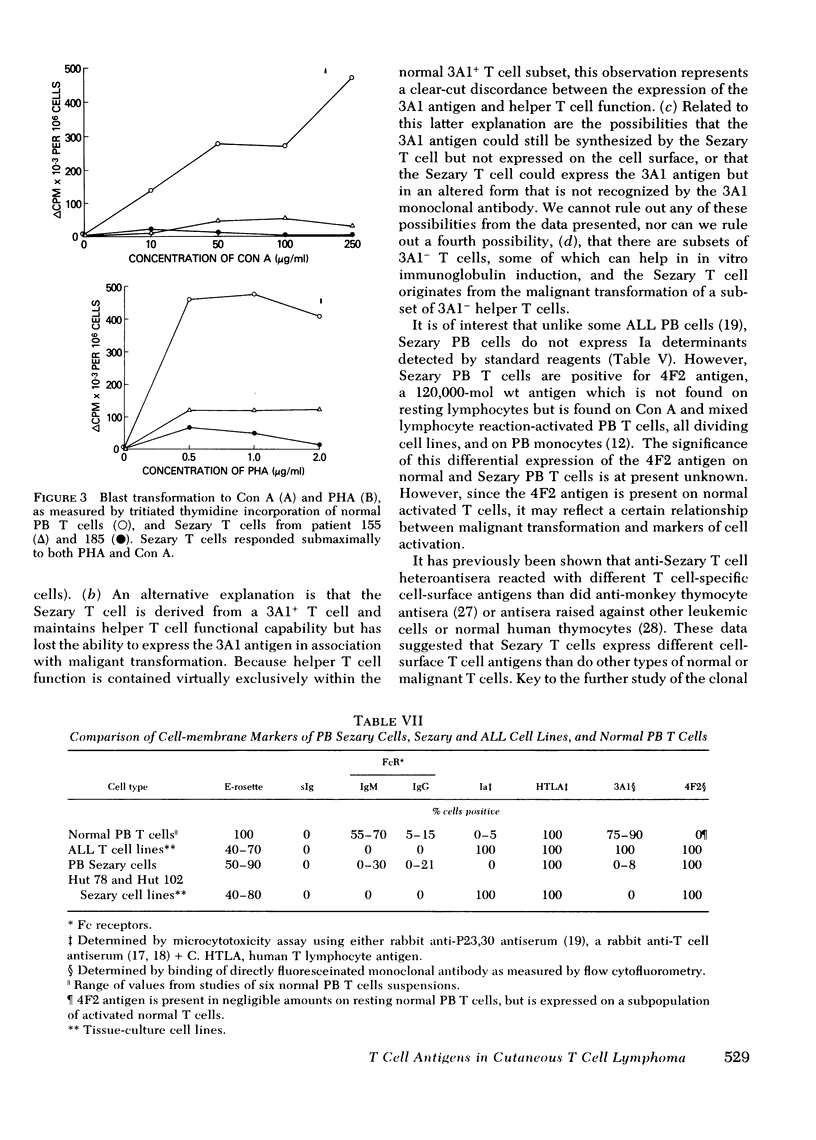
Similar to 3A1- normal PB T cells, 3A1- Sezary PB T cells proliferated poorly to phytohemagglutinin and concanavalin A. However, 3A1- Sezary T cells were able to provide T cell help towards pokeweed mitogen-induced in vitro B cell immunoglobulin synthesis, an immunoregulatory function limited to 3A1+ T cells in normal subjects.
Thus, the 3A1 antigen is present on 85% of normal PB T cells, and on most T-acute lymphoblastic leukemia lines tested; in contrast the 3A1 antigen is not present on the majority of circulating malignant Sezary PB T cells nor on T cell lines derived from malignant Sezary T cells. The lack of expression of the 3A1 antigen may be associated with malignant transformation of T cells in CTCL and may be an important marker for tracing the clonal origin of the malignant Sezary T cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. K., Metzgar R. S. Detection and partial characterization of human T and B lymphocyte membrane antigens with antisera to HSB and SB cell lines. J Immunol. 1978 Jan;120(1):262–271. [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Boumsell L., Bernard A., Coppin H., Richard Y., Penit C., Rouget P., Lemerle J., Dausset J. Human T cell differentiation antigens and correlation of their expression with various markers of T cell maturation. J Immunol. 1979 Nov;123(5):2063–2067. [PubMed] [Google Scholar]
- Broder S., Bunn P. A., Jr Cutaneous T-cell lymphomas. Semin Oncol. 1980 Sep;7(3):310–331. [PubMed] [Google Scholar]
- Broder S., Edelson R. L., Lutzner M. A., Nelson D. L., MacDermott R. P., Durm M. E., Goldman C. K., Meade B. D., Waldmann T. A. The Sézary syndrome: a malignant proliferation of helper T cells. J Clin Invest. 1976 Dec;58(6):1297–1306. doi: 10.1172/JCI108585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Indications of the thymus-derived nature of the proliferating cells in six patients with Sézary's syndrome. N Engl J Med. 1973 Aug 16;289(7):341–344. doi: 10.1056/NEJM197308162890703. [DOI] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Lamberg S. I. Report of the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas. Cancer Treat Rep. 1979 Apr;63(4):725–728. [PubMed] [Google Scholar]
- Bunn P. A., Jr, Whang-Peng J., Carney D. N., Schlam M. L., Knutsen T., Gazdar A. F. DNA content analysis by flow cytometry and cytogenetic analysis in mycosis fungoides and Sézary syndrome. J Clin Invest. 1980 Jun;65(6):1440–1448. doi: 10.1172/JCI109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H. T., Sundharadas G. Production of chemotactic activity in mixed leukocyte cultures: maximum effect caused by H-2I region disparity. J Immunol. 1979 Nov;123(5):2189–2193. [PubMed] [Google Scholar]
- Devita V. T., Jr, Serpick A. A., Carbone P. P. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970 Dec;73(6):881–895. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- Donlon J. A., Jaffe E. S., Braylan R. C. Terminal deoxynucleotidyl transferase activity in malignant lymphomas. N Engl J Med. 1977 Sep 1;297(9):461–464. doi: 10.1056/NEJM197709012970901. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Haynes B. F., Schroer J. A., Fauci A. S. Production of monoclonal antibodies reacting with peripheral blood mononuclear cell surface differentiation antigens. J Immunol. 1980 Mar;124(3):1237–1244. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. X. Heterogeneity of concanavalin A-generated suppressor cells of the pokeweed mitogen-induced plaque-forming cell response of human peripheral blood lymphocytes. J Immunol. 1978 Aug;121(2):559–565. [PubMed] [Google Scholar]
- Haynes B. F., Mann D. L., Hemler M. E., Schroer J. A., Shelhamer J. H., Eisenbarth G. S., Strominger J. L., Thomas C. A., Mostowski H. S., Fauci A. S. Characterization of a monoclonal antibody that defines an immunoregulatory T cell subset for immunoglobulin synthesis in humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2914–2918. doi: 10.1073/pnas.77.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzner M., Edelson R., Schein P., Green I., Kirkpatrick C., Ahmed A. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975 Oct;83(4):534–552. doi: 10.7326/0003-4819-83-4-534. [DOI] [PubMed] [Google Scholar]
- Mann R. B., Jaffe E. S., Berard C. W. Malignant lymphomas--a conceptual understanding of morphologic diversity. A review. Am J Pathol. 1979 Jan;94(1):105–192. [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Blaese R. M., Altman L. C. Lack of effector cell function in chronic lymphocytic leukemia, a B-cell malignancy, and the Sézary syndrome, a T-cell malignancy. Clin Immunol Immunopathol. 1978 Jul;10(3):306–314. doi: 10.1016/0090-1229(78)90186-1. [DOI] [PubMed] [Google Scholar]
- Niaudet P., Greaves M. Diversity of cell surface polypeptides that are recognized by heteroantisera to human T cells. J Immunol. 1980 Mar;124(3):1203–1208. [PubMed] [Google Scholar]
- Schechter G. P., Bunn P. A., Fischmann A. B., Matthews M. J., Guccion J., Soehnlen F., Munson D., Minna J. D. Blood and lymph node T lymphocytes in cutaneous T cell lymphoma: evaluation by light microscopy. Cancer Treat Rep. 1979 Apr;63(4):571–574. [PubMed] [Google Scholar]
- Schlossman S. F., Chess L., Humphreys R. E., Strominger J. L. Distribution of Ia-like molecules on the surface of normal and leukemic human cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1288–1292. doi: 10.1073/pnas.73.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERASAKI P. I., MCCLELLAND J. D. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964 Dec 5;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Yoshida T., Edelson R., Cohen S., Green I. Migration inhibitory activity in serum and cell supernatants in patients with Sezary syndrome. J Immunol. 1975 Mar;114(3):915–918. [PubMed] [Google Scholar]


