Abstract
BACKGROUND
Loss-of-function mutations in the gene KCNQ1 encoding the Kv7.1 K+ channel cause long QT syndrome type 1 (LQT1), whereas gain-of-function mutations are associated with short QT syndrome as well as familial atrial fibrillation (FAF). However, KCNQ1 mutation pleiotropy, which is capable of expressing both LQT1 and FAF, has not been demonstrated for a discrete KCNQ1 mutation. The genotype–phenotype relationship for a family with FAF suggests a possible association with the LQT1 p.Arg231Cys-KCNQ1 (R231C-Q1) mutation.
OBJECTIVE
The purpose of this study was to determine whether R231C-Q1 also can be linked to FAF.
METHODS
The R231C-Q1 proband with AF underwent genetic testing for possible mutations in 10 other AF-linked genes plus KCNH2 and SCN5A. Sixteen members from five other R231C-positive LQT1 families were genetically tested for 21 single nucleotide polymorphisms (SNPs) to determine if the FAF family had discriminatory SNPs associated with AF. R231C-Q1 was expressed with KCNE1 (E1) in HEK293 cells, and Q1E1 currents (IQ1E1) were analyzed using the whole-cell patch-clamp technique.
RESULTS
Genetic analyses revealed no additional mutations or discriminatory SNPs. Cells expressing WT-Q1 and R231C-Q1 exhibited some constitutively active IQ1E1 and smaller maximal IQ1E1 compared to cells expressing WT-Q1.
CONCLUSION
Constitutively active IQ1E1 and a smaller peak IQ1E1 are common features of FAF-associated and LQT1-associated mutations, respectively. These data suggest that the mixed functional properties of R231C-Q1 may predispose some families to LQT1 or FAF. We conclude that R231C is a pleiotropic missense mutation capable of LQT1 expression, AF expression, or both.
Keywords: Atrial fibrillation, Familial atrial fibrillation, Genetics, Ion channel, KCNQ1, Long QT syndrome, Long QT syndrome type 1
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 10% of the population by the age of 75 years.1 The disease can present as lone AF; however, AF usually is associated with underlying heart disease, hypertension, or hyperthyroidism. Approximately 30% of AF patients have parents with a history of AF, suggesting a genetic predis-position. 2
Loss-of-function mutations in KCNQ1 cause long QT syndrome type 1 (LQT1, MIM#192500), whereas gain-of-function mutations have been implicated in short QT syndrome (SQTS, MIM#609621) and familial atrial fibrillation (FAF, MIM#607554) (for review, see references 3–7). KCNQ1 encodes the voltage-gated K+ channel α-subunit 7.1 (Kv7.1). In the heart, KCNQ1 channels (Q1) co-assemble with KCNE1 β-subunits (E1), forming a channel that generates the slowly activating delayed rectifier K+ current IKs.8,9 Heterologous expression of Q1 and E1 generate currents (IQ1E1) that recapitulate native-like IKs.
Several groups have implicated the missense mutation annotated as p.Arg231Cys (R231C-Q1) in LQT1, druginduced long QT syndrome (LQTS) with AF, fetal bradycardia, and persistent AF where QTc prolongation was noted after cardioversion.10–15 We now report the genotype– phenotype relationships for six R231C-positive families, five of which have LQTS and one family containing four R231C-positive individuals affected by lone AF before age 50 years. Functional analyses suggest that co-expression of wild-type Q1 (WT-Q1) and R231C-Q1 exhibited a mixed functional phenotype that has both loss-of-function (LQT1) and gain-of-function (FAF) properties. This is the first study to demonstrate pleiotropy in KCNQ1 whereby a discrete missense mutation is capable of both LQT1 and FAF expressivity using genetic and functional analyses.
Methods
Study population and diagnostic criterion
Patients enrolled were probands referred for genetically linked arrhythmias or fetal bradycardia. Blood samples were obtained from family members who agreed to genetic evaluation and provided written consent. Patients underwent clinical evaluation and cardiovascular examination, including 12-lead ECG and 24-hour Holter recording. Lone AF was diagnosed in patients with AF and normal thyroid function, no hypertension, or structural disease as determined by echocardiography. Prolonged QTc was diagnosed based on prolongation of ventricular repolarization at rest in lead II corrected QT interval (QTc) >440 ms for males and >460 ms for females using the Bazett formula (QTc for R231C-Q1 subjects are given in Online Supplemental Table 1). The R231C subjects with fetal bradycardia were previously reported.11
Genetic analysis
Genomic DNA was extracted from peripheral blood leukocytes using standard methods. Mutational analyses of KCNQ1, KCNH2, KCNE1, and SCN5A genes were performed in probands. The coding regions were amplified using polymerase chain reaction and sequenced with a 3100 BigDye Terminator v3.1 sequencing kit and ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). We sequenced other AF-linked genes [KCNE2 (NM172201), KCNE3 (NM005472), KCNE5 (NM012282), NPPA (NM006172), KCNA5 (NM002234), KCNJ2 (NM00891), GJA5 (NM005266 and 181703), SCN1B (NM001037 and 199037), SCN2B (NM004588)] and SCN3B (NM018400) in the R231C-Q1 proband from the family with AF (Figure 1, Family A, subject II:1) (for review, see reference 16–20). We also compared 21 single nucleotide polymorphisms (SNPs) (including KCNE5) that alter AF susceptibility (rs1805123, rs1805120, rs1805127, rs1805124, rs2200733, rs10033464, rs35594137, rs12621643, rs1800872, rs583362, rs7193343, rs3807989, rs11708996, rs6800541, rs251253, rs5063, rs13376333, rs11047543, rs3825214, rs1801252, and rs17003955) among the families shown in Figure 1 (Online Supplemental Table 2).
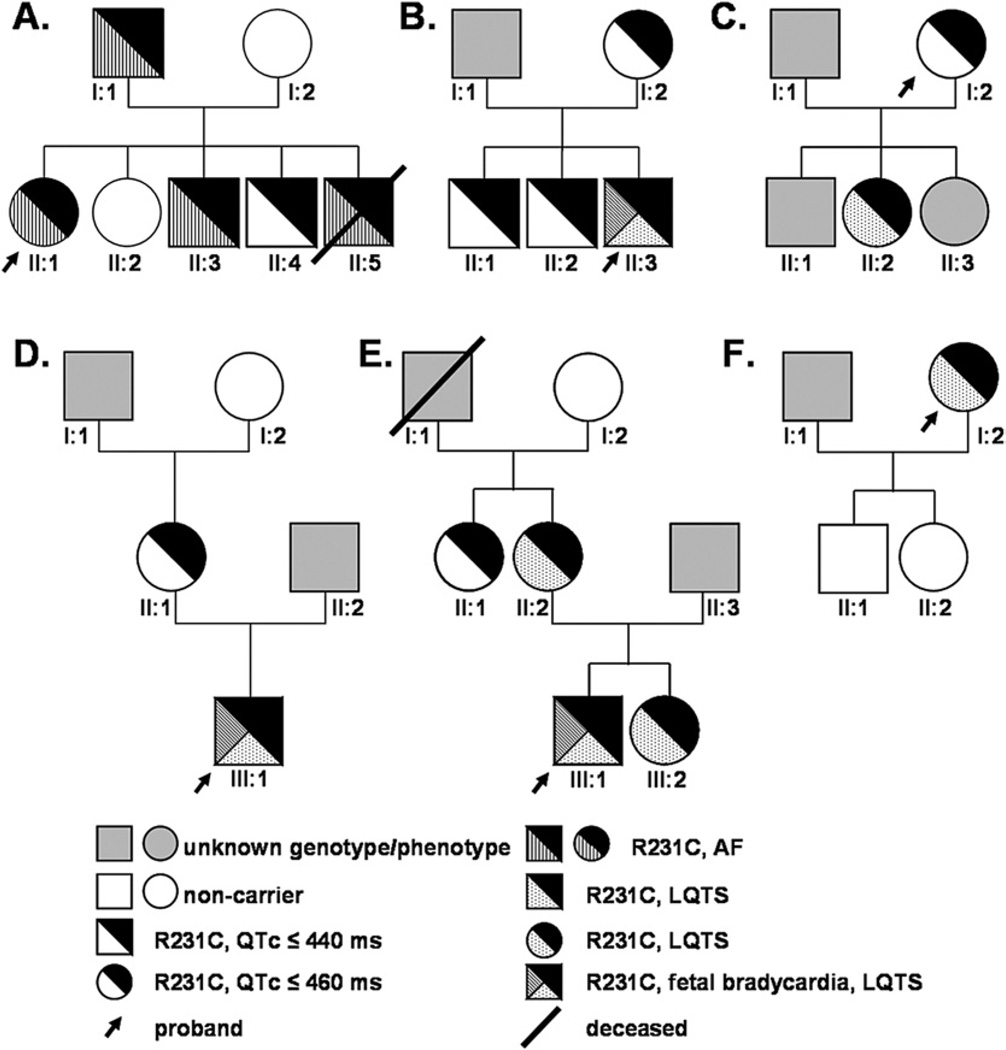
Figure 1.
Pedigrees of the six families carrying R231C-Q1. Males and females are represented as squares and circles, respectively. The different generations are denoted using roman numerals, and each individual in a generation is numbered. The genotype/phenotypes are defined in the key.
Tissue culture and electrophysiology
Mutagenesis and transfection are described in the Online Supplemental Methods. Human embryonic kidney 293 (HEK293) cells transfected with WT-Q1 (3 µg) plasmid DNA expressed small currents that did not resemble IKs. Cells expressing R231C-Q1 (3 µg) plasmid DNA also expressed small currents that appeared constitutively active (Online Supplemental Figure 1). To recapitulate IKs-like currents, we transfected cells with equal amounts of E1 (3 µg) and Q1 (3 µg) plasmid DNA (total concentration 6 µg). Because of the differences in size of E1 and Q1 cDNA, the final ratio of E1 to Q1 cDNA ratio is ~2:1. For co-expression studies with WT-Q1 and mutant Q1 cDNA, equal amounts of WT-Q1 (1.5 µg) and mutant Q1 (1.5 µg) were used to maintain the equivalent Q1 plasmid concentrations (3 µg) (see Online Supplemental Methods for electrophysiologic techniques). pCLAMP 10 software (Axon Instruments, Union City, CA, USA) was used to generate the voltage protocols, acquire current signals, and data analyses. Origin (7.0, Microcal, Northhampton, MA, USA) was used for performing Boltzmann curve fitting to the current–voltage (I–V) relations and for generating graphs. Data were fit using the following Boltzmann equation:
where IMIN = minimally activated current, IMAX = maximally activated current, V1/2 = midpoint potential for half-maximal activation, and k = slope factor. For most experiments, the holding potential was −80 mV, and the dashed line in figures is the zero current. The physiologic temperature and action potential (AP) clamp measurements were done similar to that previously described.21
Computational modeling
Computational models of human atrial and ventricular action potentials were used and are described in detail in the Online Supplemental Methods.20,22
Statistical analysis
Analysis of variance was performed on datasets. P <.05 was considered significant. We subsequently performed t-tests to identify which experimental group(s) differed from one another.
Results
R231C-Q1 is linked to LQTS, fetal bradycardia, and AF
We identified R231C-Q1 in six probands with various clinical phenotypes (Figure 1), including one with AF (Family A), three neonates with fetal bradycardia (Families B, D, E),11 one with prolonged QTc (Family F), and one who is a member of a large LQTS family previously linked to chromosome 11p15 (Family C).23 Although the Family C proband (I:2) is asymptomatic, several other family members are symptomatic for LQTS (not shown), including the baby from her niece who was diagnosed with fetal LQTS and bradycardia resulting in intrauterine death at full term.23
In Family A, AF was diagnosed in the female proband (II:1) at age 33 years, in her father (I:1) at age 18 years, and in two of her brothers (II:3, II:5) at age 30 years and 15 years, respectively. The proband was hospitalized when she was 33 years old for persistent AF that occurred during stress. After an electrical cardioversion, she has been treated by sotalol without clinical recurrences for 3 years (Online Supplemental Figure 2). Paroxysmal AF then occurred, and sotalol treatment was replaced by flecainide without clinical recurrences of AF. In contrast to the other R231C-positive LQTS families, patients of Family A do not manifest either QTc prolongation or syncope except for patient II:5, who had ventricular fibrillation 4 months after interruption of sotalol treatment and died of severe cerebral anoxic damage several days later at age 22 years. In addition, one of the R231C-positive brothers (II:4) thus far is asymptomatic for AF at age 37 years. None of the R231C- and AF-positive subjects in Family A have structural heart disease, hypertension, or hyperthyroidism consistent with a clinical diagnosis of lone AF. Furthermore, genetic testing of the proband (II:1) did not reveal any additional mutations in other AF susceptibility genes.
The members of Families B through E do not present with AF, including the proband of Family F, now age 57 years, who currently is receiving beta-blocker treatment and underwent defibrillator implantation 3 years ago following a severe LQTS-triggered cardiac event. Suspecting the possible contribution of additional genetic factors to AF development, we genotyped all the family members for 21 different SNPs that confer some AF susceptibility (Online Supplemental Table 2). We did not find any obvious differences between Family A and Families B–F that might account for their different clinical phenotypes, except for possibly rs251253, an intergenic SNP located in the region of NKX2-5 gene encoding a cardiac-specific homeobox transcription factor. Indeed, in Family A, only the son with the R231C-Q1 mutation who did not have AF carries the protective allele. All of the mutation carriers from the five additional R231C-positive LQTS families have the protective allele, with the exception of the patient II:2 from Family C (a woman who does not manifest AF at age 33 years). We conclude that R231C-Q1 is linked to LQTS, fetal bradycardia, and lone AF.
R231C-Q1 results in large constitutively activated IQ1E1
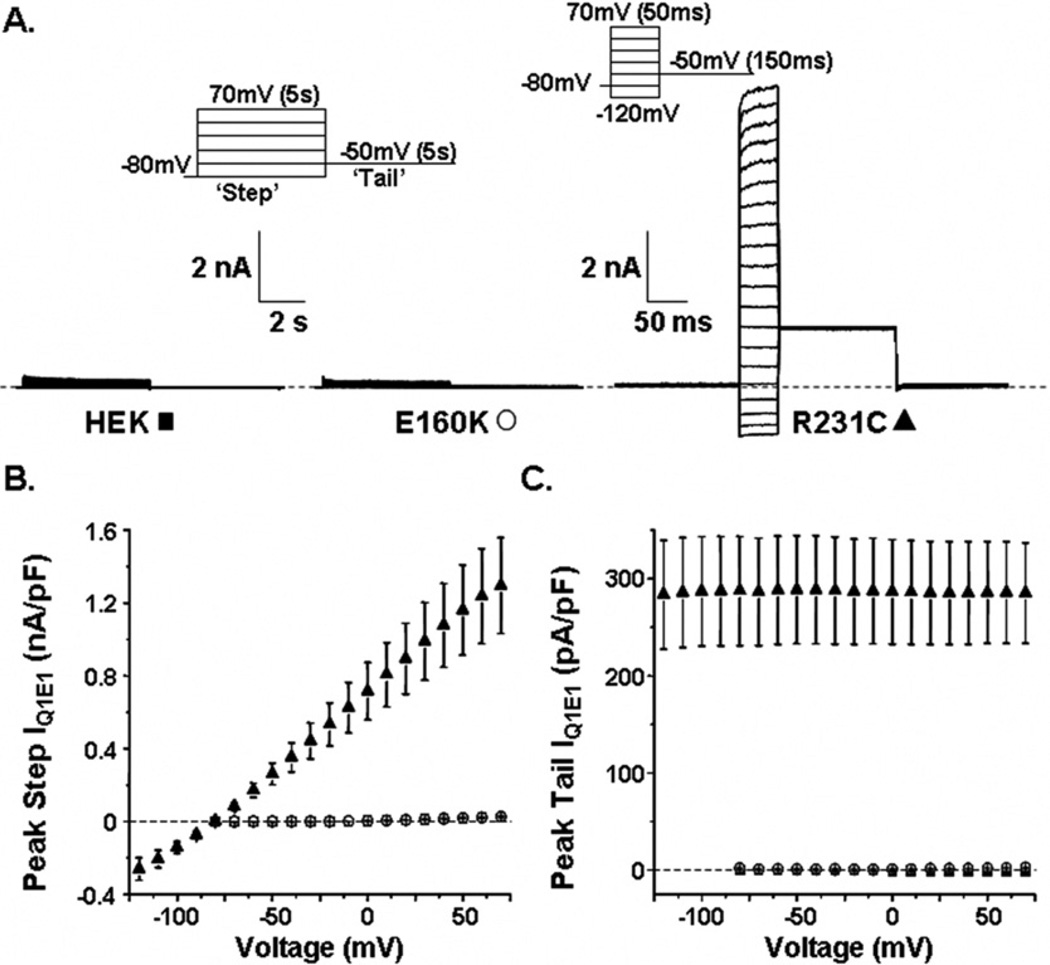
We tested the hypothesis that R231C-Q1 may generate a unique functional phenotype compared to other LQT1 mutations that typically result in a loss of function. We chose to study the LQT1 mutation E160K-Q1 as a “positive control” because studies suggest that, like most LQT1-linked mutations, E160K-Q1 causes a loss of IQ1E1.24 Figure 2A shows representative families of whole-cell current traces recorded from nontransfected HEK293 cells and from cells expressing E1 and E160K-Q1 or R231C-Q1. The currents from nontransfected cells and from cells transfected with E160K-Q1 were measured by applying “step” pulses from −80 to 70 mV in 10-mV increments for 5 seconds, followed by a “tail” pulse to −50 mV for 5 seconds. At positive step pulses, nontransfected cells expressed a small endogenous outward current that was absent during the tail pulse. As expected, cells expressing E160K-Q1 did not generate any IQ1E1. We attempted to use the same voltage protocol to measure IQ1E1 from cells expressing R231C-Q1, but R231C-Q1 was constitutively active and generated very large IQ1E1 that could not be measured reliably because of apparent changes in local [K+] (data not shown). We minimized these effects by shortening the duration of the step and tail pulses to 50 ms and 150 ms, respectively. The large constitutively active IQ1E1 measured from cells expressing R231C-Q1 was clarified by applying step pulses from −120 to 70 mV in 10-mV increments, followed by a tail pulse to −50 mV (Figure 2A). Figures 2B and 2C show the mean peak step and tail I–V relations, respectively, for nontransfected cells (n = 10), cells expressing E160K-Q1 (n = 8), and cells expressing R231C-Q1 (n = 20). Although E160K-Q1 and R231C-Q1 both are linked to LQT1, their effects on IQ1E1 are dramatically different from one another.
Figure 2.
A: Representative families of whole-cell currents recorded from nontransfected cells (HEK, ■), cells transfected with E1 and E160K-Q1 DNA (E160K, ○), or cells transfected with E1 and R231C-Q1 DNA (R231C, ▲). The corresponding voltage protocols used to measure the currents are also shown. Mean peak step (B) and tail currents (C) are plotted as a function of step voltage for nontransfected cells, cells expressing E160K-Q1, and cells expressing R231C-Q1.
Co-expression of WT-Q1 and R231C-Q1 yields a mixed functional phenotype
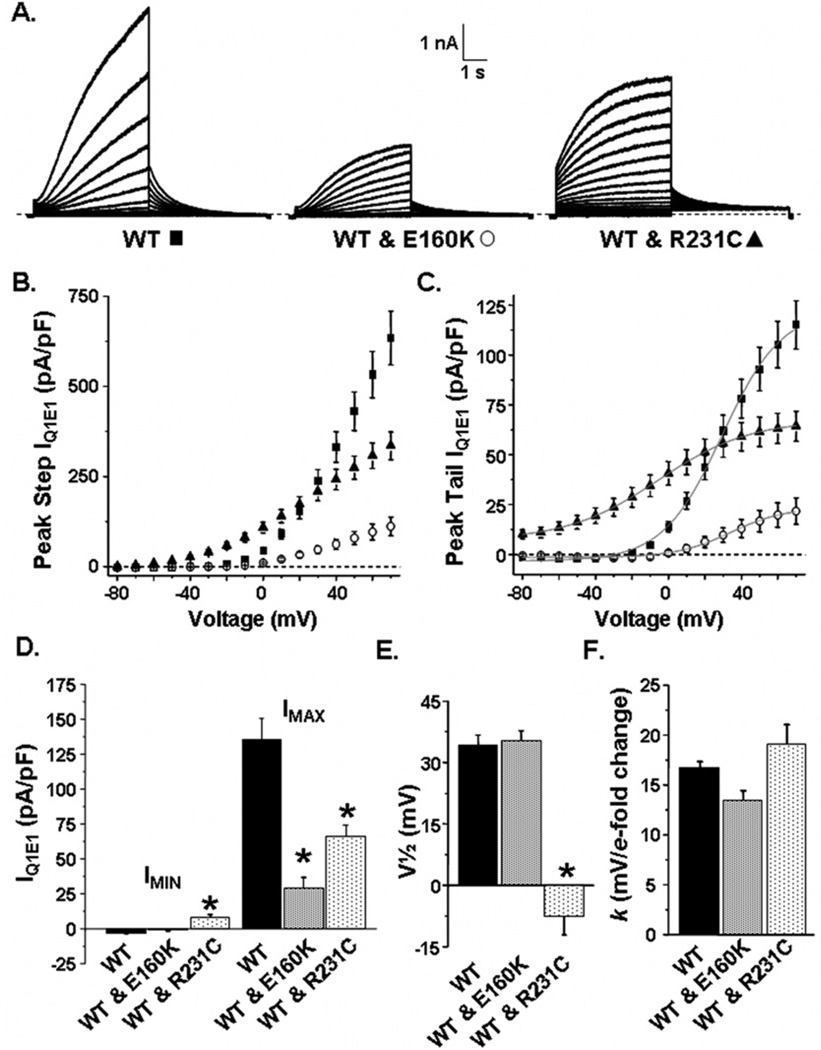
LQT1 and FAF follow a dominant inheritance pattern; therefore, we determined the effect of co-expressing WT-Q1 and E160K-Q1 or R231C-Q1. Figure 3A shows representative families of whole-cell currents measured from cells expressing E1 with WT-Q1, WT-Q1 and E160KQ1, or WT-Q1 and R231C-Q1. The currents were measured using the 5-second voltage protocol described in Figure 2. Cells expressing WT-Q1 showed IQ1E1 that resembled native IKs. Cells expressing WT-Q1 and E160K-Q1 showed IQ1E1 with similar characteristics as IKs, but the amplitudes were smaller than cells expressing WT-Q1. Cells expressing WT-Q1 and R231C-Q1 showed IQ1E1 that had a mixed functional phenotype, some constitutively active IQ1E1 and some voltage-dependent IQ1E1. Figures 3B and 3C show the mean peak I–V relations measured during the step and tail pulse, respectively, for cells expressing WT-Q1 (n = 31), WT-Q1 and E160K-Q1 (n = 13), or WT-Q1 and R231C-Q1 (n = 22). Mean peak tail IQ1E1 data were described using the Boltzmann function to calculate IMIN, IMAX, V1/2, and k for IQ1E1 activation. Figure 3D shows that cells expressing WT-Q1 and R231C-Q1 had the largest mean IMIN (which likely reflected their constitutively active component of IQ1E1), but cells expressing WT-Q1 had the largest mean IMAX (Figure 3D). Cells expressing WT-Q1 and R231C-Q1 had a more negative V1/2 (Figure 3E), but k was not different (Figure 3F).
Figure 3.
A: Representative families of currents measured from cells transfected with E1 and WT-Q1 (WT, ■), WT-Q1 and E160K-Q1 (WT & E160K, ○), or WT-Q1 and R231C-Q1 DNA (WT & R231C, ▲). Mean peak step (B) and tail currents (C) are plotted as a function of step pulse for cells expressing WT-Q1, WT-Q1 and E160K-Q1, and WT-Q1 and R231C-Q1. Mean peak tail currents were measured immediately after the step pulse and were described using the Boltzmann equation (gray line). The graphs show the calculated mean IMIN and IMAX (D), V1/2 (E), and slope constant, k (mv/e-fold change (F) for cells expressing WT-Q1, WT-Q1 and E160K-Q1, and WT-Q1 and R231C-Q1. *P <.05 vs cells expressing WT-Q1.
The data demonstrate that, compared to cells expressing WT-Q1 or R231C-Q1 (Figure 2), cells expressing WT-Q1 and R231C-Q1 had the smallest peak IQ1E1. This may be related to alterations in the cell surface expression; however, when performed, biotinylation assays did not reveal significant differences in the relative amount of Q1 at the cell surface (see Online Supplemental Figure 3).
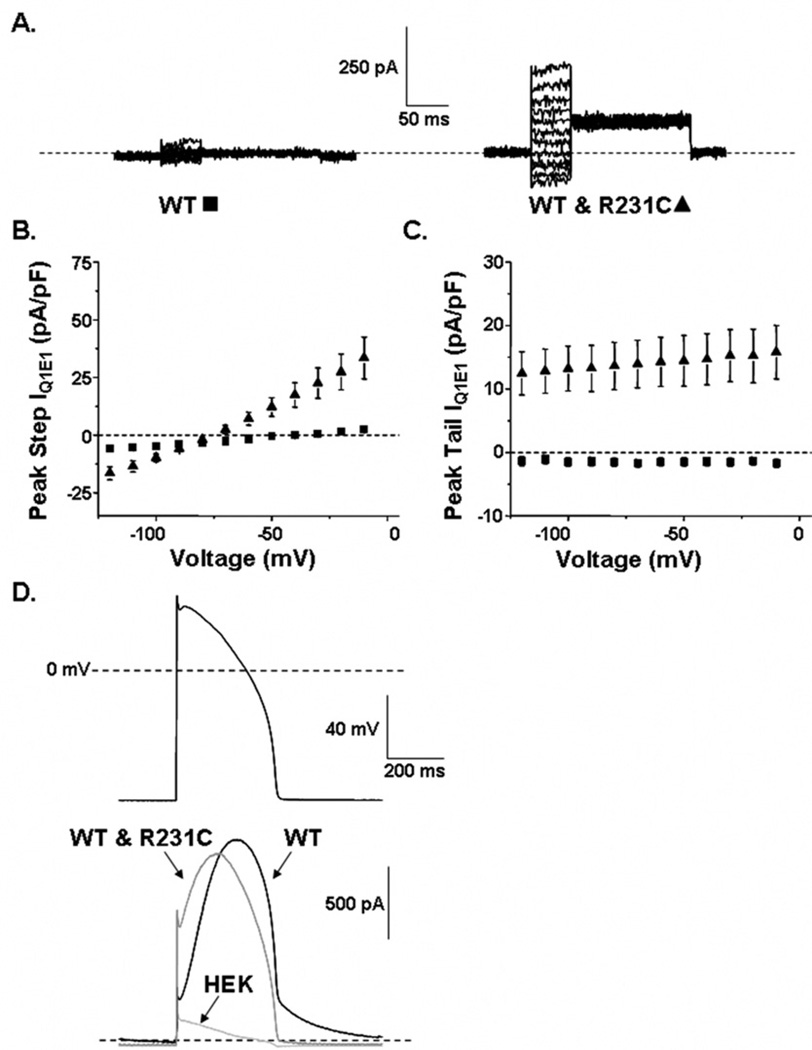
To measure the constitutively active component of IQ1E1 from cells expressing WT-Q1 and R231C-Q1, we used a voltage protocol similar to that used to measure IQ1E1 from cells expressing R231C-Q1 (Figure 2). Figure 4A shows representative families of IQ1E1 from cells expressing E1 and WT-Q1 or WT-Q1 and R231C-Q1. IQ1E1 was measured by applying 50-ms step pulses from −120 to −10 mV in 10-mV increments, followed by a 150-ms tail pulse to −50 mV. Figures 4B and 4C show the mean peak step and tail I–V relations, respectively, for cells expressing WT-Q1 (n = 11) or cells expressing WT-Q1 and R231C-Q1 (n = 13). Cells expressing WT-Q1 showed almost no IQ1E1, whereas cells expressing WT-Q1 and R231C-Q1 showed constitutively active IQ1E1.
Figure 4.
A: Representative families of whole-cell currents recorded from cells transfected with E1 and WT-Q1 DNA (WT, ■) or WT-Q1 and R231C-Q1 DNA (WT & R231C, ▲). The currents were measured by applying step pulses from −120 to −10 mV for 50 ms, followed by a tail pulse to −50 mV for 150 ms. The corresponding mean peak step (B) and tail currents (C) are plotted as a function of step voltage. D: Ventricular action potential waveform and corresponding averaged current traces measured at 37°C from nontransfected (HEK) cells and cells transfected with E1 and WT-Q1 DNA or WT-Q1 and R231C-Q1 DNA.
Thus far, the data suggest that R231C-Q1 more closely resembles an FAF mutation and not an LQT1 mutation.5,7 To understand how R231C-Q1 may contribute to an LQT1 phenotype, we measured currents from nontransfected cells, and cells expressing E1 and WT-Q1 or WT-Q1 and R231C-Q1 at 37°C using a ventricular AP waveform pulsed at 1 Hz. Figure 4D shows the AP potential waveform and representative averaged currents for 1 minute. The small current measured from nontransfected cells is a combination of endogenous HEK current and uncompensated leak and capacitative currents. Cells expressing WT-Q1 or WT-Q1 and R231C-Q1 showed large currents that both peaked during the plateau phase. Compared to cells expressing WT-Q1, cells expressing WT-Q1 and R231C-Q1 did not alter the mean peak current measured during the action potential waveform (WT-Q1 = 371 ± 162 pA/pF, n = 6; and WT-Q1 and R231C-Q1= 335 ± 171 pA/pF, n = 5, respectively), but cells expressing WT-Q1 and R231C-Q1 reduced the fraction of current that remained after repolarization to diastolic potentials (WT-Q1 = 0.14 ± 0.03, and WT-Q1 and R231C-Q1 = 0 ± 0.03). The latter effect is consistent with an LQT1 phenotype.
Discussion
This is the first study to link a known LQT1 mutation (R231C) to FAF and to show that an LQT1 mutation can generate a functional phenotype similar to the other KCNQ1-mediated FAF mutations.5,7 We sequenced most of the known AF-associated genes in the R231C-Q1 AF proband but did not identify any additional mutations. Twentyfour subjects from six R231C-Q1 families were genotyped for 21 SNPs described as being at risk or protective for AF. These analyses (see Online Supplemental Table 2) may have identified a specific allele, NKX2-5, that discriminates AF from LQTS patients. The cardiac homeobox Nkx2-5 protein is essential in cardiac development, and mutations in NKX2-5 cause various congenital heart malformations. Interestingly, Nkx2.5 regulates the transcription of NPPA, a gene in which mutations cause AF.20
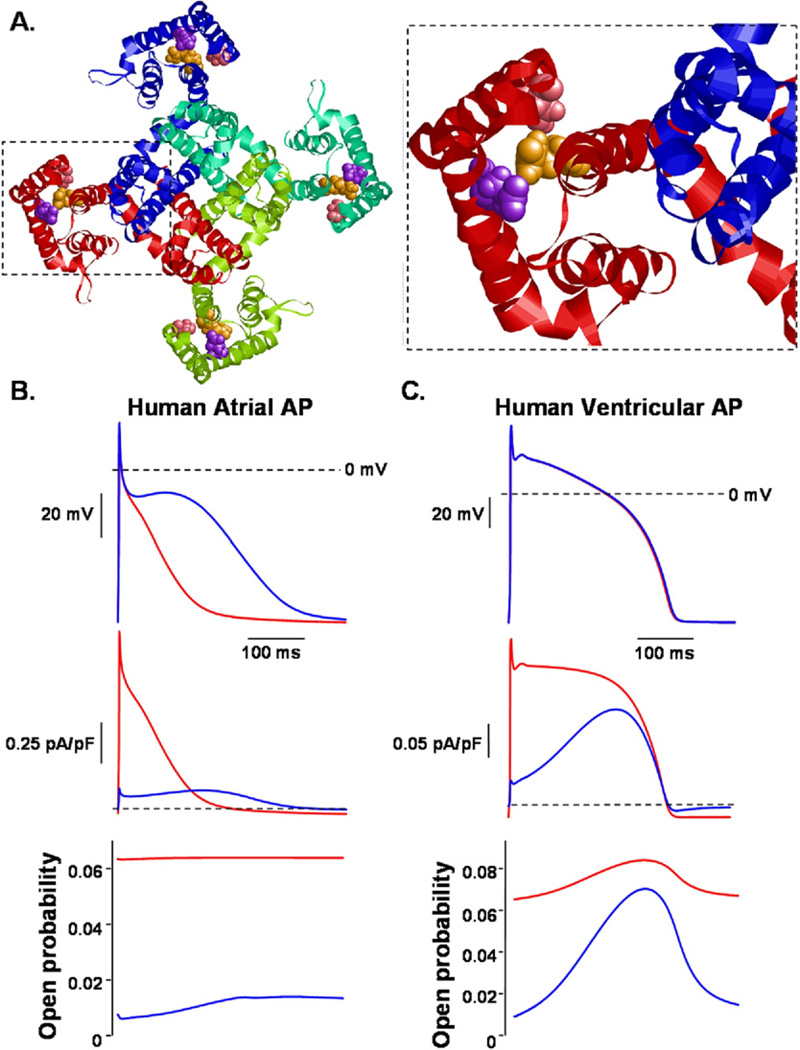
R231C-Q1 generated constitutively active IQ1E1 similar to the FAF-associated KCNQ1 mutations S140G and V141M.5,7 Restier et al25 suggest that these mutations alter charge–pair interactions between E160, R231, and R237 to cause ultraslow deactivation and generate the constitutively active IQ1E1 phenotype. A proposed structural model for a closed Q1 channel is shown in Figure 5A,26 and this model suggests that the side chains of S140 and E160 are within van der Waals contact of R231 in the closed state. Perhaps R231C-Q1 mimics the effects that S140G-Q1 or V141M-Q1 have on charge–pairing interactions to generate IQ1E1 with a constitutively active phenotype. This structural model implies that this Q1 domain is a “hotspot” for mutations associated with multiple types of arrhythmia syndromes, including LQTS, fetal sinus bradycardia, and AF.
Figure 5.
A, left: Structural model of Q1 in the closed state from the “extracellular” side looking into the pore. The backbone of each individual Q1 α-subunit is shown as a different colored ribbon. The space filled atoms for the S140 (pink), E160 (purple), and R231 (orange) residues are shown in each α-subunit. Right: Red α-subunit magnified. The side chains of S140 and E160 are predicted to be close enough to form van der Waals contacts with R231 in the closed state. B: Computational model of a human atrial action potential (AP) waveform pulsed at 1 Hz with the corresponding IKs and channel open probability. Blue traces represent control conditions. Red traces show a modification of the model that included a reduction in the number of functional channels by ~50% and a small constitutively active IKs. C: Computational model of a human ventricular AP pulsed at 1 Hz with the corresponding IKs and channel open probability. Blue traces represent control conditions. Red traces show a modification of the model that included the same reduction in functional channels and constitutively active IKs as shown in panel B.
Prior to this study, two unrelated R231C-Q1 carriers were described with AF, one was a 72-year-old woman with an increased susceptibility to drug-induced LQTS, and the other was an unrelated 58-year-old woman who showed a prolonged QT interval after cardioversion.14,15 Although it is tempting to speculate that R231C-Q1 underlies their AF phenotype, subsequent genetic analyses and the genotype– phenotype relationships of their families were not reported.
Consistent with an LQT1 phenotype, cells expressing WT-Q1 and R231C-Q1 reduced IQ1E1 during the repolarization phase of the ventricular action potential waveform (Figure 4D). Itoh et al15 showed that cells expressing WT-Q1 and R231C-Q1 reduce maximal IQ1E1 by levels similar to that reported here (~50%, Figure 3D), and that reducing IKs by ~50% prolonged the duration in a Luo-Rudy model of a ventricular AP. Interestingly, experimental evidence from undiseased human ventricular myocytes suggest that basal IKs is small, and pharmacologic block of IKs by chromanol 293B does not alter human ventricular AP duration.27,28 The existence of LQT1 suggests that IKs can contribute to human ventricular repolarization under certain physiologic conditions, for example, with sympathetic tone, and the Luo-Rudy model may be helpful for modeling such conditions.
Grandi et al22 recently developed a novel model of the human ventricular AP (the Grandi-Pasqualini-Bers [GPB] model). Similar to the experimental evidence, the model predicts only 1% to 3% prolongation with complete block of IKs in cells pulsed over a broad range of pacing cycle lengths. For didactic purposes, we modeled the mixed functional phenotype using a human atrial AP model and the GPB model (Figures 5B and 5C).20,22 Both models were adjusted to reduce the number of functional IKs channels by ~50% and contain a constitutively active IKs component at 6.25% (the predicted fraction of homomeric mutant channels assuming random co-assembly). At a 1-Hz pacing rate, the modified IKs reduced the atrial AP duration by >50% and the ventricular AP duration by <1%. Modeling the constitutively active IKs component alone, without reducing the fraction of functional channels, had a similar effect: it shortened the atrial AP by >50% and the ventricular AP by <2% (Online Supplemental Figure 4). The constitutively active component selectively alters the atrial AP duration because the atrial AP is less positive and the atrial myocytes have a larger IKs density.29 The modeling suggests that a small increase in the open probability of IKs channels will result in much larger IKs in atrial myocytes. This may explain why some AF-linked KCNQ1 mutations that increase open probability do not always shorten QT-intervals. 5,30,31 Of note, early-onset AF (age <50 years) was reported in 2% of patients with genetically proven LQT1 compared to a background population prevalence of 0.1%.32 This study raises the intriguing possibility that some of these LQT1 mutations may generate a mixed functional phenotype similar to R231C-Q1.
R231C-Q1 represents the first discrete missense mutation in the KCNQ1-encoded IKs potassium channel capable of pleiotropic expression. This is akin to the E1784K missense mutation in the SCN5A-encoded Nav1.5 sodium channel where E1784K not only is the most common SCN5A missense mutation responsible for LQTS type 3,33 but it also is the most common type 1 Brugada syndrome-associated mutation.34,35 The genetic, epigenetic, and environmental factors that drive a R231C-positive host or a E1784K-positive host to express one arrhythmia phenotype over the other remain elusive.
Study limitations
Unidentified factors may account for the different clinical phenotypes among R231C-Q1 families, and these data were obtained with a widely used heterologous overexpression system that could differ in native myocytes.
Conclusion
This is the first study to link a known LQT1 mutation to FAF. R231C-Q1 generates IQ1E1 with a mixed functional phenotype when co-expressed with WT-Q1: a constitutively active component with a smaller maximal IQ1E1. This mixed functional phenotype may predispose R231C-Q1 families to multiple clinical phenotypes, including LQTS, fetal bradycardia, and/or AF.
Supplementary Material
Acknowledgments
This work was supported by National Heart Lung and Blood Institute Grant T32 HL072743 to Daniel Bartos; National Heart Lung and Blood Institute Grant R01 HL60723 to Dr. January; National Heart Lung and Blood Institute Grant R01 HL087039 to Dr. Delisle; Région Ile-de-France fellowships to Dr. Duchatelet; European Commission Grant 221685 to Drs. Guicheney and Peat; and Fédération and Société Françaises de Cardiologie to Drs. Denjoy and Guicheney.
ABBREVIATIONS
- AF
atrial fibrillation
- AP
action potential
- E1
KCNE1
- HEK293
human embryonic kidney 293
- IKs
slowly activating delayed rectifier K+ current
- IMAX
maximally activated current
- IMIN
minimally activated current
- IQ1E1
Q1E1 current
- I–V
current–voltage
- k
slope factor
- LQT1
long QT syndrome type 1
- LQTS
long QT syndrome
- Q1
KCNQ1
- SNP
single nucleotide polymorphism
- V1/2
potential for half-maximal activation
- WT
wild type
Appendix
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.hrthm.2010.09.010.
References
- 1.Conen D, Osswald S, Albert CM. Epidemiology of atrial fibrillation. Swiss Med Wkly. 2009;39:346–352. doi: 10.4414/smw.2009.12500. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Parise H, D’Agostino RB, Sr, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 3.Lehnart SE, Ackerman MJ, Benson DW, Jr, et al. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 5.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 6.Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 7.Hong K, Piper DR, Diaz-Valdecantos A, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium channel. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 9.Sanguinetti MC, Curran ME, Zou A, Shen J, et al. Coassembly of KvLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 10.Fodstad H, Swan H, Laitinen P, et al. Four potassium channel mutations account for 73% of the genetic spectrum underlying long-QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann Med. 2004;36S:53–63. doi: 10.1080/17431380410032689. [DOI] [PubMed] [Google Scholar]
- 11.Lupoglazoff JM, Denjoy I, Villain E, et al. Long QT syndrome in neonates: conduction disorders associated with HERG mutations and sinus bradycardia with KCNQ1 mutations. J Am Coll Cardiol. 2004;43:826–830. doi: 10.1016/j.jacc.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Millat G, Chevalier P, Restier-Miron L, et al. Spectrum of pathogenic mutations and associated polymorphisms in a cohort of 44 unrelated patients with long QT syndrome. Clin Genet. 2006;70:214–227. doi: 10.1111/j.1399-0004.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- 13.Basavarajaiah S, Wilson M, Whyte G, Shah A, Behr E, Sharma S. Prevalence and significance of an isolated long QT interval in elite athletes. Eur Heart J. 2007;28:2944–2949. doi: 10.1093/eurheartj/ehm404. [DOI] [PubMed] [Google Scholar]
- 14.Zienciuk A, Szwoch M, Raczak G. Atrial fibrillation in the long QT syndrome. Kardiol Pol. 2009;67:681–684. [PubMed] [Google Scholar]
- 15.Itoh H, Sakaguchi T, Ding WG, et al. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2:511–523. doi: 10.1161/CIRCEP.109.862649. [DOI] [PubMed] [Google Scholar]
- 16.Fatkin D, Otway R, Vandenberg JI. Genes and atrial fibrillation: a new look at an old problem. Circulation. 2007;116:782–792. doi: 10.1161/CIRCULATIONAHA.106.688889. [DOI] [PubMed] [Google Scholar]
- 17.Zhang DF, Liang B, Lin J, Liu B, Zhou QS, Yang YQ. KCNE3 R53H substitution in familial atrial fibrillation. Chin Med J (Engl) 2005;118:1735–1738. [PubMed] [Google Scholar]
- 18.Ravn LS, Aizawa Y, Pollevick GD, et al. Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation. Heart Rhythm. 2008;5:427–435. doi: 10.1016/j.hrthm.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe H, Darbar D, Kaiser DW, et al. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:268–275. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham RL, Yang T, Blair M, Roden DM, Darbar D. Augmented potassium current is a shared phenotype for two genetic defects associated with familial atrial fibrillation. J Mol Cell Cardiol. 2010;48:181–190. doi: 10.1016/j.yjmcc.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Z, Gong Q, Ye B, et al. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol. 2010;48:112–121. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmyttere S, Bonduelle M, DeWolf D, Liebaers I, Lissens W. A case of term mors in utero in a chromosome 11P linked long QT family. Genetic Counsel. 1994;5:289–295. [PubMed] [Google Scholar]
- 24.Silva JR, Pan H, Wu D, et al. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc Natl Acad Sci U S A. 2009;106:11102–11106. doi: 10.1073/pnas.0904505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Restier L, Cheng L, Sanguinetti MC. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol. 2008;586:4179–4191. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang C, Tian C, Sönnichsen FD, et al. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virág L, Iost N, Opincariu M, et al. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 2001;49:790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 28.Jost N, Virág L, Bitay M, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Fermini B, Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res. 1994;28:1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- 30.Seebohm G. Kv7.1 in atrial fibrillation. Heart Rhythm. 2009;6:1154–1155. doi: 10.1016/j.hrthm.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Das S, Makino S, Melman YF, et al. Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation. Heart Rhythm. 2009;6:1146–1153. doi: 10.1016/j.hrthm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JN, Tester DJ, Perry J, Salisbury BA, Reed CR, Ackerman MJ. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008;5:704–709. doi: 10.1016/j.hrthm.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapplinger JD, Tester DJ, Salisbury BA, et al. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutation in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;1:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makita N, Behr E, Shimizu W, et al. The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J Clin Invest. 2008;118:2219–2229. doi: 10.1172/JCI34057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.