Abstract
Reduced plasma adiponectin has been associated with abnormal lipid profile, reduced left ventricle (LV) function, and the extent of coronary atherosclerosis in coronary artery disease. The aim of this study was to assess these relationships in patients with dilated cardiomyopathy (DCM) without overt heart failure. Plasma adiponectin was measured in 55 DCM patients (age, 59 ± 12 years; male, 36; body mass index [BMI], 26.9 ± 0.49 kg/m2; LV ejection fraction, 39.8% ± 1.3%; New York Heart Association class I-II) and in 40 age- and BMI-matched healthy controls. In a subset of 25 patients, myocardial blood flow (MBF) was measured at rest and during intravenous dipyridamole (0.56 mg/kg in 4 minutes) by positron emission tomography and 13N-ammonia as a flow tracer. Adiponectin was 6.6 ± 0.34 μg/mL in controls and 10.9 ± 0.85 μg/mL in DCM patients (P < .001), where it was related inversely with BMI (P = .009) and directly with brain natriuretic peptide (P = .017), high-density lipoprotein (HDL) cholesterol (P = .002), and MBF dipyridamole (P = .020). Adiponectin lesser than median value in patients was associated with higher total to HDL cholesterol ratio (4.8 ± 0.24 vs 3.9 ± 0.18, P = .009) and lower MBF reserve (1.76 ± 0.16 vs 2.43 ± 0.19, P = .01). These results could suggest that down-regulation of the adiponectin levels and reduced HDL cholesterol have a key role in causing impaired coronary function and myocardial perfusion in DCM.
1. Introduction
Adiponectin is a multifunctional protein with insulin-sensitizing effects and antiatherogenic and anti-inflammatory properties [1] resulting in important protective effects at cardiovascular level [2]. Accordingly, it has been involved in the pathogenesis of different cardiovascular diseases and has been also suggested as a potential new therapeutic target [2]. As to the peripheral circulation, the levels of this effector have been demonstrated to be a relevant biomarker with diagnostic/prognostic value in cardiovascular diseases [2].
In coronary artery disease (CAD), adiponectin has been extensively studied [3-5]; and lower levels of this cytokine have been associated with early onset [4] and severity of the disease [3-5]. A study in 1174 patients with angiographically documented CAD showed a correlation with high-density lipoprotein (HDL) cholesterol, triglycerides, and N-terminal pro–brain natriuretic peptide (BNP), suggesting that dyslipidemia could be a link between adiponectin and atherosclerosis progression [6]. Finally, Frystyk and coworkers [5], in a 10-year follow-up study in a group of healthy elderly subjects, recently showed that elevated circulating concentrations of adiponectin are associated with a lower risk for CAD independently of other well-known risk factors.
In heart failure (HF), adiponectin plasma levels increase as a function of disease severity (New York Heart Association class), correlate with BNP and tumor necrosis factor–α (TNFα) [7], and represent a predictor of death independently of other risk markers [8]. It has been suggested that a receptor-mediated “functional adiponectin resistance” may ensue in overt HF, reducing the protective effects of this adipokine [9].
Little is known about the possible involvement of adiponectin system in nonatherosclerotic cardiac diseases such as dilated cardiomyopathy (DCM) before the onset of overt HF. In this condition, an increase of inflammatory markers and abnormalities in lipid profile (reduction of HDL cholesterol and apolipoprotein A-I and -II) were observed [10]. Moreover, DCM patients may frequently show insulin resistance, suggesting a pathogenetic link between impaired glucose metabolism and left ventricle (LV) dysfunction [11]. Both lipoproteins abnormalities and insulin resistance may negatively affect macro- and microvascular structure and function [12] and have been recently related with adiponectin signal. In particular, it has been hypothesized that adiponectin may directly influence the concentration of circulating lipids and especially plasma HDL cholesterol [6,13]. Actually, HDL directly regulates endothelial function, influencing the control mechanisms of myocardial blood flow (MBF) [14]. As a matter of fact, MBF abnormalities at rest as well as in response to metabolic or pharmacologic stimulations have been observed in DCM patients with angiographically normal coronary arteries. These abnormalities were interpreted as due to endothelial/microvascular dysfunction preceding the onset of HF [15] and were demonstrated to independently predict prognosis [16].
We hypothesized that adiponectin plasma levels could be related with both an abnormal lipid and/or metabolic profile and coronary microvascular dysfunction in DCM. To test this hypothesis, adiponectin levels, as well as inflammatory markers, serum lipids, and glucose profile, were measured in patients with DCM without overt HF to avoid the confounding effects of generalized biohumoral activation. The relationship between adiponectin levels and coronary microvascular function was analyzed in a subset of patients with DCM submitted to positron emission tomography (PET) to measure MBF.
2. Materials and methods
2.1. Patients
We enrolled 55 consecutive patients (age, 59 ± 12 years) with DCM. All patients had come to medical attention because of dyspnea on effort and/or ventricular arrhythmias and/or chest pain and/or conduction disturbances, with evidence of LV systolic dysfunction at 2-dimensional (2D) echocardiographic evaluation leading to coronary angiography. Inclusion criteria were (1) LV ejection fraction (EF) less than 50% at 2D echocardiography; (2) functional New York Heart Association class I to II; (3) angiographically normal coronary arteries; (4) exclusion of vasospastic angina, congenital or valvular heart disease, hypertrophic cardiomyopathy, myocarditis, pericarditis, lung disease, and primary thyroid disease; (5) presence of sinus rhythm; and (6) exclusion of alcohol abuse or other systemic diseases.
Local ethics review committee approved the study, and the investigation conformed to the principles outlined in the Declaration of Helsinki.
Blood samples were also obtained in the same conditions in 40 age-matched healthy subjects included as a control group. All healthy subjects were asymptomatic, were not obese, had normal arterial blood pressure, had a low risk of CAD, and were free from acute diseases, as determined by an interview with a clinician. All of them had normal values for the main plasma parameters and normal leukocyte count. Furthermore, they denied the use of any drug during the 4 weeks before the study.
All subjects gave their written informed consent to be involved in the study including PET and blood sampling.
2.2. LV function and coronary functional profiles
Left ventricular EF, LV end-diastolic diameter (LVEDD, in millimeters), and LV mass index were obtained by 2D echocardiographic evaluation according to international standards. In 25 patients, coronary microvascular function was assessed by rest/stress PET MBF study. Absolute MBF was measured, using 13N-ammonia as a flow tracer, at rest and during intravenous dipyridamole (dip) (0.56 mg/kg in 4 minutes). Coronary resistance was computed in each condition as the ratio of mean aortic pressure over MBF. Myocardial blood flow and resistance reserve were obtained as the ratio of stress to rest MBF and rest to stress resistance, respectively. Both the PET protocol and the data analysis have been described elsewhere [16]. Data obtained in DCM patients were compared with those obtained in a population of 15 healthy subjects previously studied and already published [16]. These subjects (6 men, 49 ± 7 years of age) presented atypical chest pain, angiographically normal epicardial coronary arteries, normal LV function by contrast ventriculography, and no other detectable heart or systemic disease. The historical PET control group and the tested group of DCM patients submitted to PET study are slightly even if not significantly different as to age, sex, body mass index (BMI), and smoking habit.
2.3. Biohumoral profile
Adiponectin was determined on 1:500 dilution of plasma EDTA samples by using a specific immunometric assay (Linco Research, St Charles, MO): sensitivity, 0.14 ± 0.05 ng/mL; working range, 1.5 to 100 ng/mL; within-assay variability, 5.6%; and between-assay variability, 15.0%. Two monoclonal antihuman adiponectin antibodies recognizing 2 different epitopes of the molecule are used, whereas the dose-response curve was built by using, as calibrator, recombinant human adiponectin.
High–molecular weight (HMW) adiponectin was measured by enzyme-linked immunosorbent assay (ELISA) technique after pretreatment with a specific protease (ALPCO Diagnostics, Salem, NH) as previously described [17].
Plasma level of interleukin-6 (IL-6) was measured by a high-sensitivity ELISA technique (Diaclone Research, Besancon, France). Sensitivity level was 0.33 ± 0.04 pg/mL.
Plasma levels of TNFα were determined by an ELISA technique (Invitrogen, Carlsbad, CA); lower detection limit was about 0.09 pg/mL.
C-reactive protein (CRP) was measured in human serum by a high-sensitivity ELISA method (Diagnostics Biochem Canada, London, Ontario, Canada); lower detection limit was about 0.001 mg/dL.
Brain natriuretic peptide was measured with a 2-site immunoradiometric assay (Shionogi, Osaka, Japan).
Lipid, glucose, and insulin profiles were obtained by standard measurements.
2.4. Statistical analysis
All the results are reported as mean ± SEM. All the values of sample concentration were calculated by using a 4-parameter logistic function to interpolate dose-response curve.
All statistical calculations were performed with SPSS 10.5 statistical software package (SPSS, Chicago, IL). Analysis of variance followed by Fisher test was used to compare continuous variables after a logarithmic transformation of the original values if not normally distributed. Simple regression analysis was performed to correlate different variables. A P value < .05 was considered significant.
Multivariate analysis was performed to evaluate the effects of the various metabolic and inflammatory parameters on microvascular function.
3. Results
3.1. Characteristics of the study population
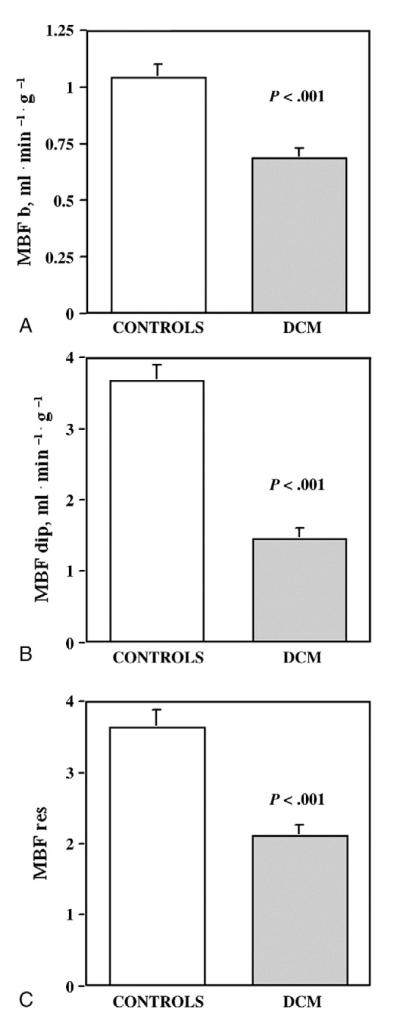
Clinical characteristics of the study population and MBF data are summarized in Table 1. Patients showed moderately depressed LV systolic function, moderately increased LV dimensions and mass, and increased BNP plasma levels. In the subgroup studied with PET, patients had significantly depressed MBF as compared with previously published values in healthy people (MBF b, 1.04 ± 0.22 mL min−1 g−1; MBF dip, 3.67 ± 0.86 mL min−1 g−1; MBF reserve, 3.63 ± 0.97) (P < .001, DCM patients vs healthy people) [16] (Fig. 1). As to the treatment regimens, angiotensin-converting enzyme inhibitors were being taken by 89% of patients, β-blockers by 56%, digitalis by 31%, diuretics by 64%, amiodarone by 24%, and statins by 13%. Cardiovascular risk profile of the study population is shown in Table 2. Overall, 92% of patients had at least one abnormal metabolic risk factor; but only 9% had evidence of metabolic syndrome according to published criteria [18].
Table 1.
Clinical and MBF data at enrollment
| Mean values ± SEM | |
|---|---|
| Age, y | 58.7 ± 1.3 |
| Men, % | 67% |
| LVEF, % | 39.8 ± 1.3 |
| LVEDD, mm | 60.4 ± 1.0 |
| LVEDP, mm Hg | 12.9 ± 1.3 |
| LV mass index, g/m2 | 144.2 ± 5.8 |
| MBF b, mL min−1 g−1 | 0.69 ± 0.13 |
| MBF dip, mL min−1 g−1 | 1.46 ± 0.13 |
| MBF reserve | 2.11 ± 0.14 |
| Res bas, mm Hg mL−1 min−1 g−1 | 151.54 ± 8.55 |
| Res dip, mm Hg mL−1 min−1 g−1 | 78.63 ± 7.68 |
| Res reserve | 2.14 ± 0.15 |
LVEDP indicates LV end-diastolic pressure; bas, baseline; res, microvascular resistance.
Fig. 1.
Myocardial blood flow variables in DCM patients and in controls. Myocardial blood flow b (A), MBF dip (B), and MBF reserve (C) are significantly reduced in DCM patients with respect to controls [16].
Table 2.
Cardiovascular risk factors at enrollment
| Mean value ± SEM |
Range | % Abnormala |
|
|---|---|---|---|
| SBP, mm Hg | 133.1 ± 2.5 | 100-175 | 38.2 |
| DBP, mm Hg | 75.4 ± 1.5 | 52-115 | 10.9 |
| Total cholesterol, mg/dL | 197.0 ± 5.9 | 105-335 | 44.4 |
| HDL cholesterol, mg/dL | 47.2 ± 1.6 | 28-79 | 35.2 |
| LDL cholesterol, mg/dL | 125.5 ± 5.6 | 50-225 | 28.3 |
| Total cholesterol to HDL …cholesterol |
4.3 ± 0.16 | 2.3-7.8 | 37.0 |
| Plasma triglycerides, mg/dL | 114.6 ± 11.0 | 41-473 | 16.7 |
| BMI, kg/m2 | 26.9 ± 0.49 | 19.5-37.8 | 17.0 |
| Insulin, μIU/mL | 9.7 ± 0.58 | 2.8-23.8 | 0 |
| Glucose, mg/dL | 108.6 ± 4.2 | 67-252 | 42.6 |
| HOMA index | 2.7 ± 0.49 | 0.65-7.9 | 22.6 |
SBP indicates systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; HOMA, Homeostasis Model Assessment.
Systolic blood pressure ≥140; DBP ≥90; total cholesterol >200 mg/dL; LDL cholesterol >150 mg/dL; HDL cholesterol <35 (male) and <39 (female) mg/dL; triglycerides >150 mg/dL; BMI >30 kg/m2; insulin >27 μIU/mL; glucose >100 mg/dL; HOMA index <3.8.
3.2. Relationships between adiponectin, inflammatory profile, cardiovascular risk profile, LV function, and MBF
Plasma adiponectin values in DCM patients were significantly higher than those found in healthy controls (10.9 ± 0.85 vs 6.6 ± 0.34 μg/mL, mean ± SEM; P < .001). High–molecular weight adiponectin was 2.51 ± 0.24 and 1.76 ± 0.15 μg/mL (P = .036) in DCM patients and in controls, respectively. Inflammatory markers were increased in DCM patients with respect to controls: IL-6 was 1.49 ± 0.26 in DCM patients and 0.78 ± 0.41 pg/mL in controls (P < .001), whereas CRP was 0.34 ± 0.08 and 0.11 ± 0.033 mg/dL, respectively (P = not significant [NS]). Tumor necrosis factor–α was 7.3 ± 0.21 in DCM group and 7.2 ± 0.45 pg/mL in controls (P = NS).
In DCM patients, adiponectin levels were not related with inflammatory markers or echocardiographic LV functional parameters. However, a significant direct correlation was found between adiponectin and BNP plasma concentration (r = 0.321, P = .017). Among cardiovascular risk factors, adiponectin was negatively correlated with BMI (P = .009, standard error of estimate [SEE]=0.516) (Fig. 2A) and positively correlated with HDL cholesterol (P = .003, SEE = 0.507) (Fig. 2B). In the subgroup studied with PET, adiponectin was positively correlated with MBF dip (P = .0197, SEE = 0.495) (Fig. 2C). Similarly to total adiponectin, HMW adiponectin positively correlated with HDL cholesterol; but no correlation was found with either BMI or MBF parameters. The DCM patients were subdivided into 2 groups according to the median value of plasma adiponectin concentration (9.1 μg/mL; interquartile range, 7.625). Clinical, LV functional, metabolic, and inflammatory markers are compared in the 2 groups in Table 3. The treatment regimens are similar in the 2 groups of patients. The patients with plasma adiponectin lower than the median value (group 1) had lower HDL cholesterol and significantly higher total to HDL cholesterol ratio and BMI as compared with patients with plasma adiponectin higher than the median value (group 2). Moreover, lower adiponectin levels in DCM were associated with an evident impairment of coronary microvascular function as demonstrated by depressed MBF values and reduced MBF reserve (Fig. 3). Finally, multivariate analysis indicates that adiponectin plasma levels (P = .05) are the factors mainly affecting MBF dip values in DCM patients with respect to HDL cholesterol, IL-6, and BMI.
Fig. 2.
Relationship between adiponectin, and metabolic and MBF variables. In patients with DCM, adiponectin correlates negatively with BMI (A), and positively with HDL cholesterol (B) and MBF dip (C). Logarithmic transformation of the original values was performed when variables were not normally distributed.
Table 3.
Clinical, functional, cardiovascular risk, and inflammatory markers as a function of plasma adiponectin
| Group 1 | Group 2 | Significance | |
|---|---|---|---|
| Age, y | 56.6 ± 2.2 | 60.9 ± 1.3 | NS |
| SBP, mm Hg | 129.9 ± 3.4 | 136.5 ± 3.7 | NS |
| DBP, mm Hg | 74.4 ± 1.9 | 76.4 ± 2.4 | NS |
| LV function data | |||
| LVEF% | 39.6 ± 1.9 | 39.9 ± 1.9 | NS |
| LVEDD, mm | 61.9 ± 1.5 | 58.9 ± 1.4 | NS |
| LVEDP, mm | 10.6 ± 1.4 | 15.1 ± 2.1 | NS |
| LV mass index, g/m2 | 140.0 ± 6.8 | 149.3 ± 10.0 | NS |
| BNP, pg/mL | 53.1 ± 12.9 | 163.9 ± 42.4 | NS |
| Cardiovascular risk factors | |||
| Total cholesterol, mg/dL | 202.4 ± 8.1 | 191.6 ± 9.7 | NS |
| HDL cholesterol, mg/dL | 44.4 ± 2.1 | 50.1 ± 2.3 | P = .07 |
| LDL cholesterol, mg/dL | 131.5 ± 8.1 | 119.5 ± 7.3 | NS |
| Total to HDL cholesterol | 4.8 ± 0.24 | 3.9 ± 0.18 | P = .009 |
| Plasma triglycerides, mg/dL | 118.8 ± 12.7 | 110.4 ± 18.1 | NS |
| BMI, kg/m2 | 27.9 ± 0.69 | 25.9 ± 0.65 | P = .037 |
| Glucose, mg/dL | 108.0 ± 6.1 | 109.0 ± 5.9 | NS |
| HOMA index | 2.82 ± 0.27 | 2.61 ± 0.39 | NS |
| Inflammatory markers | |||
| IL-6, pg/mL | 1.2 ± 0.27 | 1.8 ± 0.45 | NS |
| TNFα, pg/mL | 7.3 ± 0.19 | 7.4 ± 0.42 | NS |
| CRP, mg/dL | 0.22 ± 0.052 | 0.52 ± 0.17 | NS |
Fig. 3.
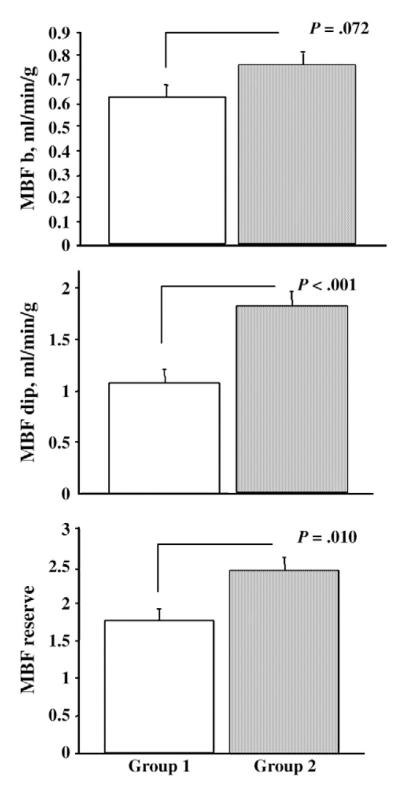
Myocardial blood flow and adiponectin. Myocardial blood flow b (top), MBF dip (middle), and MBF reserve (bottom) are severely reduced in patients of group 1 (ie, with adiponectin lower than the median value, 9.1 μg/mL; interquartile range, 7.625) with respect to patients of group 2 (ie, with adiponectin higher than the median value). Mean values and SEM are shown.
4. Discussion
The present study clearly indicates that, in the DCM population, subjects with relatively lower plasma levels of adiponectin show reduced HDL cholesterol and a severe impairment of myocardial perfusion.
Our data strongly suggest that a relative down-regulation of the adiponectin signal and reduced HDL cholesterol may be involved in the pathogenesis of coronary endothelial/microvascular dysfunction in DCM.
4.1. Adiponectin, CAD, LV dysfunction, and HF
It is well known that low levels of adiponectin are associated with increased risk of CAD [5]. In patients with established CAD, reduced adiponectin levels are associated with disease severity and increased risk of acute coronary events [3]. To explain this apparent protective role of adiponectin on coronary atherosclerotic damage, different mechanisms have been hypothesized mainly including the anti-inflammatory and metabolic properties of this adipokine [1].
In patients with CAD, adiponectin strongly correlates with triglycerides (negative correlation) and HDL cholesterol (positive correlation), even after adjustment for many influencing factors such as age, sex, BMI, history of hypertension or diabetes, and smoking habit [6]. These observations suggested that the antiatherosclerotic actions of adiponectin could be due to its effects on lipids. Adiponectin is associated with key enzymes in lipid metabolism [19,20] and may directly influence the concentrations of circulating lipids in particular HDL cholesterol [13].
In patients with CAD, increased levels of adiponectin are associated with LV dysfunction and plasma levels of BNP[7,21], possibly expressing a compensatory response to prevent further deterioration in systolic function, thus contributing to preserving myocardial function [22]. When LV dysfunction progresses and HF ensues, adiponectin levels increase proportionally and correlate with markers of disease severity and mortality [7,8], suggesting that in HF the adiponectin signal might lose its efficacy because of a “functional adiponectin resistance” [9].
4.2. DCM, coronary microvascular dysfunction, and adiponectin
A potential pathogenetic role for functional abnormalities of the coronary macro- and microcirculation has been recently hypothesized in DCM [23] as well as in other cardiovascular disorders [24]. Different clinical studies have demonstrated a clear impairment of coronary endothelial function in DCM as part of a systemic alteration of the vascular endothelium [25]. Endothelial dysfunction is able to strongly limit the coronary vasodilatory response to stress. This condition is potentially able to cause an imbalance between myocardial oxygen demand and blood flow delivery, setting the “scene” for possible myocardial ischemia even in the absence of overt CAD. Actually, myocardial flow and metabolic patterns characteristic of ischemic myocardial dysfunction have been described in clinical DCM [26] and in experimental models [27]. The MBF abnormalities can be documented early in the natural history of DCM [15] and are able to predict the unfavorable evolution of the disease toward progressive ventricular dysfunction and HF [16].
Among the possible mechanisms leading to abnormal coronary macro- and microvascular function in DCM, preliminary evidences suggest a role for abnormal lipid profile. In a recent study from our group [10], patients with DCM, as compared with a matched population of healthy controls, were characterized by lower HDL levels associated with increase in CRP. The PET flow studies in non-DCM populations demonstrated that lower plasma HDL cholesterol was associated with reduced MBF response to both sympathetic and pharmacologic stimulation at the coronary microvascular level [28].
Because of the known relationships between adiponectin, lipid, coronary atherosclerosis, and LV function in CAD, it can be hypothesized that a similar pathogenetic link may exist with myocardial and vascular dysfunction in DCM. No data exist in this population because published data are mainly related to patients with severe HF where the effects of neurohumoral activation might confound the observations. Accordingly, in the present study, we carefully selected a population with LV dysfunction and angiographically normal coronary arteries without overt HF. In this very selected cohort of patients, adiponectin levels were significantly increased with respect to a control group of healthy individuals. Although adiponectin was inversely related with BMI, as expected [29,30], and directly related with BNP levels, no correlation with inflammatory markers was found, probably because of the complex reciprocal interplay between adiponectin and inflammatory mediators. These findings are very similar to those described in CAD patients or in patients with cardiovascular risk factors without HF. Present results underline the similarity between DCM, CAD, and ischemic LV dysfunction and the potential protective role of adiponectin also in this population. Changes in HMW adiponectin reflected similar changes in total adiponectin concentrations. The lower significance level of correlations between this active form and lipid profile or myocardial flow parameters may be due to methodological features of the HMW assay [17]. This observation is in tune with previously found data in HF patients [8].
The main observation of this study, however, is that in DCM patients lower levels of adiponectin were associated with reduced HDL cholesterol and evidence of severe impairment of MBF and MBF reserve. By contrast, patients with lower or higher levels of adiponectin did not differ in any other LV functional, metabolic, or inflammatory parameter. This finding strongly indicates that in DCM adiponectin exerts protective functions on the coronary vessels and that a relative down-regulation of its signal is associated with severe endothelial/microvascular dysfunction. The resulting depression of myocardial perfusion and impairment of coronary vasodilating capability during stress may significantly contribute to disease progression and worse prognosis [16].
4.3. Limitations of the study
The major limitation of the present study is the relatively low number of patients submitted to PET, which may not be optimal for a multivariate analysis to assess independent predictors of MBF abnormalities. However, the PET subgroup was representative of the whole DCM group for all the analyzed variables and also superimposable to previously published populations of DCM patients evaluated by PET.
Another limitation is the lack at present of a prolonged follow-up, which could have provided evidence of the prognostic impact of observed abnormalities in lipid profile and adiponectin levels as compared with the known prognostic value of microvascular variables.
4.4. Conclusions
Because coronary endothelial/microvascular dysfunction has been recently recognized as a key pathogenetic factor of progressive LV dysfunction not only in DCM but also in other cardiovascular disorders, the understanding of the pathogenetic mechanisms involved may reveal new therapeutic targets to prevent HF. In this contest, adiponectin pathway is gaining increasing attention. Although low adiponectin influences the concentrations of HDL cholesterol, both these factors are associated with endothelial dysfunction [13,31], suggesting synergistic actions. As a matter of fact, the known beneficial effects of statin and fibrate therapy in CAD have been reproduced in DCM and appear to be related with increase in adiponectin levels [32].
References
- [1].Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–6. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hara K, Yamauchi T, Imai Y, et al. Reduced adiponectin level is associated with severity of coronary artery disease. Int Heart J. 2007;48:149–53. doi: 10.1536/ihj.48.149. [DOI] [PubMed] [Google Scholar]
- [4].Hashimoto N, Kanda J, Nakamura T, et al. Association of hypoadiponectinemia in men with early onset of coronary heart disease and multiple coronary artery stenoses. Metabolism. 2006;55:1653–7. doi: 10.1016/j.metabol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [5].Frystyk J, Berne C, Berglund L, et al. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–6. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- [6].vonEynatten M, Hamann A, Twardella D, et al. Relationship of adiponectin with markers of systemic inflammation, atherogenic dyslipidemia, and heart failure in patients with coronary heart disease. Clin Chem. 2006;52:853–9. doi: 10.1373/clinchem.2005.060509. [DOI] [PubMed] [Google Scholar]
- [7].Nakamura T, Funayama H, Kubo N, et al. Association of hyperadiponectinemia with severity of ventricular dysfunction in congestive heart failure. Circ J. 2006;70:1557–62. doi: 10.1253/circj.70.1557. [DOI] [PubMed] [Google Scholar]
- [8].Tsutamoto T, Tanaka T, Sakai H, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007;28:1723–30. doi: 10.1093/eurheartj/ehm154. doi:10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- [9].Kintscher U. Does adiponectin resistance exist in chronic heart failure? Eur Heart J. 2007;28:1676–7. doi: 10.1093/eurheartj/ehm233. [DOI] [PubMed] [Google Scholar]
- [10].Sampietro T, Neglia D, Bionda A, et al. Inflammatory markers and serum lipids in idiopathic dilated cardiomyopathy. Am J Cardiol. 2005;96:1718–20. doi: 10.1016/j.amjcard.2005.07.093. [DOI] [PubMed] [Google Scholar]
- [11].Witteles RM, Tang WH, Jamali AH, et al. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol. 2004;44:78–81. doi: 10.1016/j.jacc.2004.03.037. [DOI] [PubMed] [Google Scholar]
- [12].Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–7. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- [13].vonEynatten M, Schneider JG. The role of adiponectin in atherosclerosis: do lipids tip the scales? Future Cardiol. 2005;1:775–84. doi: 10.2217/14796678.1.6.775. [DOI] [PubMed] [Google Scholar]
- [14].Norata GD, Catapano AL. Molecular mechanisms responsible for the antiinflammatory and protective effect of HDL on the endothelium. Vasc Health Risk Manag. 2005;1:119–29. doi: 10.2147/vhrm.1.2.119.64083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Neglia D, De Maria R, Masi S, et al. Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. Circulation. 1995;92:796–804. doi: 10.1161/01.cir.92.4.796. [DOI] [PubMed] [Google Scholar]
- [16].Neglia D, Michelassi C, Trivieri MG, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–93. doi: 10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- [17].Caselli C, Melaiu O, Maltinti M, et al. A methodological reappraisal of total and high molecular weight adiponectin determination in human peripheral circulation: comparison of four immunometric assays. Clin Chem Lab Med. doi: 10.1515/CCLM.2010.104. (in press) [DOI] [PubMed] [Google Scholar]
- [18].Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American heart association Conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- [19].von Eynatten M, Schneider JG, Humpert PM, et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: an association independent of systemic inflammation and insulin resistance. Diabetes Care. 2004;27:2925–9. doi: 10.2337/diacare.27.12.2925. [DOI] [PubMed] [Google Scholar]
- [20].Schneider JG, von Eynatten M, Schiekofer S, et al. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care. 2005;28:2181–6. doi: 10.2337/diacare.28.9.2181. [DOI] [PubMed] [Google Scholar]
- [21].Cavusoglu E, Chopra V, Battala V, et al. Baseline plasma adiponectin levels as a predictor of left ventricular systolic dysfunction in patients referred for coronary angiography. Am J Cardiol. 2008;101:1073–8. doi: 10.1016/j.amjcard.2007.12.008. [DOI] [PubMed] [Google Scholar]
- [22].Shibata R, Izumiya Y, Sato K, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–74. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neglia D, L’Abbate A. Coronary microvascular dysfunction and idiopathic dilated cardiomyopathy. Pharmacol Rep. 2005;57:151–5. [PubMed] [Google Scholar]
- [24].Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- [25].Treasure CB, Vita JA, Cox DA, et al. Endothelium-dependent dilation of the coronary microvascolature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–9. doi: 10.1161/01.cir.81.3.772. [DOI] [PubMed] [Google Scholar]
- [26].Neglia D, De Caterina A, Marraccini P, et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–8. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- [27].Lionetti V, Guiducci L, Simioniuc A, et al. Mismatch between uniform increase in cardiac glucose uptake and regional contractile dysfunction in pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H2747–56. doi: 10.1152/ajpheart.00592.2007. [DOI] [PubMed] [Google Scholar]
- [28].Prior JO, Schindler TH, Facta AD, et al. Determinants of myocardial blood flow response to cold pressor testing and pharmacologic vasodilation in healthy humans. Eur J Nucl Med Mol Imaging. 2007;34:20–7. doi: 10.1007/s00259-006-0193-4. [DOI] [PubMed] [Google Scholar]
- [29].Orio F, Jr, Palomba S, Cascella T, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2619–23. doi: 10.1210/jc.2002-022033. [DOI] [PubMed] [Google Scholar]
- [30].Panidis D, Kourtis A, Farmakiotis D, et al. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1790–6. doi: 10.1093/humrep/deg353. [DOI] [PubMed] [Google Scholar]
- [31].Shimabukuro M, Higa N, Asahi T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–40. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- [32].Nakamura T, Kodama Y, Takano H, et al. Increase in circulating levels of adiponectin after treatment with statin and fibrate in patients with coronary artery disease and hyperlipidemia. Atherosclerosis. 2007;193:449–51. doi: 10.1016/j.atherosclerosis.2006.08.028. [DOI] [PubMed] [Google Scholar]