Abstract
When mutations in nuclear lamins were first identified in skeletal and cardiac muscle diseases, the molecular events underlying pathogenesis were mere points of speculation. As more and more unrelated diseases were linked to lamins and other nuclear envelope proteins, nuclear structure and disease became an increasingly prominent research focus. Today, the disease mechanisms remain unresolved, but incredible progress has occurred. Nuclear envelope dysfunction is not only associated with altered nuclear activity, but also impaired structural dynamics and aberrant cell signaling. Building on these findings, small molecules are being discovered in animal models that may become effective therapeutic agents.
Introduction
Since their discovery more than 35 years ago as constituents of the nuclear lamina (Gerace et al., 1978), the nuclear lamins have been the subject of intense speculation regarding their possible roles in almost everything that happens in the nucleus. Early studies focused on biochemistry and cell biology with the goal of achieving a basic understanding of the principles governing nuclear organization. The nuclear envelope entered the medical realm in the mid-1990s when mutations in emerin were identified in patients with Emery-Dreifuss muscular dystrophy (EDMD) (Bione et al., 1994). The LMNA gene, encoding all A-type nuclear lamins, was linked to EDMD a few years later (Bonne et al., 1999), and since then links between nuclear structure and human disease have been studied extensively in labs throughout the world.
With nearly 15 diseases attributed to LMNA mutations, including a range of dystrophic and progeroid syndromes, and mutations in genes encoding associated nuclear envelope proteins causing an overlapping set of diseases, the questions and experimental approaches have evolved. Why do alterations in nuclear envelope proteins confer disease? What are the mechanisms underlying disease pathology? Do A-type lamins have a role in normal aging? Can effective therapies be developed for these debilitating diseases? While a range of exciting discoveries have been made in the last decade, there remain a great many unknowns. Here, we seek to frame the current questions, propose possible paths toward mechanistic understanding and briefly evaluate the therapeutic possibilities that are starting to emerge. Given the amount of interest and momentum in the lamin field, it is feasible that therapies to rescue the pathogenic consequences of misbehaved nuclear structural components will be developed in the not-too-distant future.
Nuclear lamins
The nuclear envelope is comprised of two membranes, the outer nuclear membrane, which is continuous with the endoplasmic reticulum, and the inner nuclear membrane, which associates with the nuclear lamina. Nuclear pore complexes perforate the nuclear envelope to allow transport between the cytoplasm and nucleus. The nuclear lamina is primarily composed of nuclear lamins, which were originally identified as lamins A, B and C (Gerace et al., 1978). These proteins constitute the only class of intermediate filament proteins in the nucleus and form associated filamentous structures that underlie the nuclear envelope and interact with neighboring proteins (Gerace and Huber, 2012). Lamins A and C, as well as two other variants (C2 and AΔ10), are classed as A-type lamins and are encoded by the LMNA gene through alternative splicing. Three different lamin B family members (B-type lamins) are encoded by two genes (lamin B1 by LMNB1 and lamins B2 and B3 by LMNB2).
A- and B-type lamins have fundamentally different properties, perhaps most importantly by virtue of their different isoelectric points, which dictate that B-type lamins stay associated with the nuclear envelope during mitosis while A-type lamins become soluble. Expression patterns differ as well, with B-type lamins expressed in most or all cell types and A-type lamins expressed during cell differentiation in many different developmental lineages (Rober et al., 1989). At the cellular level, both classes of proteins have been ascribed structural roles in the nucleus as well as a range of other activities including coordination of transcription and replication. While specific functions of A-type lamins remain somewhat elusive, a number of recent discoveries point to key interactions between lamins and cell proliferation, differentiation and stress response pathways.
Both A- and B-type lamins undergo post-translational processing based on a C-terminal CaaX motif that dictates a series of modifications (Weber et al., 1989); only lamin C avoids this by virtue of alternative splicing of the LMNA transcript that lacks the C-terminus. As a first step, the cysteine residue is farnesylated. Next, proteolytic processing leads to cleavage after the cysteine residue, followed by a carboxymethylation of the new C-terminal residue. Many membrane associated proteins, including Ras undergo this processing event. However, in the case of lamin A, isoprenylation is a transient event since a second proteolytic event mediated by the zinc metalloproteinase, Zmpste24, leads to excision of another 15 amino acids. Due to this cleavage, mature lamin A lacks the modified cysteine. This process is clearly important to pathologic states, since laminopathies are linked to altered processing of lamin A as well as loss of function mutations in ZMPSTE24.
The reasons for farnesylation of lamin A remain to be elucidated despite extensive efforts. Until recently, thinking has been that the transient farnesylation event was needed, through association of the hydrophobic farnesyl group with the nuclear envelope, to provide initial recruitment of lamin A to the nuclear periphery (Hennekes and Nigg, 1994). After assembly into filaments, farnesylation of lamin A may no longer be required. Consistent with this hypothesis, the nucleus has been shown to be the site of both lamin A carboxymethylation and proteolytic cleavage by ZMPSTE24. However, several recent studies using mice and/or cells engineered to express mutant forms of lamin A indicate that farnesylation is not required for recruitment (Davies et al., 2011). For instance, when only a non-farnesylated version of lamin A is expressed, normal localization of the lamin A variant to the nuclear periphery was observed (Davies et al., 2010; Lee et al., 2010), although mice generated in this manner develop cardiomyopathy (see below). In addition, mice expressing only lamin C (not farnesylated) or only a mature (pre-processed) lamin A are (surprisingly) normal and have apparently correct localization of the respective protein to the nuclear periphery . While these studies do not preclude a more subtle role for lamin A processing in filament assembly or envelope association, they raise serious questions about the importance of these events in the mouse and provide an interesting puzzle to be pieced together by future studies.
Diseases linked to mutations in nuclear structure proteins
The number of different diseases linked to mutations in LMNA, at least 15 by now, surpasses that of any other human gene. It is hard to establish absolute numbers, however, since many of the associated syndromes have overlapping pathologies. Nevertheless, the range of tissues and functions that can be adversely affected by mutation in LMNA is striking (Table 1). Diseases include the aforementioned Emery-Dreifuss muscular dystrophy (EDMD2/3) (Bonne et al., 1999) and a second muscular dystrophy (Limb-girdle, LGMD1B) (Muchir et al., 2000), which affects different skeletal muscle groups. Patients with both forms of muscular dystrophy also present with dilated cardiomyopathy, which is often the cause of mortality. Other LMNA mutations lead to dilated cardiomyopathy (CDM1A) without skeletal muscle involvement (Fatkin et al., 1999). Finally a form of congenital muscular dystrophy has more recently been linked to mutations in LMNA (Quijano-Roy et al., 2008), as well as Heart-hand syndrome, which couples a range of cardiac defects to brachydactyly (Renou et al., 2008).
Table 1.
Diseases Caused by Mutations in Genes Encoding Lamins and Lamin-Associated Proteins
| Striated Muscle Diseases | Gene Mutated |
|---|---|
| Emery-Dreifuss muscular dystrophy | LMNA, EDMD, SYNE1, SYNE2, TMEM43, TMPO |
| Limb-girdle muscular dystrophy | LMNA |
| Dilated cardiomyopathy | LMNA, EDMD, SYNE1, SYNE2, TMEM43, TMPO |
| Cogenital muscular dystrophy | LMNA |
| Heart-hand syndrome | LMNA |
| Lipodstrophy | |
| Dunnigan-type familial partial lipodystrophy | LMNA |
| Mandibuloacral dysplasia | LMNA, ZMPSTE24 |
| Lipoatrophy | LMNA |
| Partial Lipodystrophy | LMNB2 |
| Premature Aging | |
| Atypicial Werner Syndrome | LMNA |
| Hutchinson-Gilford Progeria Syndrome | LMNA |
| Restrictive Dermopathy | LMNA, ZMPSTE24 |
| Atypical Progeria Syndrome | BANF1 |
| Peripheral Nerve Disorders | |
| Charcotte-Marie Tooth Syndrome | LMNA |
| Adult-onset leukodystrophy | LMNB1 |
| Spinocerebellular ataxia type 8 | SYNE1 |
| Bone Diseases | |
| Buschke-Ollendorff Sydrome | LEMD3 |
| Melorheostosis | LEMD3 |
| Osteopoikilosis | LEMD3 |
| Greenberg skeletal dysplasia | LBR |
| Other | |
| Pelger-Huet Anomaly | LBR |
| Arthrogryposis | SYNE2 |
Pathology associated with LMNA mutations is not restricted to striated muscle tissue, as other diseases confer loss of adipose tissue, including Dunnigan-type familial partial lipodystrophy (FPLD2) (Shackleton et al., 2000), Mandibuloacral dysplasia (MAD) (Novelli et al., 2002), generalized lipoatrophy (Caux et al., 2003), Restrictive dermopathy (RD) (Navarro et al., 2004) and other overlapping disorders. Highlighting the importance of Lamin A processing, mutations resulting in loss of ZMPSTE24 function, which result in partially processed lamin A, lead to both MAD and RD (Agarwal et al., 2003; Navarro et al., 2005). However, links between lamin A processing and pathology extend beyond mutations in ZMPSTE24 and connect with another set of disorders termed progeroid, which give rise to the appearance of premature aging. The most noted of these is Hutchinson-Gilford progeria syndrome (HGPS), a severe disorder for which symptoms, including cachexia, alopecia and atherosclerosis become apparent shortly after birth. Death results from heart attack or stroke usually before the patient reaches the age of 20. The most common LMNA mutation leading to HGPS, G608G, does not affect coding sequence but instead creates a cryptic splice site leading to removal of 50 amino acids in the C-terminus of lamin A (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003), resulting in a protein named progerin. A similar splicing mutant has been identified that leads to removal of an extra 40 amino acids (90 total) in a patient diagnosed with RD (Navarro et al., 2004), leading to speculation that RD is a more severe version of HGPS, although the two diseases do not overlap entirely. This splicing event removes the cleavage site for ZMPSTE24, creating a permanently farnesylated protein that likely causes a dominant gain-of-function toxicity. Other mutations in LMNA that do not obviously affect C-terminal splicing lead to HGPS as well as other generally less severe progeroid pathologies (Cao and Hegele, 2003; Chen et al., 2003; Verstraeten et al., 2006). Finally, with regard to LMNA mutations, homozygous loss of lamin A function leads to Charcot-Marie Tooth Syndrome, characterized by loss of peripheral nerve myelination (De Sandre-Giovannoli et al., 2002).
Before leaving A-type lamins, it is worth noting the interesting connections that have arisen with cancer progression (Butin-Israeli et al., 2012). Most laminopathies are not associated with cancer, but an increasing range of tumors are characterized by down-regulation of A-type lamin expression (Broers et al., 1993; Kaufmann, 1992) although results differ in tumor types. Recalling that this family of lamins is expressed in differentiated cells, but not stem cells, speculation has developed that A-type lamins may act as tumor suppressors, perhaps by blocking de-differentiation into a more stem cell-like state. A-type lamins have also been ascribed roles in regulating cell proliferation and the DNA damage response, either of which could be linked to cancer progression (Redwood et al., 2011). Among these activities, A-type lamins are required to stabilize the retinoblastoma tumor suppressor protein (Johnson et al., 2004). This may be relevant because the one tumor described in HGPS patients (the sample size is quite small) is an early onset osteosarcoma (Shalev et al., 2007), one of the most common tumors linked to homozygous mutation of the Rb locus (Friend et al., 1986). Although progerin can stabilize pRb levels (Nitta et al., 2006), the HGPS patient with osteosarcoma had a rare T623S LMNA mutation that has not been tested with regard to pRb stability (Shalev et al., 2007).
Mutations in genes encoding other nuclear envelope proteins are also associated with disease (described in more detail in (Mendez-Lopez and Worman, 2012)). In addition to emerin and LMNA, mutations in SYNE1 and SYNE2 (encoding nesprin-1 and nesprin-2), TMEM43 (encoding LUMA) and TMPO (encoding LAP2alpha) are all associated with dilated cardiomyopathy and muscular dystrophy (Liang et al., 2011; Taylor et al., 2005; Zhang et al., 2007). These genes encode proteins that all interact as part of the linker of nucleoskeleton and cytoskeleton (LINC) complex, suggesting that altered LINC function may underlie striated muscle pathology (Puckelwartz and McNally, 2011). Unrelated SYNE1 and SYNE2 mutations are also linked to autosomal recessive spinocerebellular ataxia type 8 and autosomal recessive arthrogyrposis, respectively (Attali et al., 2009; Gros-Louis et al., 2007). LEMD3, encoding MAN1, an LEM domain containing protein, is also associated with disease, with mutations linked to a series of disorders associated with increased bone density (Hellemans et al., 2004). In addition, mutations in BANF1, encoding the nuclear envelope protein BAF that binds DNA and is involved in chromatin organization and nuclear envelope assembly, are associated with Atypical progeria (Puente et al., 2011).
Not to be left out, LMNB1 and LMNB2 mutations are both linked to rare diseases. Autosomal dominant mutations in LMNB1 lead to adult-onset leukodystrophy, which is characterized by central nervous system demyelination (Padiath et al., 2006). In the case of LMNB2, individuals with heterozygous mutations are susceptible to acquired partial lipodystrophy, likely triggered by one of several autoimmune diseases (Hegele et al., 2006). Finally, the lamin B receptor (LBR), which interacts with B-type lamins and may serve to help link them to the nuclear envelope and chromatin, is also a target for mutation in two syndromes: homozygous mutations in LBR cause Greenberg skeletal dysplasia (Waterham et al., 2003), whereas heterozygous mutations are associated with Pelger-Huet anomaly, a benign condition characterized by altered chromatin organization in granulocytes (Best et al., 2003; Hoffmann et al., 2002). Given the rate of new discoveries of disease association with nuclear structural factors, it is fair to speculate that new diseases will continue to emerge.
Disease mechanisms: Mouse models lead the way
How could altered function of nuclear structural components lead to such a wide range of diseases? In the beginning, there were few connections between lamins and known disease mechanisms; however, lamins were known to be important for a wide range of nuclear functions, including replication and transcription. Many of the initial ideas were based on changes observed at the level of cell biology. For instance, the shape of the nucleus was found to be disrupted in fibroblasts lacking A-type lamins, with enhanced nuclear deformation and sensitivity to mechanical stress (Lammerding et al., 2004). Emerin-deficit cells have similar properties and reduced mechanical stress could explain part of the pathology associated with diseases such as dilated cardiomyopathy and muscular dystrophy, where affected tissues are under regular strain (Lammerding et al., 2005). However, cells isolated from human and mouse tissue from the various laminopathies, all display abnormal nuclear structure. These phenotypes range from abnormal nuclear shape to nuclear blebbing and even dispersal of DNA into the cytoplasm. While these observations may relate to disease, they do not clearly differentiate one laminopathy from another and researchers have turned to more detailed assessments of cellular function to generate more recent hypotheses.
Theories to explain the pathology associated with nuclear structure defects have emerged largely from two areas: (1) an extensive set of mouse models and, more recently, (2) studies of stem cells expressing a range of mutant forms of A-type lamins. An informative starting point for the former was the generation of mice lacking A-type lamins (Sullivan et al., 1999). In addition to being cachexic, these mice present with a subset of the pathologies associated with LMNA mutation, including muscular dystrophy, dilated cardiomyopathy and Charcot-Marie Tooth Syndrome and succumb to the cardiac phenotype at about six weeks of age. Lmna+/− heterozygous mice also develop the cardiac pathology, although at a slower rate, and mice expressing two different LMNA alleles associated with striated muscle disease recapitulate at least some of the human phenotypes (Arimura et al., 2005; Mounkes et al., 2005). One assertion arising from these findings is that the muscle and peripheral myelination diseases result from reduced A-type lamin function. This is not surprising for Charcot-Marie Tooth Syndrome, which is a recessive disorder in humans (De Sandre-Giovannoli et al., 2002). However, both dominant and recessive mutations have been identified in the muscle pathologies and one possibility is that autosomal dominant alleles have a dominant negative effect, interfering with intermediate filament assembly or some other property of A-type lamins. Haploinsufficiency also likely explains the onset of disease in many cases.
It should be noted that the Lmna−/− mouse described originally may in fact not be a null allele of the gene (Sullivan et al., 1999). Recent evidence suggests that this mouse expresses a still incompletely characterized, truncated 54kD protein derived from a splicing event, that bypasses the removed exons (Jahn et al., 2012). While the dust has not settled from this finding, most data suggest that the lamin A variant expressed in this mouse is hypomorphic. Interestingly, another Lmna−/− model has been derived through disruption with a reporter gene and this mouse presents with defective development of heart liver and somites leading to death before weaning (Kubben et al., 2011). This latter mouse is more consistent with a homozygous LMNA nonsense mutation that resulted in the complete absence of A-type lamins and was associated with the death of a newborn patient (van Engelen et al., 2005). Clearly, these findings call for some re-evaluation of studies performed in the Lmna−/− mouse despite its past and continued value to the field.
One recent, highly informative mouse model was engineered to homozygously express a non-farnesylated version of lamin A in the absence of lamin C (Davies et al., 2010). These mice were expected to resemble the phenotype of mice lacking ZMPSTE24 (see below), but instead present with cardiomyopathy. The investigators sought to determine whether the cardiac pathology was attributable to gain-of-function toxicity or a hypomorphic, reduced function of the lamin A variant. To distinguish, they generated a mouse expressing a non-farnesylated allele over a null, finding that this mouse has a more severe phenotype, consistent with further reduced lamin A function. If the pathology were a result of toxicity, the heterozygous mouse would have had a less severe cardiac phenotype. These findings are consistent with the data that cardiomyopathy derives from reduced lamin A function.
While striated muscle pathology represents one cluster of mouse LMNA models, progeria characterizes the other. In this case, the data is generally supportive of a model whereby lamin A variants with processing defects show dominant onset of a subset of features associated with Hutchinson-Gilford progeria syndrome. These models are covered in detail in a recent review (Zhang et al., 2012). Recall that the primary human lesion associated with Hutchinson-Gilford progeria syndrome is a heterozygous G608G mutation that creates a splicing defect and leads to permanently farnesylated lamin A. Of the many different HGPS models, much debate centers around which ones are the best to develop mechanistic explanations and therapies for human patients. Most of the models, including Lmna mutants and Zmpste24−/−, present with a subset of phenotypes characteristic of progeroid mice, including cachexia, reduced bone density and rib fractures, loss of subcutaneous fat, kyphosis, alopecia and premature death. However, a model generated to express human progerin from a BAC clone does not exhibit these phenotypes, instead displaying arterial smooth muscle defects (Varga et al., 2006). While the differences are unknown, both types of models may have advantages. For instance, the BAC progerin model mimics atherosclerosis, which by leading to heart attacks and strokes results in mortality in most patients. Therefore, studies in this mouse explore effects on what may be the most important pathology in children with disease. However, the rapid presentation and wider array of phenotypes in the other mice offer clear advantages as well. Of note, some of the progeria model mice display cardiac defects more consistent with dilated cardiomyopathy (Davies et al., 2010; Yang et al., 2011). One point worth considering is that a LMNA mutation could lead to gain-of-function toxicity for some phenotypes and loss-of-function for others.
In the next two sections, we focus in on the two classes of LMNA-associated disease about which we understand the most: striated muscle disease and progeroid disorders. The exciting progress in these two areas has led to possible therapeutic approaches.
Disease mechanisms and possible therapies for LMNA-associated striated muscle diseases
Interesting findings have emerged on several fronts with respect to LMNA-associated dilated cardiomyopathy with conduction defects and muscular dystrophies. While these findings do not yet come together in a neat package, continued studies may begin to generate such a composite understanding. The fact that LMNA mutants leading to EDMD2/3 so closely resemble X-linked EDMD, caused by Emerin mutations, must be considered for any mechanistic disease model. Unlike A-type lamins, emerins reside in the inner and outer nuclear membranes, interacting with lamins in the former case and with microtubules in the latter. Lamin A/C binding to emerin is required for its localization to the nuclear envelope (Vaughan et al., 2001). This raises the possibility that emerins might be a conduit by which the nuclear lamina communicates with the cytoskeleton. However, no clear understanding has emerged as to how and why the lamin A/C-emerin interaction is important in skeletal and cardiac muscle.
The linker of nucleoskeleton and cytoskeleton (LINC) protein complex, consisting on SUN1 and 2 as well as Nesprin 1 and 2, also connect A-type lamins to the cytoskeleton with Sun proteins directly interacting with lamin A/C at the inner nuclear membrane and Nesprins in the lumen (Mejat and Misteli, 2010). Nesprins cross the outer nuclear membranes and connect to the cytoskeleton in the cytoplasm. In addition to linking the nucleo- and cytoskeleton, LINC complexes have a wide range of cellular functions, including in cell division, centrosome-nucleus association, nuclear migration and positioning. Disruption of any of these activities could contribute to disease progression. An interesting recent study has implicated SUN1 in disease progression, albeit through an unexpected mechanism. In Lmna−/− mice, SUN1 is dramatically overexpressed and directed to the Golgi, presumably after nuclear occupancy sites are saturated (Chen et al., 2012a). RNAi-mediated knockdown of SUN1 rescued nuclear defects in cell culture and knockout of SUN1 significantly extended the survival of Lmna−/− mice. While many questions remain unresolved, this report suggests that one significant problem associated with reduced A-type lamin function is SUN1-mediated toxicity in the Golgi.
Another intermediate filament factor, desmin, serves as a linking factor between lamins and many cytoplasmic structures in striated muscle cells. Desmin mutations can result in desmin-related myopathies (DRM), which are characterized by cardiac and skeletal muscle weakness with a highly variability of presentation. Inherent in DRM at the cellular level is disruption of desmin filaments and accumulation of desmin-containing protein aggregates. Interestingly, cardiomyoctes from Lmna−/− mice display disrupted desmin networks and elevated protein levels (Nikolova et al., 2004). This may not be the case for skeletal muscle as electron micrographs of muscle biopsies from human patients failed to detect abnormal desmin localization (Frock et al., 2012; Piercy et al., 2007). The authors of this study also looked at murine embryonic stem cells transfected with a human EDMD mutation and differentiated into cardiomyocytes, finding no defects in desmin localization. These latter findings appear to differ from the in vivo studies described above and may suggest that knockout of A-type lamins, as opposed to expression of an EDMD missense mutation, is required to induce abnormal desmin localization. Alternatively, the cell culture model may not recapitulate events regarding desmin.
Myoblasts generated from Lmna−/− mice are reported to have differentiation defects, suggesting that reduced regenerative potential of adult stem cells may combine with increased damage to myofibers from mechanical stress sensitivity to explain the rapid onset of dystrophic pathology (Frock et al., 2006). Interestingly, a small percentage of Lmna−/− myoblasts respond normally to differentiation signals whereas a majority fail to induce the differentiation program. The majority of proliferating Lmna−/− myoblasts also display reduced levels of both MyoD and desmin. Stable transfection of desmin rescued the differentiation defects of these cells, implying that reduced desmin levels during the proliferation phase may in part be responsible for the inability of cells to respond to differentiation cues. Stable expression of MyoD also rescued differentiation defects. With respect to EDMD mutations, MyoD transformed human patient fibroblasts were reported to differentiate normally (Piercy et al., 2007). Again, the differences may be attributable to the relative severity of the LMNA mutation or they may have been suppressed in the latter case due to artificially high MyoD levels (Frock et al., 2006; Piercy et al., 2007).
In recent years, it has become apparent that Lmna mutations can lead to altered activation of major signal transduction pathways in the cell (Figure 1). While the mechanisms connecting the nuclear envelope to cell signaling have not been fully elucidated, the findings are important since (1) altered signaling can be linked to pathological progression and (2) in some cases small molecules are available as therapeutic options to correct signaling defects. In cardiac tissue, three different branches of the MAP kinase signaling pathways have been found to be aberrantly activated in a mouse model homozygously expressing the human LMNA H222P mutant associated with dilated cardiomyopathy (Muchir et al., 2007b; Muchir et al., 2012). One of these, the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway was also upregulated in Emerin-deficient mice, while the Jun N-terminal kinase (JNK) pathway was not elevated and the p38α pathway remains to be tested (Muchir et al., 2007a). Elevated ERK1/2 phosphorylation has also been detected in human cancer cells lines where A-type lamin or Emerin expression was inhibited by an siRNA approach and in cardiac tissue from Lmna−/− mice (Frock et al., 2012; Muchir et al., 2009b). In Lmna−/− hearts, aberrant phosphorylation could be corrected by restoration of lamin A expression specifically in cardiomyocytes, indicating that the defects are cell autonomous (Frock et al., 2012). Finally, at least elevated p38α phosphorylation has been detected in heart tissue from human dilated cardiomyopathy patients (Muchir et al., 2012).
Figure 1. Signaling pathways disrupted by LMNA mutations.
Recent years have seen several discoveries of signal transduction pathways that are altered in LMNA mutant backgrounds associated with gain-of-function toxicity, loss-of-function or both. A list of pathways are provided that are described in detail in the text.
A variety of MAP kinase inhibitors have been generated and many have been tested in the clinic for other disease indications. Worman and colleagues have tested several of these in LmnaH222p/H222p mice, finding that inhibition of each branch of the Map kinase pathway either delays onset or progression of cardiac symptoms (Muchir et al., 2009a; Muchir et al., 2012; Wu et al., 2010). Given that some of these inhibitors appear to be relatively well tolerated in humans, these findings lead to a potential therapeutic route for dilated cardiomyopathies associated with LMNA mutation. Potential benefits for muscular dystrophy have not been assessed.
How does LMNA mutation lead to activation of the MAP kinase pathways? While the answer to this question remains to be determined, ideas have emerged. For instance, MAP kinases are known to be activated by mechanical stress and reduced A-type lamin function is associated with impaired activation of mechanosensitive genes in cardiomyocytes. A second, more direct model has potentially emerged that involves direct interaction between ERK1/2 and A-type lamins in the nucleus. ERK1/2 is reported to interact with lamin A and the retinoblastoma protein pRb at the nuclear periphery. Stabilization of pRb by A-type lamins is important to maintain normal cell cycle control (Nitta et al., 2006). Upon serum stimulation of quiescent cells, ERK1/2 phosphorylates c-Fos releasing it to stimulate Ap-1 activation and also dislodges pRb from A-type lamins, leading to pRb phosphorylation and E2F activation (Gonzalez et al., 2008; Ivorra et al., 2006; Rodriguez et al., 2010). It is unclear presently how disruption of the ERK1/2-A-type lamin interaction by LMNA mutation affects ERK1/2 activation but this question needs to be investigated.
Equally unclear are the pathways downstream of MAP kinases that mediate cardiac pathology. Two possibilities have emerged. The first involves an observation that connexins are mis-localized in mice expressing a different mutant associated with DCM (N195K). Here, connexin 43 was found to be mis-localized and not associated with gap junctions, a finding that could explain conduction defects associated with altered A-type lamin function (Mounkes et al., 2005). Expression of another DCM mutant (E82K) was found to lead to downregulation and mis-localization of connexin 43 in neonatal myocytes (Sun et al., 2010). Finally, a recent study has demonstrated mis-localization of connexin 43 in heart cardiomyocytes of Lmna−/− mice (Frock et al., 2012). Re-expression of lamin A rescued aberrant ERK1/2 phosphorylation and restored connexin 43 localization. Given that connexins are known substrates of ERK1/2, the possibility exists that aberrant activity of this pathway disrupts normal connexin43 localization and interferes with cardiac conduction (Chen et al., 2012b).
Two recent studies point to the involvement of another major signal transduction pathway in LMNA-related cardiac and skeletal muscle disease. In Lmna−/− mice, the mTORC1 pathway was found to be upregulated in cardiac and skeletal muscle, leading at least in the heart to impaired autophagy (Ramos et al., 2012). Reduced mTORC1 signaling by the specific kinase inhibitor rapamycin, led to enhanced cardiac function and survival with indications of improved skeletal muscle function, although the latter possibility needs to be more fully explored. A similar study conducted in the LmnaH222P/H222P mouse led to highly overlapping findings, suggesting that aberrant mTORC1 signaling may be a common feature of this class of laminopathies (Choi et al., 2012). Among several upstream activators of mTORC1 are ERK1/2 MAP kinases and one possibility is that increased mTORC1 signaling occurs by this mechanism. However, there are numerous upstream activators of mTORC1 that need to be more fully explored. The possibility of testing rapamycin as a treatment for LMNA-associated DCM is intriguing since the drug has been tested in a wide range of clinical trials and is approved for multiple disease indications. However, there are side effects such as dyslipidemia and impaired insulin signaling that, while generally manageable, must be considered for treatment of cardiac disease. Elevated mTORC1 signaling, which is classically associated with increased protein translation and cell growth, is already linked to forms of cardiac hypertrophy. However, general levels of translation do not appear to be elevated in the Lmna−/− heart (Ramos et al., 2012), suggesting that other pathways are offsetting the translational effects of mTORC1 in this scenario. Interestingly, rapamycin has been reported to improve autophagic flux and suppress nuclear blebbing in fibroblasts expressing progerin, indicating that suppression of the mTOR pathway may be efficacious in LMNA-associated progeria models as well (Cao et al., 2011).
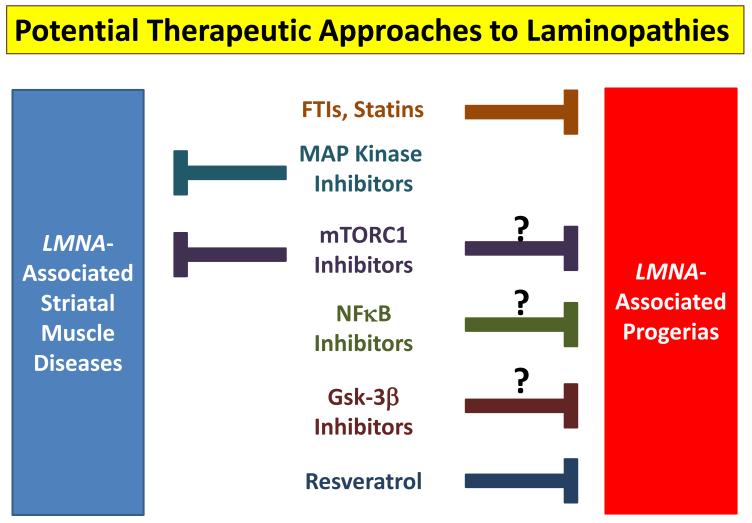
Given the remarkable progress in this cluster of LMNA-associated diseases, it has been possible to move from identification of LMNA mutations in EDMD and DCM to possible therapeutic approaches in under two decades (Figure 2). Whether the current drugs will prove efficacious in humans remains to be seen. Even if this is not the case, new candidate therapeutic approaches will surely continue to emerge.
Figure 2. Potential therapeutic approaches to laminopathies.
Several small molecules have been proposed as treatments for laminopathies. The major ones are listed with arrows indicating the diseases to which they may have efficacy. Question marks indicate that animal data has yet to be presented. Notably, FTIs have been tested in human children with HGPS with promising initial results (Gordon et al., 2012).
Disease mechanisms and possible therapies for LMNA-associated progerias
Although very rare, progeria syndromes have long been of great interest based in part on the hypothesis that by learning the mechanisms underlying their pathology, insights will be made into the normal aging process. This assumption is yet to be validated and researchers in the aging field have a wide range of viewpoints. One thing is clear. The studies into LMNA-associated progerias have yielded major biological insights and provided hope that therapeutic approaches can be developed to slow the impact of these very severe syndromes. In this section, the latest findings in progeria and lamin A processing will be discussed.
A large body of work suggests that HGPS mutants in LMNA at least in part confer toxicity by virtue of being permanently farnesylated. Several deformations of the nucleus were found in cells expressing progerin or other non-farnesylated versions of lamin A, and several studies indicated that these phenotypes could be rescued by a class of drugs that inhibit farnesyltransferases (Young et al., 2006). These drugs were initially generated based on their ability to block Ras farnesylation and the promise that that would inhibit tumor progression. While cancer studies continue, their development has been fortuitous to the study HGPS. Not only do they rescue cellular defects but they have beneficial properties when delivered to HGPS mouse models, extending survival and improving other physiological readouts including bone and cardiovascular defects (Capell et al., 2008; Yang et al., 2008b). These findings, together with the fact that FTIs have good safety profiles in the clinic, were cause for great optimism, leading to the first clinical trial in human patients with HGPS. Initial findings were recently reported, showing variable rates of improvement in vascular function, enhanced bone rigidity and improved sensorineural hearing in 25 patients treated with Ionafarnib for at least two years (Gordon et al., 2012).
One reason FTIs may have limited potency is that lamin a variants can become geranylgeranylated, especially when farnesyltransferase activity is blocked (Varela et al., 2008). This has led to the assumption that blocking the HMG-CoA reductase pathway upstream, in a manner that inhibits both lamin A modifications, might have enhanced efficacy. Consistently, combined treatment of Zmpste24−/− mice with two such agents, statins and aminobisphosphonates, enhances survival and improves several pathologies. Another potential approach has emerged in a mouse that is genetically engineered to have the exact G608G mutation (G609G in mice) (Osorio et al., 2011). As in the human case, alternative splicing leads to progerin production and progeroid phenotypes. Interestingly, treatment of the mice with a morpholino-based therapy therapy that prevents pathogenic splicing, delays pathology and extends survival suggesting an alternative therapeutic approach.
Genetic studies support the toxicity of farnesylated lamin A in progerias. For instance, mice lacking Zmpste24 develop progeroid features linked to the toxicity of an unprocessed lamin A, since deletion of one copy of LMNA in this background improves the range of phenotypes (Fong et al., 2004). Extensive studies by Young and colleagues have further elucidated the role of farnesylation in vivo. Mice engineered to express a non-farnesylated version of progerin still develop progeroid features, albeit at a slower rate (Yang et al., 2008a). However, mice expressing a non-farnesylated version of prelamin A do not develop progeroid features as described earlier (Davies et al., 2010). One possible interpretation of these studies in that farnesylation may be required for toxicity in the case of prelamin A but that the 50 amino acid deletion in progerin also contributes to disease progression.
Several lines of evidence implicate enhanced DNA damage and/or an impaired DNA damage response pathway in the etiology of HGPS. HGPS cells have higher levels of reactive oxygen species and greater rates of basal DNA damage (Viteri et al., 2010). These findings are likely connected since a reduction in ROS by exposure to n-acetylcysteine reduces double strand break formation. These alterations lead in part to enhanced activation of DNA response pathways, including enhanced ATM and RAD3-associated foci, that may adversely affect cell cycle proliferation. An interesting and unusual feature of HGPS cells is persistent basal levels of phosphorylated γH2AX foci marking double strand breaks that also stain positive for Xeroderma pigmentosum group A protein (XPA) (Liu et al., 2008), a component of nucleotide excision repair. No other related factors are upregulated, suggesting that the foci have an abnormal set of repair proteins and the type of DNA damage in HGPS cells may have unique features.
Cells from mice lacking Zmpste24 also exhibit a significant delay in recruitment of 53BP1 to sites of DNA repair after induction of double strand breaks (Liu et al., 2005). p53 targets such as GADD45, p21 and ATF3 were also elevated and deletion of p53 was sufficient to rescue some of the progeroid phenotypes of the Zmpste24−/− mouse (Varela et al., 2005). While p53 targets were not elevated in HGPS fibroblasts, inactivation of the transcription factor was sufficient to suppress premature senescence (Kudlow et al., 2008). More recent data indicates that ATM and NEMO pathways become activated and promote NF-κB dependent inflammation in both Zmpste24−/− and LmnaG609G/G609G mice (Osorio et al., 2012). Genetic and pharmacological interventions of these pathways slow progeroid pathology and enhance survival. These findings are particularly interesting since (1) they suggest that NF-κB inhibitors may be effective therapeutic agents and (2) enhanced inflammation may be a major driving of normal aging processes. Furthermore, the tissues affected by altered lamin A processing have remained unresolved. Progeria involves systemic pathology and one possibility is that defects in every tissue cause cell autonomous phenotypes. More likely, defects in a smaller set of tissues lead to systemic responses that impact the whole organism. Enhanced NF-κB signaling could mediate such a systemic effect.
A more straightforward approach to understanding the role of A-type lamins in DNA damage responses may involve loss-of-function studies. In contrast to progeroid models, loss of A-type lamins leads to 53BP1 degradation by the proteasome (Gonzalez-Suarez et al., 2009). In its absence, repair of double strand breaks proceeds more slowly, hindering effective non-homologous end joining (Redwood et al., 2011). Homologous recombination is also compromised through a transcriptional mechanism by which enhanced proteasome dependent degradation of pRb and p107 leads to repression of RAD51 and BRCA1 (Redwood et al., 2011). It remains unclear why enhanced protein turnover of pRb and 53BP1 occur in the absence of A-type lamins, but the hypothesis has been put forward that A-type lamins may have a general role in promoting the stability of several nuclear regulatory factors through keeping proteasome-dependent degradation in check (Parnaik et al., 2011). It should also be noted that many of these properties may explain why loss of A-type lamin expression could have tumor promoting properties.
In addition to impaired DNA damage response pathways, telomere dysregulation may also contribute to progeroid pathology. HGPS fibroblasts in culture experience faster telomere shortening and progerin expression in normal fibroblasts recapitulates this phenotype, as well as enhancing formation of signal free ends (Decker et al., 2009). Enhanced telomere attrition may contribute to proliferation defects and early senescence, since telomerase expression restores both properties in fibroblasts (Benson et al., 2010; Kudlow et al., 2008). One role of telomerase may be to enhance resolution of DNA damage foci, which were found to localize in regions near telomeres (Benson et al., 2010). The mechanisms by which this might occur and the extent to which altered telomere dynamics promotes progeroid pathology remains to be determined.
Given that HGPS (and other laminopathies) primarily affect tissues of mesenchymal origin, altered mesenchymal stem cell function may be a major site of progerin-induced dysfunction. Gene expression profiling in fibroblasts expressing progerin provide support for this assertion as Notch signaling was found to be highly enhanced (Scaffidi and Misteli, 2008). Elevated Notch activity associated with progerin expression was found to promote expression of a range of differentiation markers in human mesenchymal stem cells. As a possible mechanism, progerin was found to disrupt nuclear matrix association of SKIP, a co-activator of Notch genes, leading to its release into the nucleoplasm and activation of targets. Reduced mesenchymal stem cell function could promote a subset of progeroid phenotypes in vivo, but this remains to be tested.
The Wnt/β-catenin pathway is altered in a variety of laminopathies as well. In both Zmpste24−/− and HGPS mice, reduced β-catenin levels were detected and cell proliferation defects could be rescued by inhibition of Gsk-3β, leading to β-catenin stabilization (Espada et al., 2008; Hernandez et al., 2010). Notably the Wnt pathway may be disrupted in mice lacking emerin as well (Markiewicz et al., 2006; Tilgner et al., 2009). Given that the Wnt pathway may have critical roles in maintaining adult stem cell function with age, the role of this pathway in laminopathies needs further interrogation.
Adult stem cells may also be impaired in progeroid laminopathies due to impaired SIRT1 function. A recent study has demonstrated that A-type lamins interact with the protein deacetylase and that preprocessed lamin A disrupts this association in Zmpste24−/− cells, leading to reduced deacetylase activity and rapid in vivo stem cell depletion (Liu et al., 2012). Treatment of mice with resveratrol restores SIRT1 activity, reduces the pathology and extends survival, indicating that enhancing SIRT1 activity may be another therapeutic approach in progeroid disorders associated with LMNA mutation.
Conclusions
Since the identification of diseases caused by mutations in genes encoding for nuclear lamina proteins in 1999, research has been dedicated toward understanding the molecular mechanisms leading to these specific phenotypes. Understanding how the nuclear lamina interacts with structural proteins, chromatin, transcription factors, and other signaling partners will likely give us an understanding of mechanistic links to disease. At this moment, the puzzle is starting to come together, but the overall picture of how lamins regulate all of these pathways and how this regulation leads to disease is still underway. Understanding the mechanisms by which mutations in lamins cause these rare diseases will provide molecular insight into other common conditions that laminopathies model (such as muscle diseases, cardiomyopathy, aging?). Additionally, since mutations in the nuclear lamina result in rapid aging-like disease, defining the role of the nuclear lamina in regulating normal human longevity will be of great importance.
Acknowledgments
The authors would like to apologize to those scientists whose studies were not referenced due to space limitations and also acknowledge the editorial contributions of Juniper Pennypacker. Lamin-related research in the lab of B.K.K. is supported by a grant from the National Institute of Aging (R01 AG024287). K.H.S. is supported by a Ruth L. Kirschstein NRSA Postdoctoral Fellowship. B.K.K. is an Ellison Medical Foundation Senior Scholar in Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, Fromes Y, Toussaint M, Mura AM, Keller DI, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Human molecular genetics. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, Glick B, Nevo Y, Ben-Neriah Z, Melki J. Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Human molecular genetics. 2009;18:3462–3469. doi: 10.1093/hmg/ddp290. [DOI] [PubMed] [Google Scholar]
- Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci. 2010;123:2605–2612. doi: 10.1242/jcs.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best S, Salvati F, Kallo J, Garner C, Height S, Thein SL, Rees DC. Lamin B-receptor mutations in Pelger-Huet anomaly. British journal of haematology. 2003;123:542–544. doi: 10.1046/j.1365-2141.2003.04621.x. [DOI] [PubMed] [Google Scholar]
- Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- Broers JL, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS, Ramaekers FC. Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am J Pathol. 1993;143:211–220. [PMC free article] [PubMed] [Google Scholar]
- Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends in genetics : TIG. 2012;28:464–471. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Hegele RA. LMNA is mutated in Hutchinson-Gilford progeria (MIM 176670) but not in Wiedemann-Rautenstrauch progeroid syndrome (MIM 264090) J Hum Genet. 2003;48:271–274. doi: 10.1007/s10038-003-0025-3. [DOI] [PubMed] [Google Scholar]
- Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Science translational medicine. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, Conneely KN, Qu X, San H, Ganesh SK, et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15902–15907. doi: 10.1073/pnas.0807840105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux F, Dubosclard E, Lascols O, Buendia B, Chazouilleres O, Cohen A, Courvalin J-C, Laroche L, Capeau J, Vigouroux C, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88:1006–1013. doi: 10.1210/jc.2002-021506. [DOI] [PubMed] [Google Scholar]
- Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Stewart CL, Jeang KT. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell. 2012a;149:565–577. doi: 10.1016/j.cell.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, Botha EG, Garg A, Hanson NB, Martin GM, et al. LMNA mutations in atypical Werner’s syndrome. Lancet. 2003;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- Chen SC, Kennedy BK, Lampe PD. Phosphorylation of connexin43 on S279/282 may contribute to laminopathy-associated conduction defects. Exp Cell Res. 2012b doi: 10.1016/j.yexcr.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JC, Muchir A, Wu W, Iwata S, Homma S, Morrow JP, Worman HJ. Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Science translational medicine. 2012;4:144ra102. doi: 10.1126/scitranslmed.3003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BS, Barnes RH, 2nd, Tu Y, Ren S, Andres DA, Spielmann HP, Lammerding J, Wang Y, Young SG, Fong LG. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Human molecular genetics. 2010;19:2682–2694. doi: 10.1093/hmg/ddq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BS, Coffinier C, Yang SH, Barnes RH, 2nd, Jung HJ, Young SG, Fong LG. Investigating the purpose of prelamin A processing. Nucleus. 2011;2:4–9. doi: 10.4161/nucl.2.1.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, et al. Lamin A truncation in Hutchison-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, Tazir M, Kassouri N, Szepetowski P, Hammadouche T, Vandenberghe A, Stewart CL, et al. Homozygous defects in LMNA, encoding lamin A/C nuclear envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet. 2002;70:726–736. doi: 10.1086/339274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker ML, Chavez E, Vulto I, Lansdorp PM. Telomere length in Hutchinson-Gilford progeria syndrome. Mechanisms of ageing and development. 2009;130:377–383. doi: 10.1016/j.mad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et al. Recurrent de novo point mutations in lamin A cause Hutchison-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada J, Varela I, Flores I, Ugalde AP, Cadinanos J, Pendas AM, Stewart CL, Tryggvason K, Blasco MA, Freije JM, et al. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J Cell Biol. 2008;181:27–35. doi: 10.1083/jcb.200801096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJJ, Spudich S, De Girolami U, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- Fong LG, Ng JK, Meta M, Cote N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, et al. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Frock RL, Chen SC, Da DF, Frett E, Lau C, Brown C, Pak DN, Wang Y, Muchir A, Worman HJ, et al. Cardiomyocyte-specific expression of lamin a improves cardiac function in Lmna-/- mice. PloS one. 2012;7:e42918. doi: 10.1371/journal.pone.0042918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978;79:546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. Journal of structural biology. 2012;177:24–31. doi: 10.1016/j.jsb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol. 2008;183:653–666. doi: 10.1083/jcb.200805049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16666–16671. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet. 2007;39:80–85. doi: 10.1038/ng1927. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, Rodger NW, Durrington PN. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. American journal of human genetics. 2006;79:383–389. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, Janssens K, Menten B, Van Roy N, Vermeulen SJ, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- Hennekes H, Nigg EA. The role of isoprenylation in membrane attachment of nuclear lamins. A single point mutation prevents proteolytic cleavage of the lamin A precursor and confers membrane binding properties. J Cell Sci. 1994;107(Pt 4):1019–1029. doi: 10.1242/jcs.107.4.1019. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Roux KJ, Wong ES, Mounkes LC, Mutalif R, Navasankari R, Rai B, Cool S, Jeong JW, Wang H, et al. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Developmental cell. 2010;19:413–425. doi: 10.1016/j.devcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Dreger CK, Olins AL, Olins DE, Shultz LD, Lucke B, Karl H, Kaps R, Muller D, Vaya A, et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huet anomaly) Nat Genet. 2002;31:410–414. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O’Connor JE, Burke B, Andres V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn D, Schramm S, Schnolzer M, Heilmann CJ, de Koster CG, Schutz W, Benavente R, Alsheimer M. A truncated lamin A in the Lmna −/− mouse line: implications for the understanding of laminopathies. Nucleus. 2012;3:463–474. doi: 10.4161/nucl.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci, USA. 2004;101:9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH. Expression of nuclear envelope lamins A and C in human myeloid leukemias. Cancer Res. 1992;52:2847–2853. [PubMed] [Google Scholar]
- Kubben N, Voncken JW, Konings G, van Weeghel M, van den Hoogenhof MM, Gijbels M, van Erk A, Schoonderwoerd K, van den Bosch B, Dahlmans V, et al. Post-natal myogenic and adipogenic developmental: defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2011;2:195–207. doi: 10.4161/nucl.2.3.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlow BA, Stanfel MN, Burtner CR, Johnston ED, Kennedy BK. Suppression of proliferative defects associated with processing-defective lamin A mutants by hTERT or inactivation of p53. Molecular biology of the cell. 2008;19:5238–5248. doi: 10.1091/mbc.E08-05-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Chang SY, Trinh H, Tu Y, White AC, Davies BS, Bergo MO, Fong LG, Lowry WE, Young SG. Genetic studies on the functional relevance of the protein prenyltransferases in skin keratinocytes. Human molecular genetics. 2010;19:1603–1617. doi: 10.1093/hmg/ddq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WC, Mitsuhashi H, Keduka E, Nonaka I, Noguchi S, Nishino I, Hayashi YK. TMEM43 mutations in Emery-Dreifuss muscular dystrophy-related myopathy. Annals of neurology. 2011;69:1005–1013. doi: 10.1002/ana.22338. [DOI] [PubMed] [Google Scholar]
- Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, et al. Resveratrol Rescues SIRT1-Dependent Adult Stem Cell Decline and Alleviates Progeroid Features in Laminopathy-Based Progeria. Cell metabolism. 2012;16:738–750. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, et al. Genomic instability in laminopathy-based premature aging. Nature medicine. 2005;11:780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Rusinol AE, Sinensky MS, Liu J, Shell SM, Zou Y. Involvement of xeroderma pigmentosum group A (XPA) in progeria arising from defective maturation of prelamin A. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:603–611. doi: 10.1096/fj.07-8598com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejat A, Misteli T. LINC complexes in health and disease. Nucleus. 2010;1:40–52. doi: 10.4161/nucl.1.1.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Lopez I, Worman HJ. Inner nuclear membrane proteins: impact on human disease. Chromosoma. 2012;121:153–167. doi: 10.1007/s00412-012-0360-2. [DOI] [PubMed] [Google Scholar]
- Mounkes LC, Kozlov SV, Rottman JN, Stewart CL. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Human molecular genetics. 2005;14:2167–2180. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- Muchir A, Bonne G, van der Kooi AJ, van Meegan M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamin A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- Muchir A, Pavlidis P, Bonne G, Hayashi YK, Worman HJ. Activation of MAPK in hearts of EMD null mice: similarities between mouse models of X-linked and autosomal dominant Emery Dreifuss muscular dystrophy. Human molecular genetics. 2007a;16:1884–1895. doi: 10.1093/hmg/ddm137. [DOI] [PubMed] [Google Scholar]
- Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, Bonne G, Worman HJ. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007b;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Human molecular genetics. 2009a;18:241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Wu W, Choi JC, Iwata S, Morrow J, Homma S, Worman HJ. Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Human molecular genetics. 2012;21:4325–4333. doi: 10.1093/hmg/dds265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Wu W, Worman HJ. Reduced expression of A-type lamins and emerin activates extracellular signal-regulated kinase in cultured cells. Biochimica et biophysica acta. 2009b;1792:75–81. doi: 10.1016/j.bbadis.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C, De Sandre-Giovannoli A, Bernard R, Boccaccio I, Boyer A, Genevieve D, Hadj-Rabia S, Gaudy-Marqueste C, Smith HS, Vabres P, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganisation and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- Navarro CL, Cadinanos J, De Sandre-Giovannoli A, Bernard R, Courrier S, Boccaccio I, Boyer A, Kleijer WJ, Wagner A, Giuliano F, et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Human molecular genetics. 2005;14:1503–1513. doi: 10.1093/hmg/ddi159. [DOI] [PubMed] [Google Scholar]
- Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta RT, Jameson SA, Kudlow BA, Conlan LA, Kennedy BK. Stabilization of the retinoblastoma protein by A-type nuclear lamins is required for INK4A-mediated cell cycle arrest. Mol Cell Biol. 2006;26:5360–5372. doi: 10.1128/MCB.02464-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D’Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;7:426–431. doi: 10.1086/341908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio FG, Barcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JM, Lopez-Otin C. Nuclear lamina defects cause ATM-dependent NF-kappaB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012;26:2311–2324. doi: 10.1101/gad.197954.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio FG, Navarro CL, Cadinanos J, Lopez-Mejia IC, Quiros PM, Bartoli C, Rivera J, Tazi J, Guzman G, Varela I, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Science translational medicine. 2011;3:106ra107. doi: 10.1126/scitranslmed.3002847. [DOI] [PubMed] [Google Scholar]
- Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- Parnaik VK, Chaturvedi P, Muralikrishna B. Lamins, laminopathies and disease mechanisms: possible role for proteasomal degradation of key regulatory proteins. Journal of biosciences. 2011;36:471–479. doi: 10.1007/s12038-011-9085-2. [DOI] [PubMed] [Google Scholar]
- Piercy RJ, Zhou H, Feng L, Pombo A, Muntoni F, Brown SC. Desmin immunolocalisation in autosomal dominant Emery-Dreifuss muscular dystrophy. Neuromuscular disorders : NMD. 2007;17:297–305. doi: 10.1016/j.nmd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Puckelwartz M, McNally EM. Emery-Dreifuss muscular dystrophy. Handbook of clinical neurology / edited by PJ Vinken and GW Bruyn. 2011;101:155–166. doi: 10.1016/B978-0-08-045031-5.00012-8. [DOI] [PubMed] [Google Scholar]
- Puente XS, Quesada V, Osorio FG, Cabanillas R, Cadinanos J, Fraile JM. Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am J Hum Genet. 2011;88:650–656. doi: 10.1016/j.ajhg.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano-Roy S, Mbieleu B, Bonnemann CG, Jeannet PY, Colomer J, Clarke NF, Cuisset JM, Roper H, De Meirleir L, D’Amico A, et al. De novo LMNA mutations cause a new form of congenital muscular dystrophy. Annals of neurology. 2008;64:177–186. doi: 10.1002/ana.21417. [DOI] [PubMed] [Google Scholar]
- Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, Mackay VL, An EH, Strong R, Ladiges WC, et al. Rapamycin Reverses Elevated mTORC1 Signaling in Lamin A/C-Deficient Mice, Rescues Cardiac and Skeletal Muscle Function, and Extends Survival. Science translational medicine. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood AB, Perkins SM, Vanderwaal RP, Feng Z, Biehl KJ, Gonzalez-Suarez I, Morgado-Palacin L, Shi W, Sage J, Roti-Roti JL, et al. A dual role for A-type lamins in DNA double-strand break repair. Cell cycle. 2011;10:2549–2560. doi: 10.4161/cc.10.15.16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou L, Stora S, Yaou RB, Volk M, Sinkovec M, Demay L, Richard P, Peterlin B, Bonne G. Heart-hand syndrome of Slovenian type: a new kind of laminopathy. Journal of medical genetics. 2008;45:666–671. doi: 10.1136/jmg.2008.060020. [DOI] [PubMed] [Google Scholar]
- Rober RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Calvo F, Gonzalez JM, Casar B, Andres V, Crespo P. ERK1/2 MAP kinases promote cell cycle entry by rapid, kinase-independent disruption of retinoblastoma-lamin A complexes. J Cell Biol. 2010;191:967–979. doi: 10.1083/jcb.201004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24:153–156. doi: 10.1038/72807. [DOI] [PubMed] [Google Scholar]
- Shalev SA, De Sandre-Giovannoli A, Shani AA, Levy N. An association of Hutchinson-Gilford progeria and malignancy. Am J Med Genet A. 2007;143A:1821–1826. doi: 10.1002/ajmg.a.31803. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LP, Wang L, Wang H, Zhang YH, Pu JL. Connexin 43 remodeling induced by LMNA gene mutation Glu82Lys in familial dilated cardiomyopathy with atrial ventricular block. Chinese medical journal. 2010;123:1058–1062. [PubMed] [Google Scholar]
- Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR, Carniel E, Di Lenarda A, Sinagra G, Boucek MM, et al. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Human mutation. 2005;26:566–574. doi: 10.1002/humu.20250. [DOI] [PubMed] [Google Scholar]
- Tilgner K, Wojciechowicz K, Jahoda C, Hutchison C, Markiewicz E. Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of beta-catenin. J Cell Sci. 2009;122:401–413. doi: 10.1242/jcs.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen BG, Muchir A, Hutchison CJ, van der Kooi AJ, Bonne G, Lammens M. The lethal phenotype of a homozygous nonsense mutation in the lamin A/C gene. Neurology. 2005;64:374–376. doi: 10.1212/01.WNL.0000149763.15180.00. [DOI] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- Varela I, Pereira S, Ugalde AP, Navarro CL, Suarez MF, Cau P, Cadinanos J, Osorio FG, Foray N, Cobo J, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nature medicine. 2008;14:767–772. doi: 10.1038/nm1786. [DOI] [PubMed] [Google Scholar]
- Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, Capell BC, Cheng J, Faddah D, Perkins S, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, Wehnert M, Morris GE, Whitfield WGF, Hutchison CJ. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- Verstraeten VL, Broers JL, van Steensel MA, Zinn-Justin S, Ramaekers FC, Steijlen PM, Kamps M, Kuijpers HJ, Merckx D, Smeets HJ, et al. Compound heterozygosity for mutations in LMNA causes a progeria syndrome without prelamin A accumulation. Human molecular genetics. 2006;15:2509–2522. doi: 10.1093/hmg/ddl172. [DOI] [PubMed] [Google Scholar]
- Viteri G, Chung YW, Stadtman ER. Effect of progerin on the accumulation of oxidized proteins in fibroblasts from Hutchinson Gilford progeria patients. Mechanisms of ageing and development. 2010;131:2–8. doi: 10.1016/j.mad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, Mooyer P, Noort Gv G, Kelley RI, Wilcox WR, Wanders RJ, Hennekam RC, Oosterwijk JC. Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3 beta-hydroxysterol delta 14-reductase deficiency due to mutations in the lamin B receptor gene. American journal of human genetics. 2003;72:1013–1017. doi: 10.1086/373938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Plessman U, Traub P. Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett. 1989;257:411–414. doi: 10.1016/0014-5793(89)81584-4. [DOI] [PubMed] [Google Scholar]
- Wu W, Shan J, Bonne G, Worman HJ, Muchir A. Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Biochimica et biophysica acta. 2010;1802:632–638. doi: 10.1016/j.bbadis.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Andres DA, Spielmann HP, Young SG, Fong LG. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J Clin Invest. 2008a;118:3291–3300. doi: 10.1172/JCI35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Chang SY, Ren S, Wang Y, Andres DA, Spielmann HP, Fong LG, Young SG. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Human molecular genetics. 2011;20:436–444. doi: 10.1093/hmg/ddq490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Qiao X, Fong LG, Young SG. Treatment with a farnesyltransferase inhibitor improves survival in mice with a Hutchinson-Gilford progeria syndrome mutation. Biochimica et biophysica acta. 2008b;1781:36–39. doi: 10.1016/j.bbalip.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SG, Meta M, Yang SH, Fong LG. Prelamin A farnesylation and progeroid syndromes. The Journal of biological chemistry. 2006;281:39741–39745. doi: 10.1074/jbc.R600033200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kieckhaefer JE, Cao K. Mouse models of laminopathies. Aging cell. 2012 doi: 10.1111/acel.12021. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Human molecular genetics. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]