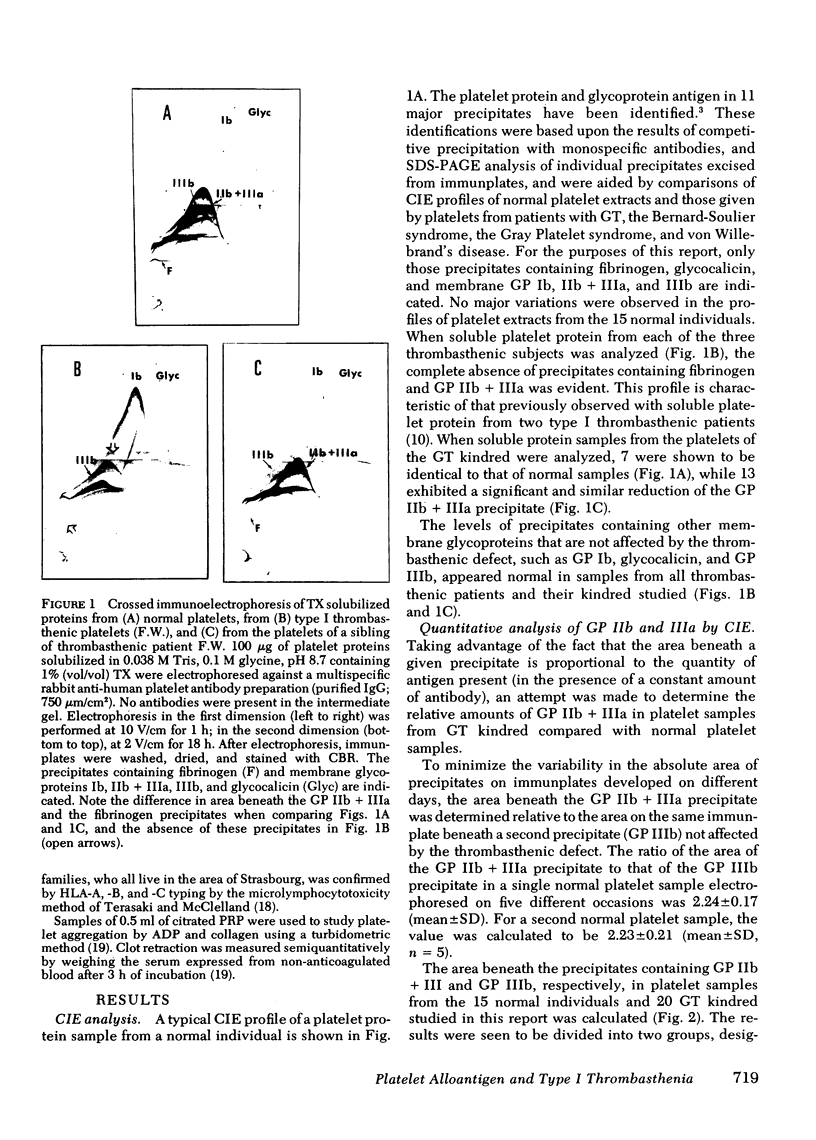
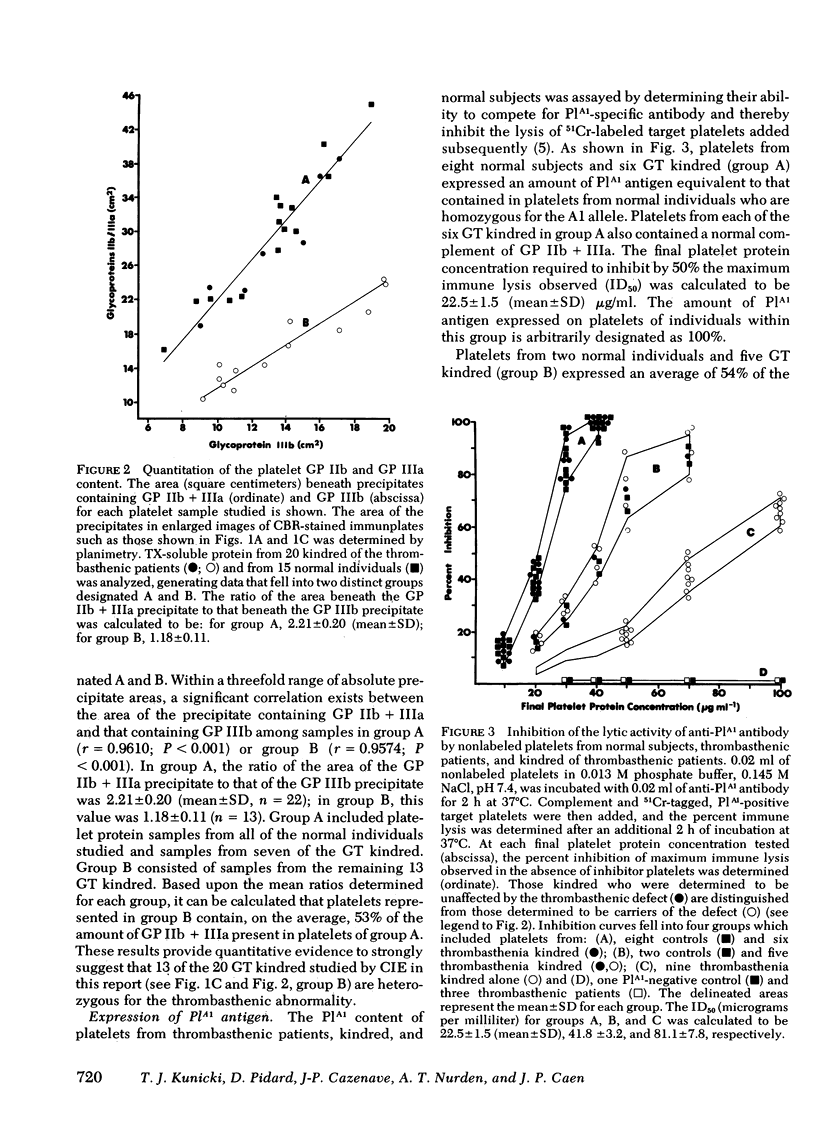
Abstract
The hereditary of the human platelet alloantigen, PlA1, has been studied in Glanzmann's thrombasthenia. The PlA1 content of platelets from three patients, 20 kindred of these patients, including parents and siblings, and 15 unrelated normal individuals was determined using immunologic techniques based on the release of 51Cr from labeled platelets. The amount of membrane glycoproteins (GP) IIb and IIIa in the platelets of these individuals was determined by quantitative crossed immunoelectrophoresis of Triton X-100 soluble proteins using a multispecific rabbit antibody raised against normal platelets. Platelets from the three thrombasthenic patients contained neither detectable GP IIb and GP IIIa nor detectable PlA1 antigen. Platelets from seven kindred with normal amounts of GP IIb and GP IIIa contained PlA1 antigen levels identical to those detected in platelets of normal individuals. Platelets from 13 kindred, including each parent studied, were shown to contain an amount of GP IIb and GP IIIa equivalent to 53% of that amount detected on normal platelets. Platelets from the same individuals expressed amounts of PlA1 antigen that were either 54.0 +/- 4.1 (mean +/- SD) or 28.0 +/- 2.7% of that present on platelets of normal individuals homozygous for the Al allele. The results presented in this report provide evidence that the expression of the thrombasthenic glycoprotein abnormality and the inheritance of PlA1 antigen are controlled by different genes. These results further suggest that lack of expression of the PlA1 antigen on thrombasthenic platelets results from the decrease or absence of the glycoprotein carrier of the PlA1 determinant, previously shown to be GP IIIa.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cazenave J-P, Guccione M. A., Packham M. A., Mustard J. F. Effects of cephalothin and penicillin G on platelet function in vitro. Br J Haematol. 1977 Jan;35(1):135–152. doi: 10.1111/j.1365-2141.1977.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Degos L., Dautigny A., Brouet J. C., Colombani M., Ardaillou N., Caen J. P., Colombani J. A molecular defect in thrombasthenic platelets. J Clin Invest. 1975 Jul;56(1):236–240. doi: 10.1172/JCI108074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen I., Nurden A., Bjerrum O. J., Solum N. O., Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980 Mar;65(3):722–731. doi: 10.1172/JCI109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen I., Solum N. O. Further studies on the protein composition and surface structure of normal platelets and platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. Thromb Res. 1978 Nov;13(5):845–855. doi: 10.1016/0049-3848(78)90189-5. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hardisty R. M. Disorders of platelet function. Br Med Bull. 1977 Sep;33(3):207–212. doi: 10.1093/oxfordjournals.bmb.a071437. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Aster R. H. Deletion of the platelet-specific alloantigen PlA1 from platelets in Glanzmann's thrombasthenia. J Clin Invest. 1978 May;61(5):1225–1231. doi: 10.1172/JCI109038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Aster R. H. Isolation and immunologic characterization of the human platelet alloantigen, P1A1. Mol Immunol. 1979 Jun;16(6):353–360. doi: 10.1016/0161-5890(79)90100-7. [DOI] [PubMed] [Google Scholar]
- Lévy J. M., Mayer G., Sacrez R., Ruff R., Francfort J. J., Rodier L. Thrombasthénie de Glanzmann-Naegeli. Etude d'un groupe ethnique à forte endogamie. Ann Pediatr (Paris) 1971 Feb 2;18(2):129–137. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Muller J. Y., Patereau C., Soulier J. P. Thrombasthénie de Glanzmann antigène PLA1 et anticorps anti-Glanzmann. Rev Fr Transfus Immunohematol. 1978 Dec;21(5):1069–1078. doi: 10.1016/s0338-4535(78)80004-x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974 Oct;28(2):253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. The different glycoprotein abnormalities in thrombasthenic and Bernard-Soulier platelets. Semin Hematol. 1979 Jul;16(3):234–250. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- SHULMAN N. R., MARDER V. J., HILLER M. C., COLLIER E. M. PLATELET AND LEUKOCYTE ISOANTIGENS AND THEIR ANTIBODIES: SEROLOGIC PHYSIOLOGIC AND CLINICAL STUDIES. Prog Hematol. 1964;4:222–304. [PubMed] [Google Scholar]
- Shulman N. R., Aster R. H., Leitner A., Hiller M. C. IMMUNOREACTIONS INVOLVING PLATELETS. V. POST-TRANSFUSION PURPURA DUE TO A COMPLEMENT-FIXING ANTIBODY AGAINST A GENETICALLY CONTROLLED PLATELET ANTIGEN. A PROPOSED MECHANISM FOR THROMBOCYTOPENIA AND ITS RELEVANCE IN "AUTOIMMUNITY". J Clin Invest. 1961 Sep;40(9):1597–1620. doi: 10.1172/JCI104383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. Diminished major antigen in Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Biol Chem. 1980 May 10;255(9):4320–4327. [PubMed] [Google Scholar]
- TERASAKI P. I., MCCLELLAND J. D. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964 Dec 5;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- VAN DER WEERDT C. M., VEENHOVEN-VONRIESZ L. E., NIJENHUIS L. E., VAN LOGHEM J. THE ZW BLOOD GROUP SYSTEM IN PLATELETS. Vox Sang. 1963 Sep-Oct;8:513–530. doi: 10.1111/j.1423-0410.1963.tb04179.x. [DOI] [PubMed] [Google Scholar]
- VAN LOGHEM JJ J., DORFMEIJER H., VAN HART M., SCHREUDER F. Serological and genetical studies on a platelet antigen (Zw). Vox Sang. 1959 Apr;4(2):161–169. doi: 10.1111/j.1423-0410.1959.tb04032.x. [DOI] [PubMed] [Google Scholar]