Abstract
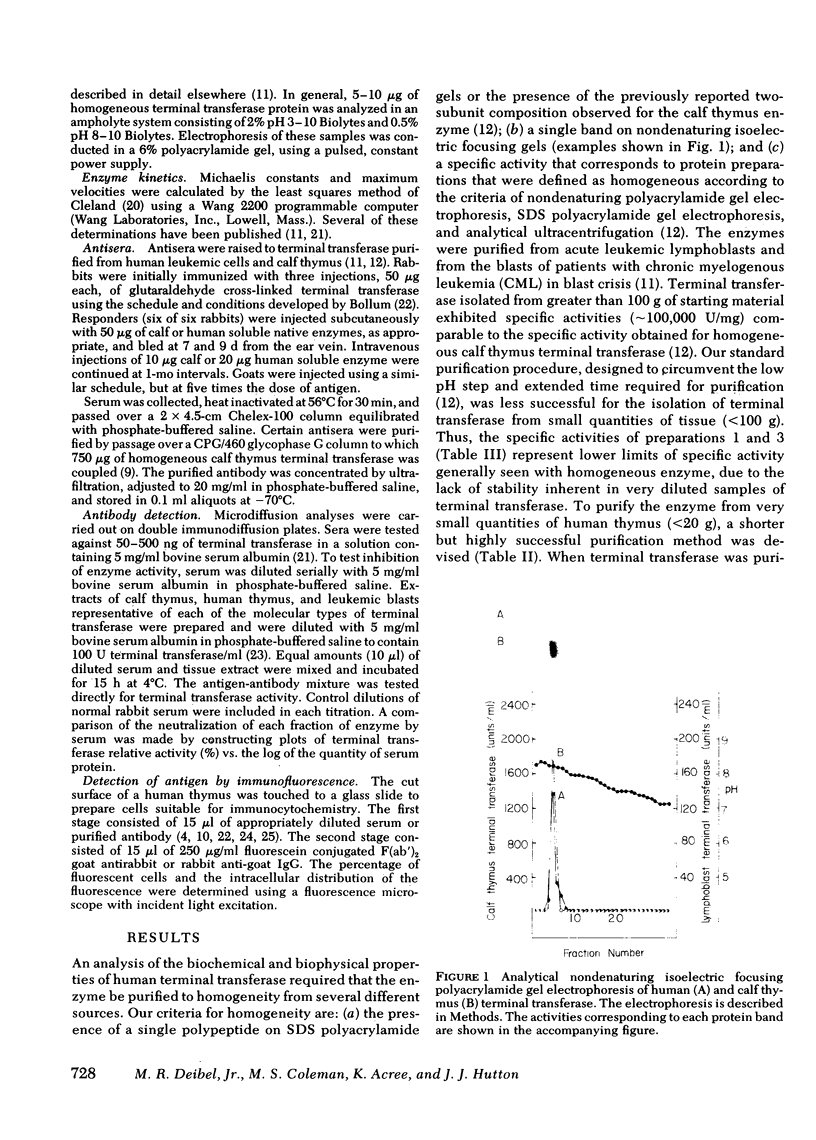
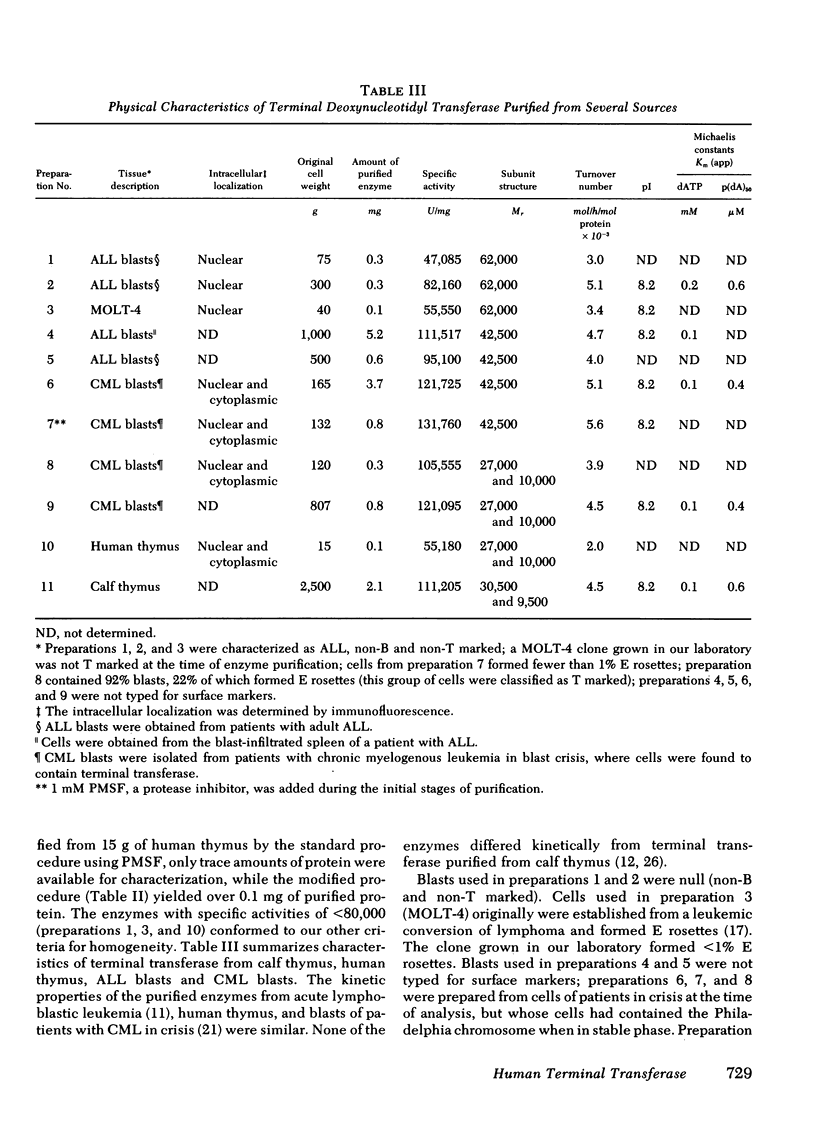
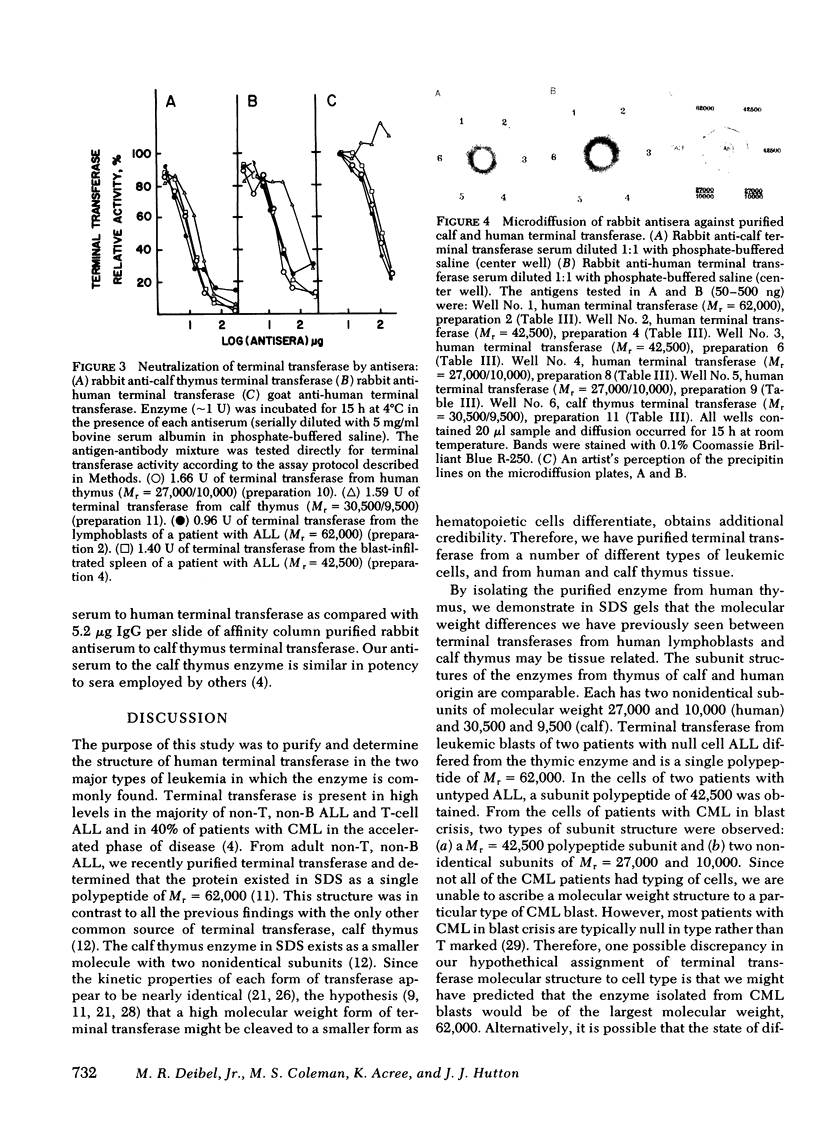
Terminal deoxynucleotidyl transferase was purified to homogeneity from the blasts of eight patients with leukemia and compared with purified transferase from normal human and calf thymus. In two cases phenylmethanesulfonylfluoride was added during purification to reduce proteolysis. Comparative kinetic analyses of the purified enzymes indicated no differences in catalytic properties. There was substantial variation in the molecular structure of terminal transferase on denaturing polyacrylamide gels: (a) a protein that migrated as a single polypeptide with Mr = 62,000 was isolated from two patients with acute lymphoblastic leukemia and from MOLT-4 cells; (b) a protein that migrated as a single polypeptide with Mr = 42,500 was isolated from two patients with acute lymphoblastic leukemia; (c) a protein that migrated as a single polypeptide with Mr = 42,500 was isolated from two patients with chronic myelogenous leukemia in blast crisis; (d) a protein that migrated as two non-identical subunits of Mr = 27,000 and 10,000, respectively, was isolated from two additional patients with chronic myelogenous leukemia in blast crisis. The subunit structure of d is characteristic of the homogeneous enzymes purified from human and calf thymus. Neutralizing and precipitating antibodies to terminal transferase from human lymphoblasts and calf thymus have been produced in rabbits and goats. Antisera directed against either human or calf antigens neutralize enzymatic activity and precipitate all forms of human terminal transferase. The multiple human forms give reactions of antigenic identity by immunodiffusion, but differ antigenically from the calf enzyme. The multiple forms of terminal transferase could represent physiological processing, artifactual degradation, or isozymes coded by several genes.
Full text
PDF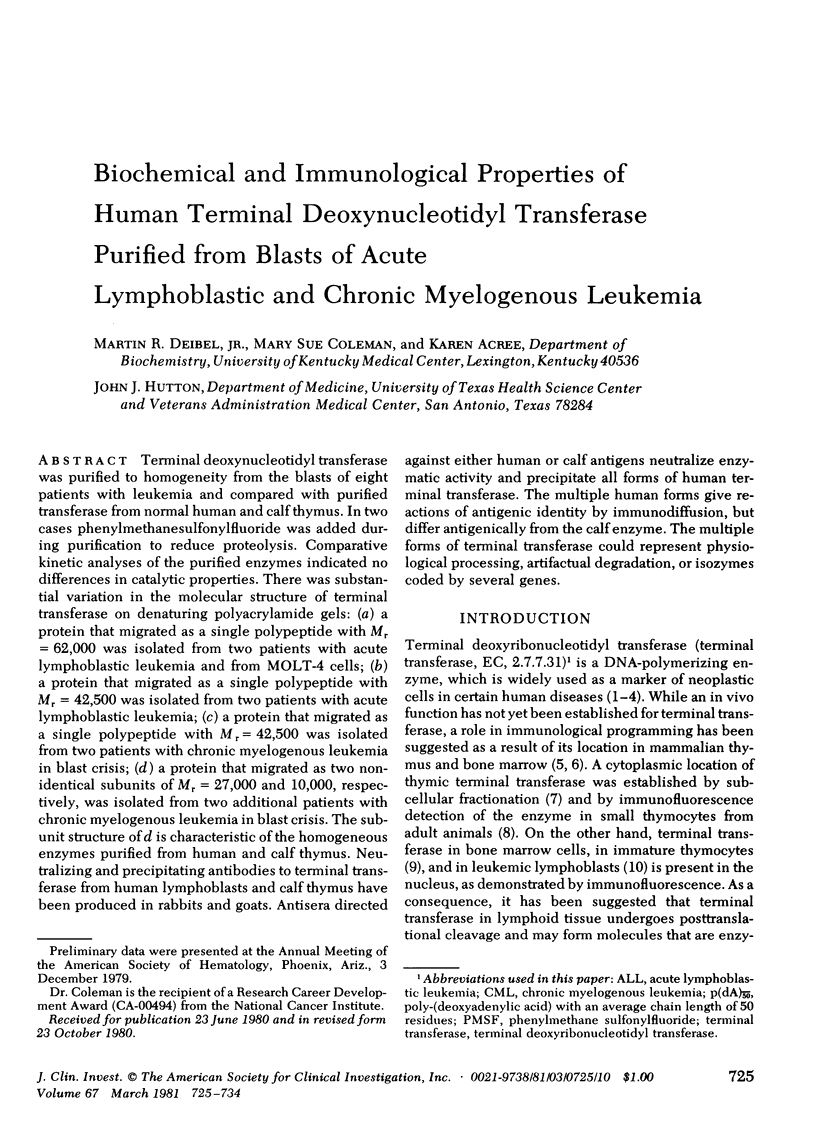






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Is terminal deoxynucleotidyl transferase a somatic mutagen in lymphocytes? Nature. 1974 Mar 29;248(447):409–411. doi: 10.1038/248409a0. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4119–4122. doi: 10.1073/pnas.72.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollum F. J., Brown M. A high molecular weight form of terminal deoxynucleotidyl transferase. Nature. 1979 Mar 8;278(5700):191–192. doi: 10.1038/278191a0. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase as a hematopoietic cell marker. Blood. 1979 Dec;54(6):1203–1215. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Doxynucleotide-polymerizing enzymes of calf thymus gland. IV. Inhibition of terminal deoxynucleotidyl transferase by metal ligands. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1041–1048. doi: 10.1073/pnas.65.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M. Development of terminal deoxynucleotidyl transferase activity in embryonic calf thymus gland. Biochem Biophys Res Commun. 1971 Jul 2;44(1):124–131. doi: 10.1016/s0006-291x(71)80167-5. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Coleman M. S. A critical comparison of commonly used procedures for the assay of terminal deoxynucleotidyl transferase in crude tissue extracts. Nucleic Acids Res. 1977 Dec;4(12):4305–4312. doi: 10.1093/nar/4.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Greenwood M. F., Hutton J. J., Bollum F. J., Lampkin B., Holland P. Serial observations on terminal deoxynucleotidyl transferase activity and lymphoblast surface markers in acute lymphoblastic leukemia. Cancer Res. 1976 Jan;36(1):120–127. [PubMed] [Google Scholar]
- Coleman M. S. Terminal deoxynucleotidyl transferase: characterization of extraction and assay conditions from human and calf tissue. Arch Biochem Biophys. 1977 Aug;182(2):525–532. doi: 10.1016/0003-9861(77)90533-1. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Biochemical properties of purified human terminal deoxynucleotidyltransferase. J Biol Chem. 1980 May 10;255(9):4206–4212. [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Purification of a high molecular weight human terminal deoxynucleotidyl transferase. J Biol Chem. 1979 Sep 10;254(17):8634–8640. [PubMed] [Google Scholar]
- Gallo R. C., Hecht S. M., Whang-Peng J., O'Hopp S. N 6 -( 2 -Isopentenyl)adenosine: the regulatory effects of a cytokinin and modified nucleoside from tRNA on human lymphocytes. Biochim Biophys Acta. 1972 Nov 9;281(4):488–500. doi: 10.1016/0005-2787(72)90149-9. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gregoire K. E., Barton R. W., Bollum F. J. Demonstration of terminal deoxynucleotidyl transferase in thymocytes by immunofluorescence. Proc Natl Acad Sci U S A. 1977 Feb;74(2):734–738. doi: 10.1073/pnas.74.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Intracellular distribution of terminal deoxynucleotidyl transferase in rat bone marrow and thymus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3993–3996. doi: 10.1073/pnas.74.9.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Bollum F. J., Granger S., Pizzolo G., Bradstock K. F., Wong L., McMichael A., Ganeshaguru K., Hoffbrand A. V. The human thymic microenvironment: an immunohistologic study. J Immunol. 1980 Jul;125(1):202–212. [PubMed] [Google Scholar]
- Janossy G., Woodruff R. K., Pippard M. J., Prentice G., Hoffbrand A. V., Paxton A., Lister T. A., Bunch C., Greaves M. F. Relation of "lymphoid" phenotype and response to chemotherapy incorporating vincristine-prednisolone in the acute phase of Ph1 positive leukemia. Cancer. 1979 Feb;43(2):426–434. doi: 10.1002/1097-0142(197902)43:2<426::aid-cncr2820430204>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- Kung P. C., Long J. C., McCaffrey R. P., Ratliff R. L., Harrison T. A., Baltimore D. Terminal deoxynucleotidyl transferase in the diagnosis of leukemia and malignant lymphoma. Am J Med. 1978 May;64(5):788–794. doi: 10.1016/0002-9343(78)90518-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCaffrey R., Harrison T. A., Parkman R., Baltimore D. Terminal deoxynucleotidyl transferase activity in human leukemic cells and in normal human thymocytes. N Engl J Med. 1975 Apr 10;292(15):775–780. doi: 10.1056/NEJM197504102921504. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Sarin P. S., Anderson P. N., Gallo R. C. Terminal deoxynucleotidyl transferase activities in human blood leukocytes and lymphoblast cell lines: high levels in lymphoblast cell lines and in blast cells of some patients with chronic myelogenous leukemia in acute phase. Blood. 1976 Jan;47(1):11–20. [PubMed] [Google Scholar]
- Stass S. A., Schumacher H. R., Keneklis T. P., Bollum F. J. Terminal deoxynucleotidyl transferase immunofluorescence of bone marrow smears: experience in 156 cases. Am J Clin Pathol. 1979 Dec;72(6):898–903. doi: 10.1093/ajcp/72.6.898. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]





