Abstract
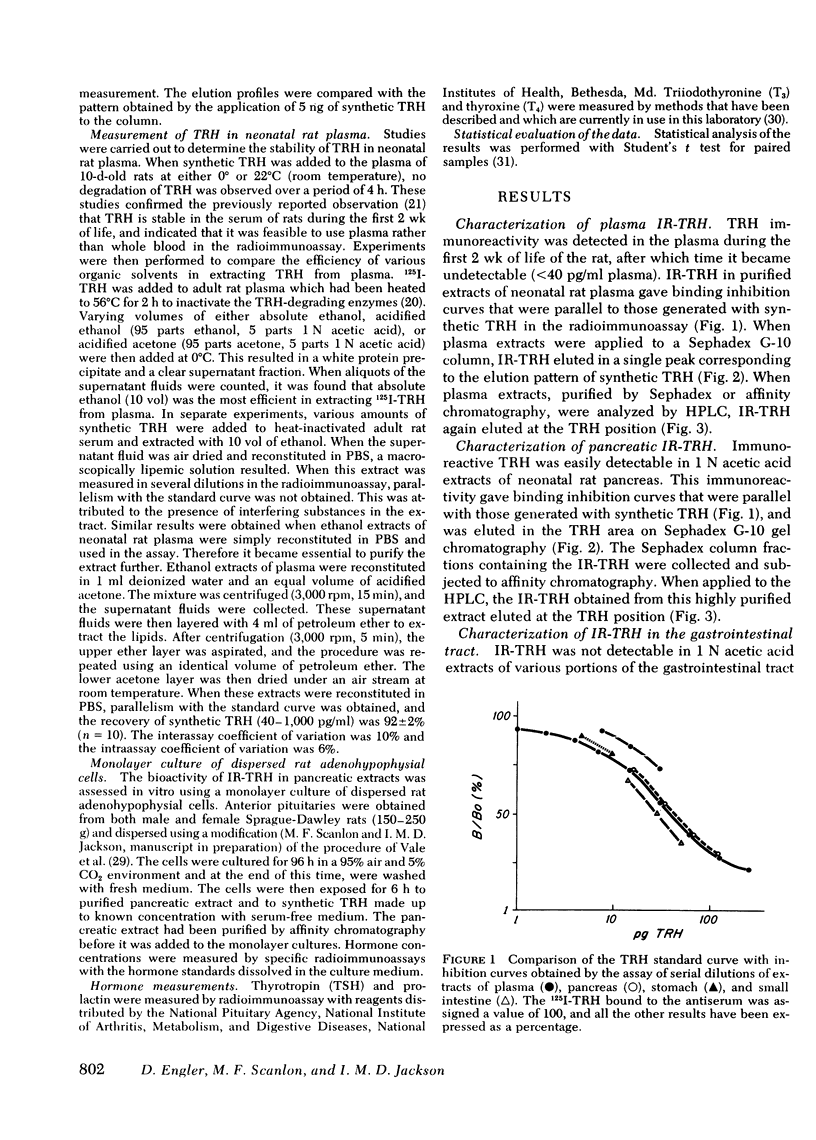
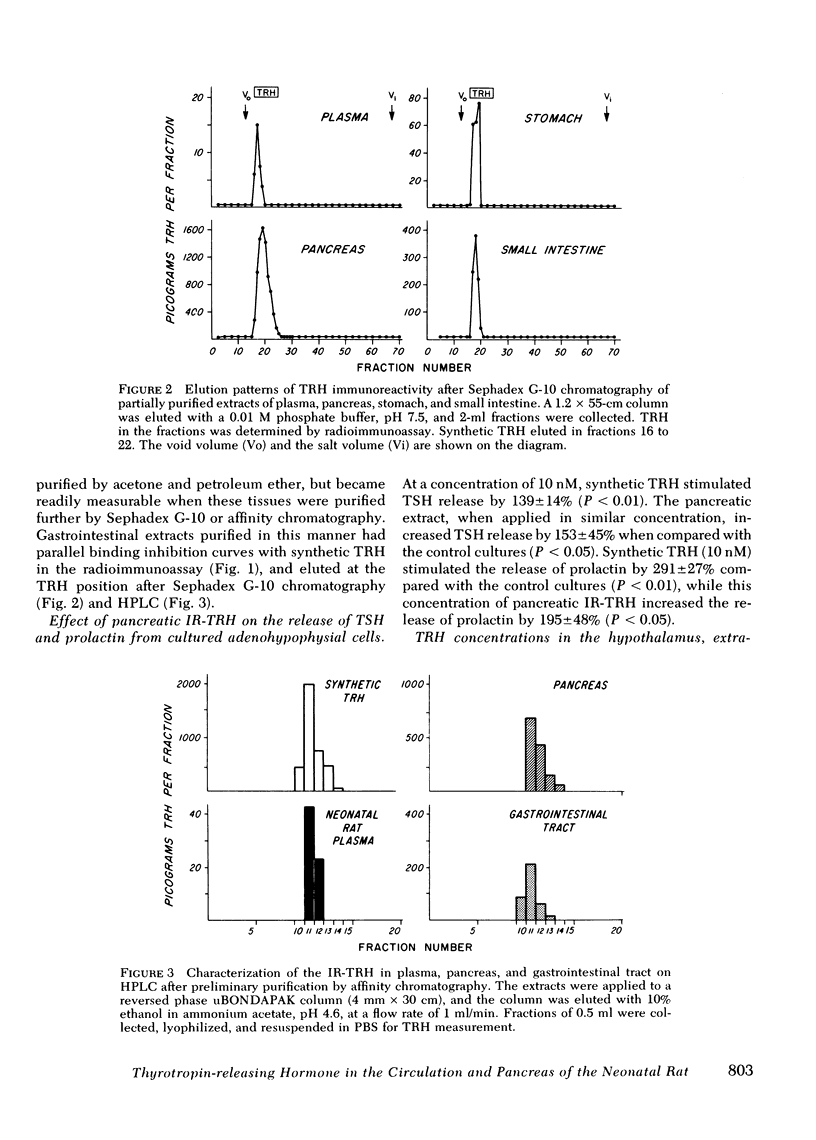
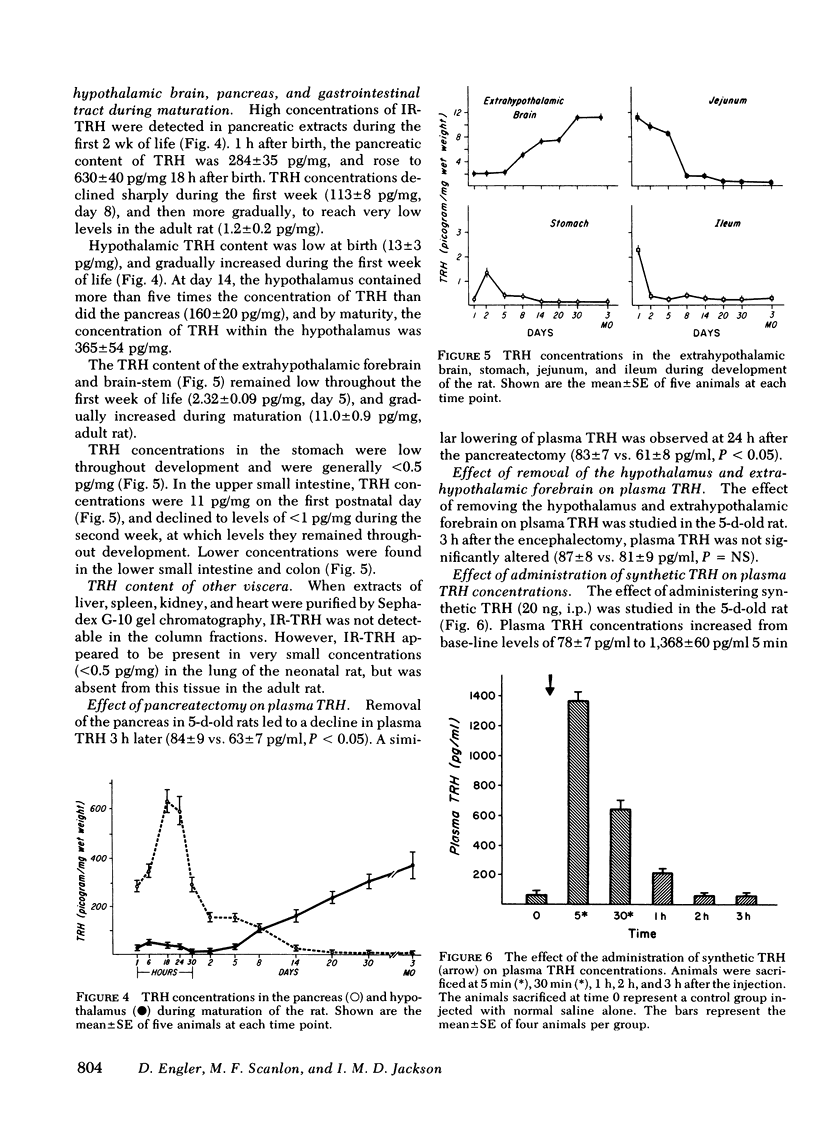
Thyrotropin-releasing hormone immunoreactivity (IR-TRH) has been detected in the circulation of the neonatal rat. This immunoreactivity was demonstrated in purified ethanol extracts of plasma, and was indistinguishable from synthetic TRH using radioimmunoassay and chromatographic criteria. To determine the source of the circulating IR-TRH, tissue concentrations of TRH were analyzed during maturation of the rat. These studies revealed that during the first 10 d of life, the pancreas contained the greatest concentration of IR-TRH of any organ (pancreas, 289±35 pg/mg; hypothalamus, 13±3 pg/mg, day 5). Thereafter, pancreatic IR-TRH concentrations declined progressively while hypothalamic concentrations gradually increased (pancreas, 1.2±0.2 pg/mg; hypothalamus, 365±54 pg/mg, adult rat). IR-TRH was also found throughout the gastrointestinal tract but was not detected in the liver, spleen, kidney, or heart. IR-TRH from the pancreas and gastrointestinal tract gave radio-immunoassay binding displacement curves that were parallel to a curve generated with synthetic TRH, and co-migrated with synthetic TRH on Sephadex G-10 and high performance liquid chromatography. In addition, IR-TRH from purified pancreatic extracts was biologically active in that it released thyrotropin and prolactin from rat adenohypophysial cells maintained in monolayer culture. When a total pancreatectomy was performed on the 5th d of life of the rat, mean plasma TRH concentrations were significantly decreased 3 h afterwards (84±9 vs. 63±7 pg/ml, P < 0.05). Neither the TRH concentrations in the brain, hypothalamus, or gastrointestinal tract, nor the pituitary-thyroid axis were affected by the pancreatectomy. However, mean plasma TRH concentrations remained unaltered 3 h after removal of the hypothalamus and extrahypothalamic brain.
From these results we conclude the following: (a) the TRH immunoreactivity in the circulation, pancreas, and gastrointestinal tract of the neonatal rat is indistinguishable from synthetic TRH; (b) pancreatic secretion provides a significant contribution to the IR-TRH in plasma, and a proportion of the circulating IR-TRH is derived from other extraneural sites. These findings therefore imply that alterations in hypothalamic and extrahypothalamic brain secretion of TRH are not reflected by changes in levels of this tripeptide in the systemic circulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alumets J., Håkanson R., Lundqvist G., Sundler F., Thorell J. Ontogeny and ultrastructure of somatostatin and calcitonin cells in the thyroid gland of the rat. Cell Tissue Res. 1980;206(2):193–201. doi: 10.1007/BF00232763. [DOI] [PubMed] [Google Scholar]
- Blázquez E., Montoya E., López Quijada C. Relationship between insulin concentrations in plasma and pancreas of oetal and weanling rats. J Endocrinol. 1970 Dec;48(4):553–561. doi: 10.1677/joe.0.0480553. [DOI] [PubMed] [Google Scholar]
- Brown M., Allen R., Villarreal J., Rivier J., Vale W. Bombesin-like activity: radioimmunologic assessment in biological tissues. Life Sci. 1978 Dec 31;23(27-28):2721–2728. doi: 10.1016/0024-3205(78)90652-5. [DOI] [PubMed] [Google Scholar]
- Brown M., Vale W. Central nervous system effects of hypothalamic peptides. Endocrinology. 1975 May;96(5):1333–1336. doi: 10.1210/endo-96-5-1333. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Samson W. K., Dobbs R. E., Orci L., Unger R. H. Glucagon-like polypeptides in canine brain. Diabetes. 1979 Jul;28(7):700–702. doi: 10.2337/diab.28.7.700. [DOI] [PubMed] [Google Scholar]
- Dupont A., Labrie F., Levasseur L., Schally A. V. Characteristics of the plasma disappearance of (3H)-thyrotropin-releasing hormone in the rat. Can J Physiol Pharmacol. 1974 Oct;52(5):1012–1019. doi: 10.1139/y74-132. [DOI] [PubMed] [Google Scholar]
- Dussault J. H., Labrie F. Development of the hypothalamic-pituitary-thyroid axis in the neonatal rat. Endocrinology. 1975 Nov;97(5):1321–1324. doi: 10.1210/endo-97-5-1321. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Asplund K., Lundqvist G. Glucagon release from the pancreas of the newborn rat. J Endocrinol. 1972 Sep;54(3):493–504. [PubMed] [Google Scholar]
- Emerson C. H., Utiger R. D. Plasma thyrotropin-releasing hormone concentrations in the rat. Effect of thyroid excess and deficiency and cold exposure. J Clin Invest. 1975 Dec;56(6):1564–1570. doi: 10.1172/JCI108238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Galbo H., Holst J. J., Schaffalitzky de Muckadell O. B. Influence of the autonomic nervous system on the release of vasoactive intestinal polypeptide from the porcine gastrointestinal tract. J Physiol. 1978 Jul;280:405–422. doi: 10.1113/jphysiol.1978.sp012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Pek S., Chance R. E. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent Prog Horm Res. 1976;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- Ghirlanda G., Bataille D., Dubois M. P., Rosselin G. Variations of the somatostatin content of gut, pancreas, and brain in the developing rat. Metabolism. 1978 Sep;27(9 Suppl 1):1167–1170. doi: 10.1016/0026-0495(78)90036-7. [DOI] [PubMed] [Google Scholar]
- Green A. R., Grahame-Smith D. G. TRH potentiates behavioural changes following increased brain 5-hydroxytryptamine accumulation in rats. Nature. 1974 Oct 11;251(5475):524–526. doi: 10.1038/251524a0. [DOI] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978 Oct 27;202(4366):390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Havrankova J., Roth J., Brownstein M. J. Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J Clin Invest. 1979 Aug;64(2):636–642. doi: 10.1172/JCI109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrankova J., Schmechel D., Roth J., Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. L., Starr J. I., Mako M. E., Blackard W. G., Rubenstein A. H. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975 Jun;55(6):1278–1283. doi: 10.1172/JCI108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. M., Papapetrou P. D., Reichlin S. Metabolic clearance of thyrotropin-releasing hormone in the rat in hypothyroid and hyperthyroid states: comparison with serum degradation in vitro. Endocrinology. 1979 May;104(5):1292–1298. doi: 10.1210/endo-104-5-1292. [DOI] [PubMed] [Google Scholar]
- Jackson I. M., Reichlin S. Thyrotropin releasing hormine (TRH): distribution in the brain, blood and urine of the rat. Life Sci. 1974 Jun 1;14(11):2259–2266. doi: 10.1016/0024-3205(74)90107-6. [DOI] [PubMed] [Google Scholar]
- Jackson I. M., Reichlin S. Thyrotropin-releasing hormone (TRH): distribution in hypothalamic and extrahypothalamic brain tissues of mammalian and submammalian chordates. Endocrinology. 1974 Sep;95(3):854–862. doi: 10.1210/endo-95-3-854. [DOI] [PubMed] [Google Scholar]
- Jackson I. M., Reichlin S. Thyrotropin-releasing hormone in the blood of the frog, Rana pipiens: its nature and possible derivation from regional locations in the skin. Endocrinology. 1979 Jun;104(6):1814–1821. doi: 10.1210/endo-104-6-1814. [DOI] [PubMed] [Google Scholar]
- Jackson I. M. TRH in the rat nervous system: identity with synthetic TRH on high performance liquid chromatography following affinity chromatography. Brain Res. 1980 Nov 10;201(1):245–248. doi: 10.1016/0006-8993(80)90794-5. [DOI] [PubMed] [Google Scholar]
- Koivusalo F., Leppäluoto J. High TRF immunoreactivity in purified pancreatic extracts of fetal and newborn rats. Life Sci. 1979 Apr 30;24(18):1655–1658. doi: 10.1016/0024-3205(79)90249-2. [DOI] [PubMed] [Google Scholar]
- Kreider M. S., Winokur A., Utiger R. D. TRH immunoreactivity in rat hypothalamus and brain: assessment by gel filtration and thin-layer chromatography. Brain Res. 1979 Jul 27;171(1):161–165. doi: 10.1016/0006-8993(79)90744-3. [DOI] [PubMed] [Google Scholar]
- Leppäluoto J., Koivusalo F., Kraama R. Thyrotropin-releasing factor: distribution in neural and gastrointestinal tissues. Acta Physiol Scand. 1978 Oct;104(2):175–179. doi: 10.1111/j.1748-1716.1978.tb06264.x. [DOI] [PubMed] [Google Scholar]
- Martino E., Lernmark A., Seo H., Steiner D. F., Refetoff S. High concentration of thyrotropin-releasing hormone in pancreatic islets. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4265–4267. doi: 10.1073/pnas.75.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya E., Seibel M. J., Wilber J. F. Thyrotropin-releasing hormone secretory physiology: studies by radioimmunoassay and affinity chromatography. Endocrinology. 1975 Jun;96(6):1413–1418. doi: 10.1210/endo-96-6-1413. [DOI] [PubMed] [Google Scholar]
- Morley J. E., Garvin T. J., Pekary A. E., Hershman J. M. Thyrotropin-releasing hormone in the gastrointestinal tract. Biochem Biophys Res Commun. 1977 Nov 7;79(1):314–318. doi: 10.1016/0006-291x(77)90097-3. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Kieffer J. D., Federico P., Mover H., Maloof F., Soodak M. Thyrotropin releasing hormone: development of inactivation system during maturation of the rat. Science. 1976 Jul 30;193(4251):403–405. doi: 10.1126/science.819993. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Kieffer J. D., Nakamura C., Mover H., Soodak M., Maloof F. The developmental pattern of thyrotropin-releasing hormone-degrading activity in the plasma of rats. Endocrinology. 1978 Nov;103(5):1849–1854. doi: 10.1210/endo-103-5-1849. [DOI] [PubMed] [Google Scholar]
- Nejad I., Bollinger J., Mitnick M. A., Sullivan P., Reichlin S. Measurement of plasma and tissue triiodothyronine concentration in the rat by radioimmunoassay. Endocrinology. 1975 Mar;96(3):773–780. doi: 10.1210/endo-96-3-773. [DOI] [PubMed] [Google Scholar]
- Oliver C., Eskay R. L., Ben-Jonathan N., Porter J. C. Distribution and concentration of TRH in the rat brain. Endocrinology. 1974 Aug;95(2):540–546. doi: 10.1210/endo-95-2-540. [DOI] [PubMed] [Google Scholar]
- Oliver C., Parker C. R., Jr, Porter J. C. Developmental changes in the degradation of thyrotrophin releasing hormone by the serum and brain tissues of the male rat. J Endocrinol. 1977 Aug;74(2):339–340. doi: 10.1677/joe.0.0740339. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Takor T. Embryology of the diffuse neuroendocrine system and its relationship to the common peptides. Fed Proc. 1979 Aug;38(9):2288–2294. [PubMed] [Google Scholar]
- Pekary A. E., Meyer N. V., Vaillant C., Hershman J. M. Thyrotropin-releasing hormone and a homologous peptide in the male rat reproductive system. Biochem Biophys Res Commun. 1980 Aug 14;95(3):993–1000. doi: 10.1016/0006-291x(80)91571-5. [DOI] [PubMed] [Google Scholar]
- Plotnikoff N. P., Prange A. J., Jr, Breese G. R., Anderson M. S., Wilson I. C. Thyrotropin releasing hormone: enhancement of dopa activity by a hypothalamic hormone. Science. 1972 Oct 27;178(4059):417–418. doi: 10.1126/science.178.4059.417. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V. On the half life of thyrotropin-releasing hormone in rats. Neuroendocrinology. 1972;9(4):250–256. doi: 10.1159/000122056. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B., Brazeau P. Depressant action of TRH, LH-RH and somatostatin on activity of central neurones. Nature. 1975 May 15;255(5505):233–235. doi: 10.1038/255233a0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig J. L., Havrankova J., Lesniak M. A., Brownstein M., Roth J. Insulin is ubiquitous in extrapancreatic tissues of rats and humans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):572–576. doi: 10.1073/pnas.77.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V. Aspects of hypothalamic regulation of the pituitary gland. Science. 1978 Oct 6;202(4363):18–28. doi: 10.1126/science.99816. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Bhathena S. J., Nompleggi D., Penhos J. C., Recant L. Studies on persistent circulating immunoreactive glucagon (IRG) and immunoreactive insulin (IRI) found in eviscerated rats with a functional liver. Diabetologia. 1978 Mar;14(3):177–184. doi: 10.1007/BF00429778. [DOI] [PubMed] [Google Scholar]
- Spindel E., Wurtman R. J. TRH immunoreactivity in rat brain regions, spinal cord and pancreas: validation by high-pressure liquid chromatography and thin-layer chromatography. Brain Res. 1980 Nov 17;201(2):279–288. doi: 10.1016/0006-8993(80)91036-7. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos T., Braverman L. E., Vagenakis A. G. Thyrotropin-releasing hormone is not required for thyrotropin secretion in the perinatal rat. J Clin Invest. 1979 Apr;63(4):588–594. doi: 10.1172/JCI109340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Vijayan E., McCann S. M. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH). Endocrinology. 1977 Jun;100(6):1727–1730. doi: 10.1210/endo-100-6-1727. [DOI] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bloom S. R., Ghatei M. A., Solcia E., Brown M. R., Pearse A. G. Bombesin-like immunoreactivity in the lung. Nature. 1978 Jun 29;273(5665):769–770. doi: 10.1038/273769a0. [DOI] [PubMed] [Google Scholar]
- Winokur A., Utiger R. D. Thyrotropin-releasing hormone: regional distribution in rat brain. Science. 1974 Jul 19;185(4147):265–267. doi: 10.1126/science.185.4147.265. [DOI] [PubMed] [Google Scholar]
- Yarbrough G. G. TRH potentiates excitatory actions of acetylcholine on cerebral cortical neurones. Nature. 1976 Oct 7;263(5577):523–524. doi: 10.1038/263523a0. [DOI] [PubMed] [Google Scholar]
- Youngblood W. W., Humm J., Kizer J. S. TRH-like immunoreactivity in rat pancreas and eye, bovine and sheep pineals, and human placenta: non-identify with synthetic Pyroglu-His-Pro-NH2 (TRH). Brain Res. 1979 Mar 9;163(1):101–110. doi: 10.1016/0006-8993(79)90154-9. [DOI] [PubMed] [Google Scholar]
- Youngblood W. W., Lipton M. A., Kizer J. S. TRH-like immunoreactivity in urine, serum and extrahypothalamic brain: non-identity with synthetic pyroglu-hist-pro-NH2 (TRH). Brain Res. 1978 Jul 28;151(1):99–116. doi: 10.1016/0006-8993(78)90953-8. [DOI] [PubMed] [Google Scholar]


