Abstract
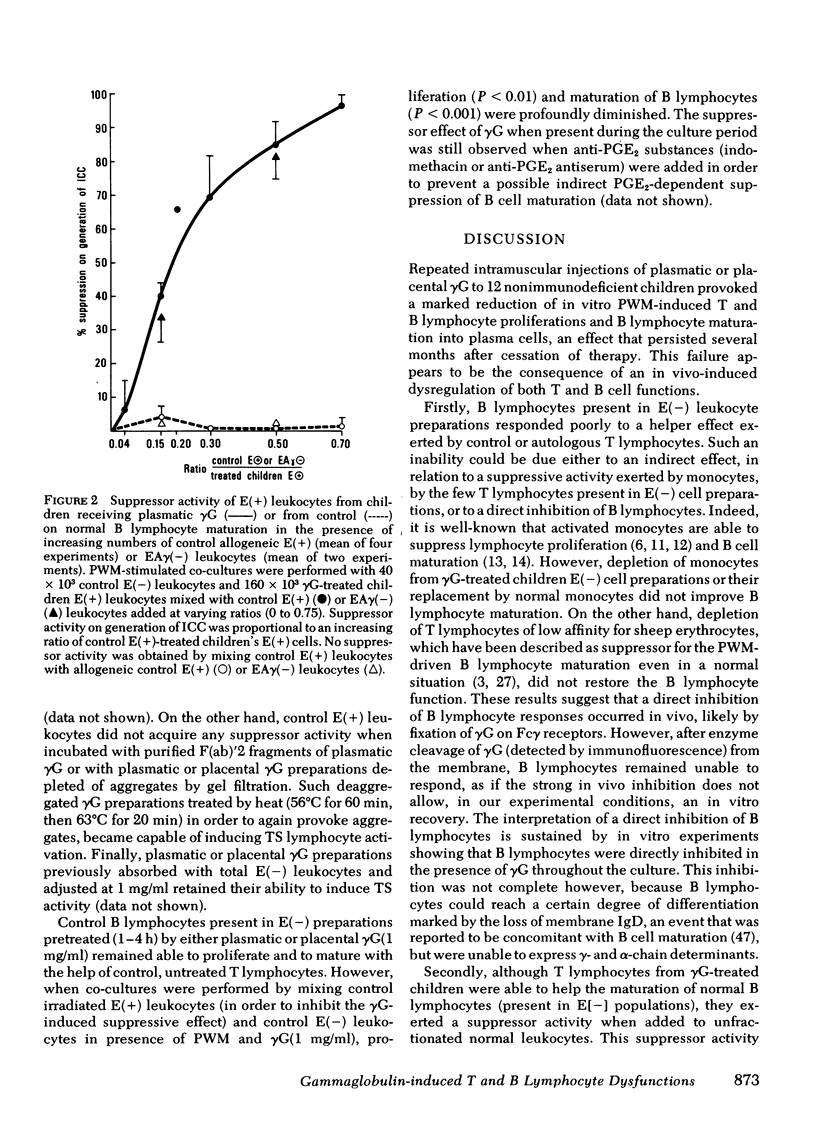
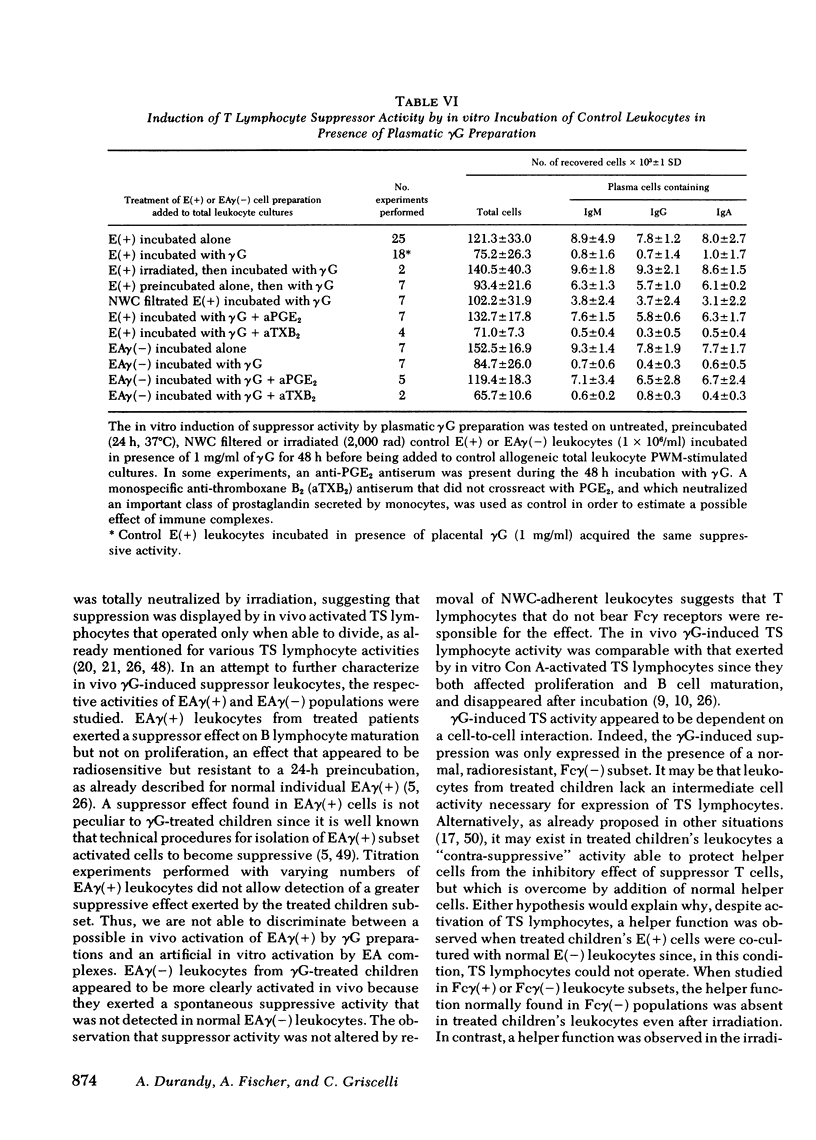
Lymphocytes obtained from nonimmuno deficient children treated with commercially available preparations of gammaglobulin failed to proliferate and to mature into plasma cells in vitro after stimulation with pokeweed mitogen. The influence of the treatment on lymphocyte functions varied according to the cell population considered. A T helper cell activity was detected in these patients but only in the cell subset bearing receptors for IgG after irradiation. T lymphocytes exerted a suppressive effect that disappeared after irradiation or incubation at 37°C. The suppressive cells were found among E rosette-forming cells depleted of leukocytes bearing receptors for IgG. Their suppressive effect was expressed only in the presence of normal radioresistant T lymphocytes that did not bear Fc receptors for IgG. Similar dysfunctions could be induced in vitro by incubation of normal T and B lymphocytes with gammaglobulin preparations. Because F(ab)′2 fragments or deaggregated preparations of gammaglobulin failed to activate T suppressor lymphocytes, this activation was likely triggered by attachment of Fc portion of denatured IgG to the corresponding membrane receptor. This activation step was prostaglandin E2-dependent, suggesting that activated monocytes were involved in the activation process. B lymphocyte responses appeared directly inhibited by attachment of denatured gammaglobulin on membrane Fc receptor. Our observations suggest that immunological effects of gammaglobulin therapy are not limited to antibody transfer, since it also induces subtle modifications of in vitro pokeweed mitogen-stimulated T and B cell responses. These modifications must be considered in interpreting results obtained in immunodeficient patients investigated under gamma-globulin therapy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballieux R. E., Heijnen C. J., Uytdehaag F., Zegers B. J. Regulation of B cell activity in man: role of T cells. Immunol Rev. 1979;45:3–39. doi: 10.1111/j.1600-065x.1979.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Bich-Thuy L. T., Brochier J. Human B cell differentiation. II. Suppression by T cells of T-dependent and T-independent plasma cell maturation. J Immunol. 1979 May;122(5):1842–1848. [PubMed] [Google Scholar]
- Bona C., Audibert F., Juy D., Chedid L. Cell suppression in PPD-induced blast specific response of human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Nov;26(2):258–266. [PMC free article] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochier J., Bonneau M., Robert M., Samarut C., Revillard J. P., Traeger J. Anti-HLA-DR alloantibodies eluted from human placental tissue. Transplant Proc. 1979 Mar;11(1):779–782. [PubMed] [Google Scholar]
- Broder S., Mann D. L., Waldmann T. A. Participation of suppressor T cells in the immunosuppressive activity of a heteroantiserum to human Ia-like antigens (p23,30). J Exp Med. 1980 Jan 1;151(1):257–262. doi: 10.1084/jem.151.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J., Colombani M. Serologic recognition of histocompatibility antigens using complement fixation. Semin Hematol. 1974 Jul;11(3):273–280. [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digeon M., Laver M., Riza J., Bach J. F. Detection of circulating immune complexes in human sera by simplified assays with polyethylene glycol. J Immunol Methods. 1977;16(2):165–183. doi: 10.1016/0022-1759(77)90051-5. [DOI] [PubMed] [Google Scholar]
- Dimitriu A., Fauci A. S. Activation of human B lymphocytes. IX. Modulation of antibody production by products of activated macrophages. J Immunol. 1978 Jun;120(6):1818–1823. [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Primary prostaglandins in human peripheral plasma by radioimmunoassay. Adv Prostaglandin Thromboxane Res. 1976;1:93–97. [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Fineman S. M., Mudawwar F. B., Geha R. S. Characteristics and mechanisms of action of the concanavalin A-activated suppressor cell in man. Cell Immunol. 1979 Jun;45(1):120–132. doi: 10.1016/0008-8749(79)90367-8. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Dosch H. M., Hastings B., Shore A. Lithium: a modulator of cyclic AMP-dependent events in lymphocytes? Science. 1979 Jan 26;203(4378):365–367. doi: 10.1126/science.216075. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscelli C., Durandy A., Guy-Grand D., Daguillard F., Herzog C., Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978 Oct;65(4):691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. II. Effect of thymopoietin, corticosteroids, and irradiation. Cell Immunol. 1977 Nov;34(1):10–18. doi: 10.1016/0008-8749(77)90224-6. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. V. Kinetics and mechanisms of suppression of plaque-forming cell responses by concanavalin A-generated suppressor cells. J Immunol. 1978 Mar;120(3):700–708. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. X. Heterogeneity of concanavalin A-generated suppressor cells of the pokeweed mitogen-induced plaque-forming cell response of human peripheral blood lymphocytes. J Immunol. 1978 Aug;121(2):559–565. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. XIII. Characterization of multiple populations of naturally occurring immunoregulatory cells of polyclonally induced in vitro human B cell function. J Immunol. 1979 Sep;123(3):1289–1298. [PubMed] [Google Scholar]
- Hayward A. R., Layward L., Lydyard P. M., Moretta L., Dagg M., Lawton A. R. Fc-receptor heterogeneity of human suppressor T cells. J Immunol. 1978 Jul;121(1):1–5. [PubMed] [Google Scholar]
- Hayward A. R., Lydyard P. M. Suppression of B lymphocyte differentiation by newborn T lymphocytes with an Fc receptor for IgM. Clin Exp Immunol. 1978 Dec;34(3):374–378. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K. Antibody regulates the cooperation of B cells with helper cells. Immunol Rev. 1980;49:79–91. doi: 10.1111/j.1600-065x.1980.tb00427.x. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Goldstone A. H., Capellaro D., Greaves M. F., Kulenkampff J., Pippard M., Welsh K. Differentiation linked expression of p28,33 (Ia-like) structures on human leukaemic cells. Br J Haematol. 1977 Nov;37(3):391–402. doi: 10.1111/j.1365-2141.1977.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaszubowski P. A., Goodwin J. S., Williams R. C., Jr Ia antigen on the surface of a subfraction of T cells that bear Fc receptors for IgG. J Immunol. 1980 Mar;124(3):1075–1078. [PubMed] [Google Scholar]
- Knapp W., Baumgartner G. Monocyte-mediated suppression of human B lymphocyte differentiation in vitro. J Immunol. 1978 Sep;121(3):1177–1183. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughter A. H., Twomey J. J. Suppression of lymphoproliferation by high concentrations of normal human mononuclear leukocytes. J Immunol. 1977 Jul;119(1):173–179. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Romanzi C. A. Loss of Fc receptors for IgG from human T lymphocytes exposed to IgG immune complexes. Nature. 1978 Apr 13;272(5654):618–620. doi: 10.1038/272618a0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig J. B., Turner M. W. Localization of Gm markers to different molecular regions of the Fc fragment. Clin Exp Immunol. 1971 May;8(5):685–700. [PMC free article] [PubMed] [Google Scholar]
- Olding L. B., Benirschke K., Oldstone M. B. Inhibition of mitosis of lymphocytes from human adults by lymphocytes from human newborns. Clin Immunol Immunopathol. 1974 Sep;3(1):79–89. doi: 10.1016/0090-1229(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Merler E. Increased prostaglandin production by human monocytes after membrane receptor activation. J Immunol. 1979 Jul;123(1):115–120. [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Prostaglandins and the immune response. Life Sci. 1977 Mar 15;20(6):903–913. doi: 10.1016/0024-3205(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Flandrin G. Identification by peroxidase staining of monocytes in surface immunofluorescence tests. J Immunol. 1974 Nov;113(5):1650–1653. [PubMed] [Google Scholar]
- Preudhomme J. L. Loss of surface IgD by human B lymphocytes during polyclonal activation. Eur J Immunol. 1977 Mar;7(3):191–193. doi: 10.1002/eji.1830070316. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Con A-inducible suppression of MLC: evidence for mediation by the TH2 + T cell subset in man. J Immunol. 1979 Apr;122(4):1335–1341. [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Rivat L., Rivat C., Daveau M., Ropartz C. Comparative frequencies of anti-IgA antibodies among patients with anaphylactic transfusion reactions and among normal blood donors. Clin Immunol Immunopathol. 1977 May;7(3):340–348. doi: 10.1016/0090-1229(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Henkart P. A. Fc receptor-mediated inhibition of murine B-lymphocyte activation. J Exp Med. 1976 Sep 1;144(3):768–775. doi: 10.1084/jem.144.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Suppression of B-cell differentiation by leukocytes from hypogammaglobulinemic patients. J Clin Invest. 1976 Jul;58(1):109–122. doi: 10.1172/JCI108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Tadakuma T., Pierce C. W. Site of action of a soluble immune response suppressor (SIRS) produced by concanavalin A-activated spleen cells. J Immunol. 1976 Sep;117(3):967–972. [PubMed] [Google Scholar]
- Waldmann T. A., Blaese R. M., Broder S., Krakauer R. S. Disorders of suppressor immunoregulatory cells in the pathogenesis of immunodeficiency and autoimmunity. Ann Intern Med. 1978 Feb;88(2):226–238. doi: 10.7326/0003-4819-88-2-226. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. Mitogen-induced changes in lymphocyte prostaglandin levels: a signal for the induction of suppressor cell activity. Cell Immunol. 1978 Nov;41(1):72–85. doi: 10.1016/s0008-8749(78)80029-x. [DOI] [PubMed] [Google Scholar]


