Abstract
The purpose of these experiments was to determine whether alterations in preproinsulin messenger (m)RNA activity could account for changes in insulin biosynthesis during fasting and refeeding. Rats were fasted 4 d and then fed for 6, 8, 24, or 48 h. With fasting, body weight decreased 25%, plasma glucose decreased from 6.1 to 2.2 mM, and pancreatic insulin content fell to 40% that of fed animals. Islet RNA decreased to 50% and protein to 55% that of control animals, while islet DNA content remained unchanged. After 6 h of refeeding, islet RNA content increased and was not significantly different from controls.
Total islet and preproinsulin mRNA activity was estimated with an mRNA-dependent wheat germ cell-free protein synthesizing system. Preproinsulin and total protein synthesis was linearly dependent upon added RNA at concentrations up to 3 μg. Preproinsulin was identified by its mobility on SDS polyacrylamide gel electrophoresis and by hybrid arrested translation of preproinsulin mRNA. After an 18-h fast, islet mRNA activity decreased 33%; after 4 d mRNA activity decreased to 66% below that of control fed animals. Preproinsulin mRNA activity was decreased, but to a lesser extent, accounting for 20% of total islet protein in fed animals and 46% in the 4-d fasted animals. Total mRNA activity returned to control values after 8 h of refeeding and increased to 150% of controls at 24 and 48 h. Preproinsulin mRNA activity increased more rapidly on refeeding. By 8 h it was 160% of controls.
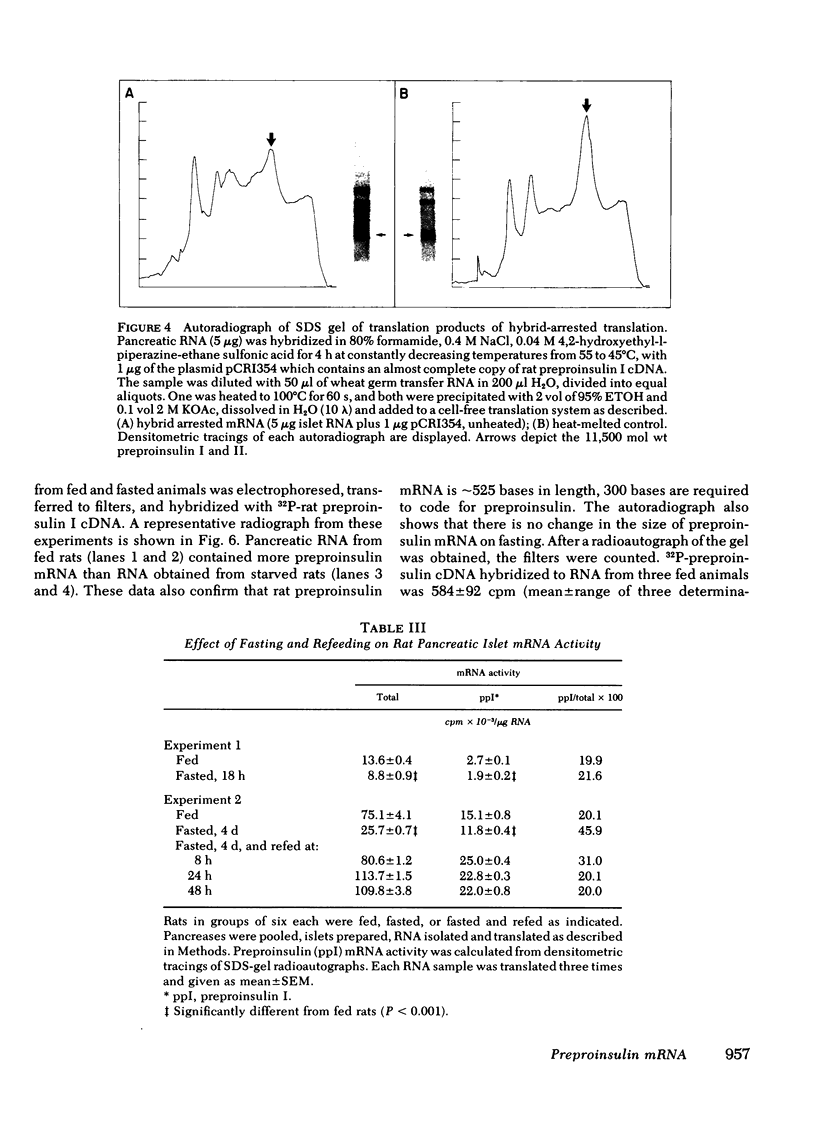
To determine whether changes in preproinsulin mRNA activity were associated with changes in the amount of preproinsulin mRNA, nucleic acid hybridization analysis was performed. Pancreatic RNA from fed and fasted animals was electrophoresed on agarose gels, transferred to diazophenylthio paper, and hybridized to 32P-labeled preproinsulin complementary (c)-DNA. This analysis demonstrated that changes in mRNA activity were associated with changes in the amount of hybridizable mRNA present. These studies are the first to demonstrate alterations of preproinsulin mRNA under any conditions, and the changes correlate with alterations in rates of insulin biosynthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert S. G., Permutt M. A. Proinsulin precursors in catfish pancreatic islets. J Biol Chem. 1979 May 10;254(9):3483–3492. [PubMed] [Google Scholar]
- Bell G. I., Swain W. F., Pictet R., Cordell B., Goodman H. M., Rutter W. J. Nucleotide sequence of a cDNA clone encoding human preproinsulin. Nature. 1979 Nov 29;282(5738):525–527. doi: 10.1038/282525a0. [DOI] [PubMed] [Google Scholar]
- Best C. H., Haist R. E., Ridout J. H. Diet and the insulin content of pancreas. J Physiol. 1939 Nov 14;97(1):107–119. doi: 10.1113/jphysiol.1939.sp003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone A. J., Howell S. L. Alterations in regulation of insulin biosynthesis in pregnancy and starvation studied in isolated rat islets of langerhans. Biochem J. 1977 Sep 15;166(3):501–507. doi: 10.1042/bj1660501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Itoh N., Sei T., Nose K., Okamoto H. Glucose stimulation of the proinsulin synthesis in isolated pancreatic islets without increasing amount of proinsulin mRNA. FEBS Lett. 1978 Sep 15;93(2):343–347. doi: 10.1016/0014-5793(78)81136-3. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T., Feigelson P. Multihormonal induction of hepatic alpha2u-globulin mRNA as measured by hybridization to complementary DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4791–4795. doi: 10.1073/pnas.74.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Cell-free modulation of proinsulin synthesis. Science. 1977 Nov 11;198(4317):620–622. doi: 10.1126/science.918657. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Wright P. H. Effect of fasting upon insulin secretion in the rat. Am J Physiol. 1967 Oct;213(4):843–848. doi: 10.1152/ajplegacy.1967.213.4.843. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Hemmaplardh D., Finch C. A., Palmiter R. D. Transferrin gene expression. Effects of nutritional iron deficiency. J Biol Chem. 1980 Jan 10;255(1):144–147. [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permutt M. A., Boime I., Chyn R., Goldford M. Isolation and partial purification of catfish pancreatic islet messenger RNA. Biochemistry. 1978 Feb 7;17(3):537–543. doi: 10.1021/bi00596a026. [DOI] [PubMed] [Google Scholar]
- Permutt M. A. Effect of glucose on initiation and elongation rates in isolated rat pancreatic islets. J Biol Chem. 1974 May 10;249(9):2738–2742. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharp D. W., Kemp C. B., Knight M. J., Ballinger W. F., Lacy P. E. The use of ficoll in the preparation of viable islets of langerhans from the rat pancreas. Transplantation. 1973 Dec;16(6):686–689. doi: 10.1097/00007890-197312000-00028. [DOI] [PubMed] [Google Scholar]
- Schimmel M., Utiger R. D. Thyroidal and peripheral production of thyroid hormones. Review of recent findings and their clinical implications. Ann Intern Med. 1977 Dec;87(6):760–768. doi: 10.7326/0003-4819-87-6-760. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F. Editorial: Errors in insulin biosynthesis. N Engl J Med. 1976 Apr 22;294(17):952–953. doi: 10.1056/NEJM197604222941713. [DOI] [PubMed] [Google Scholar]
- Tjioe T. O., Bouman P. R. Effect of fasting on the incorporation of [3H]-L-phenylalanine into proinsulin-insulin and total protein in isolated rat pancreatic islets. Horm Metab Res. 1976 Jul;8(4):261–266. doi: 10.1055/s-0028-1093651. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]





