Abstract
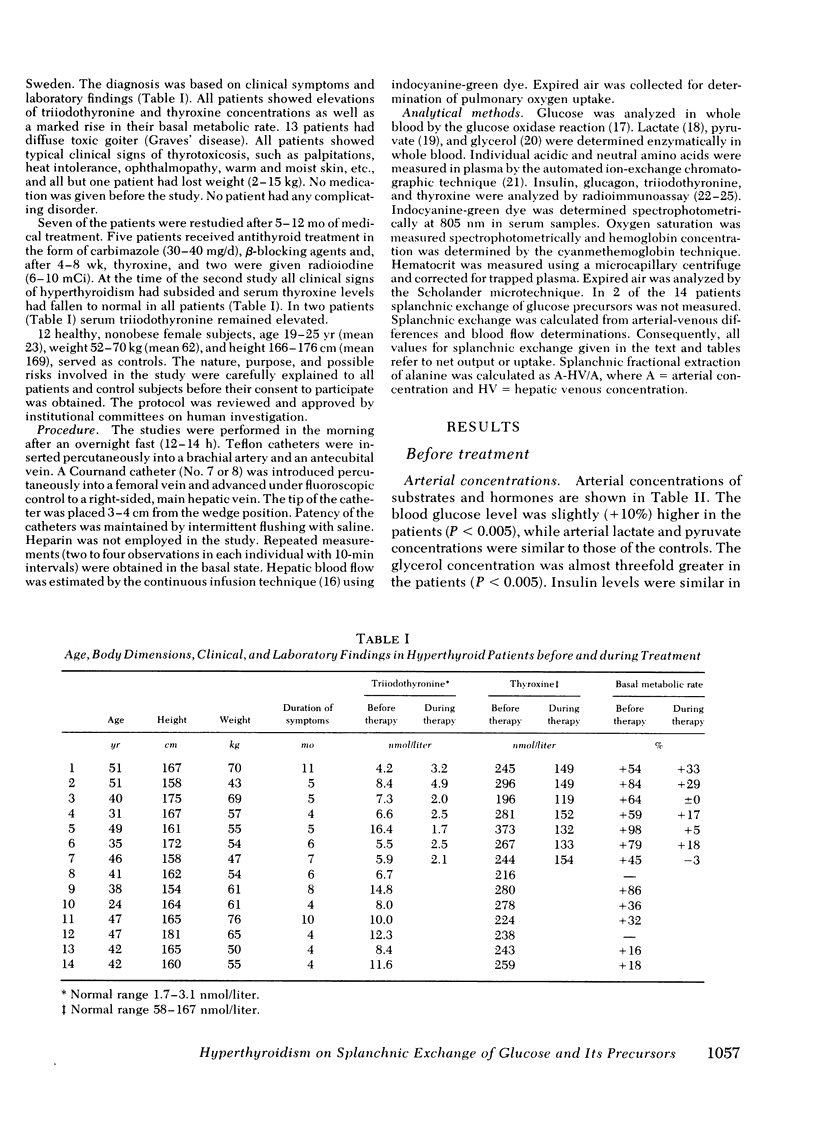
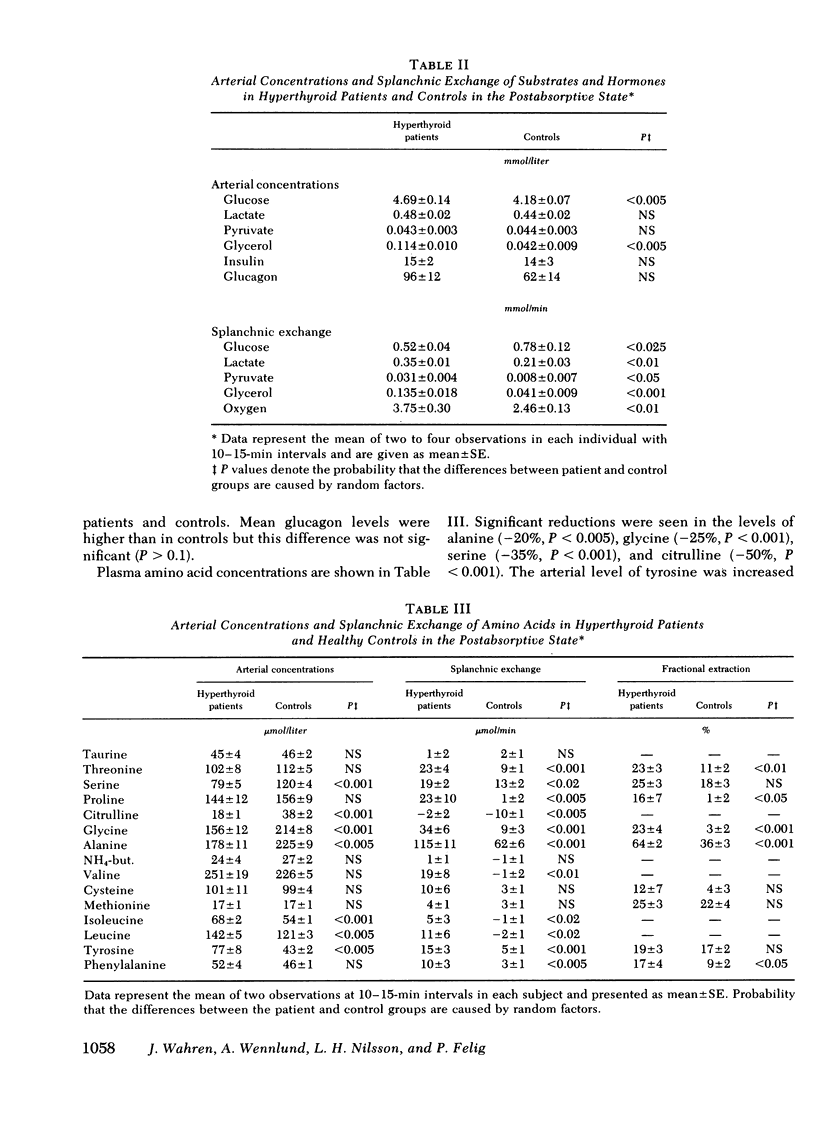
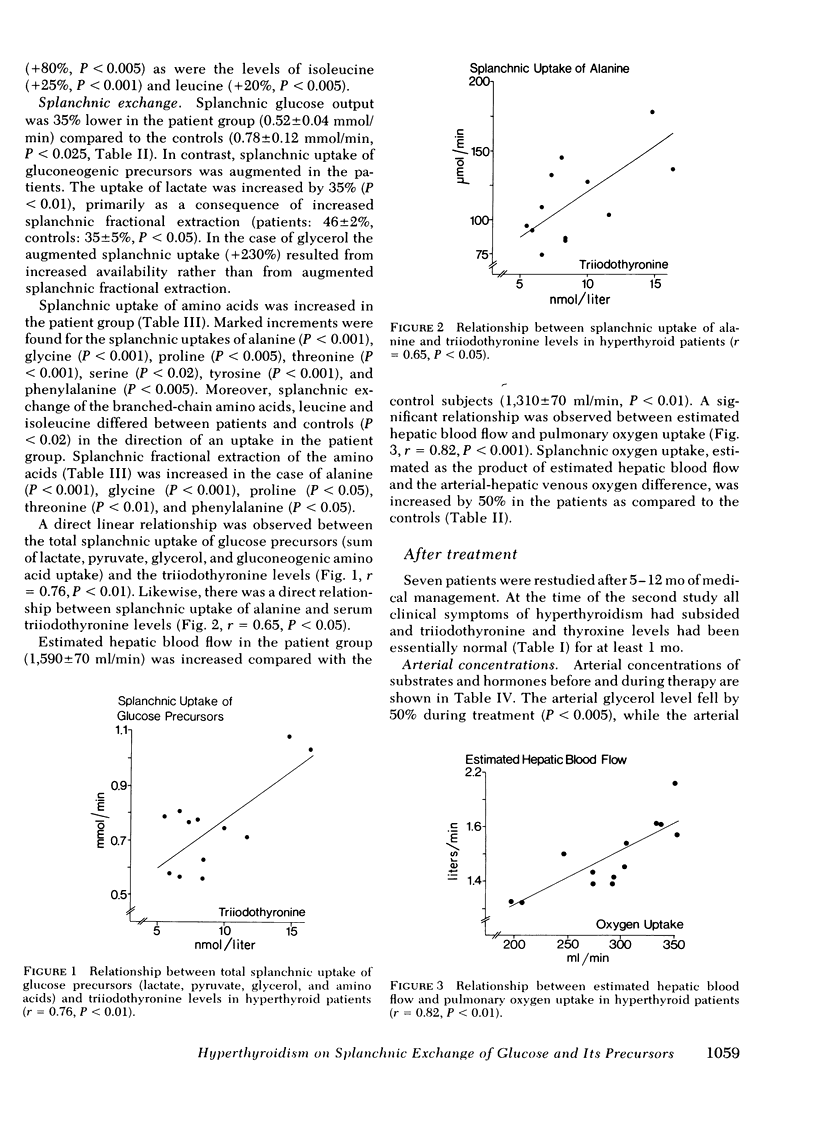
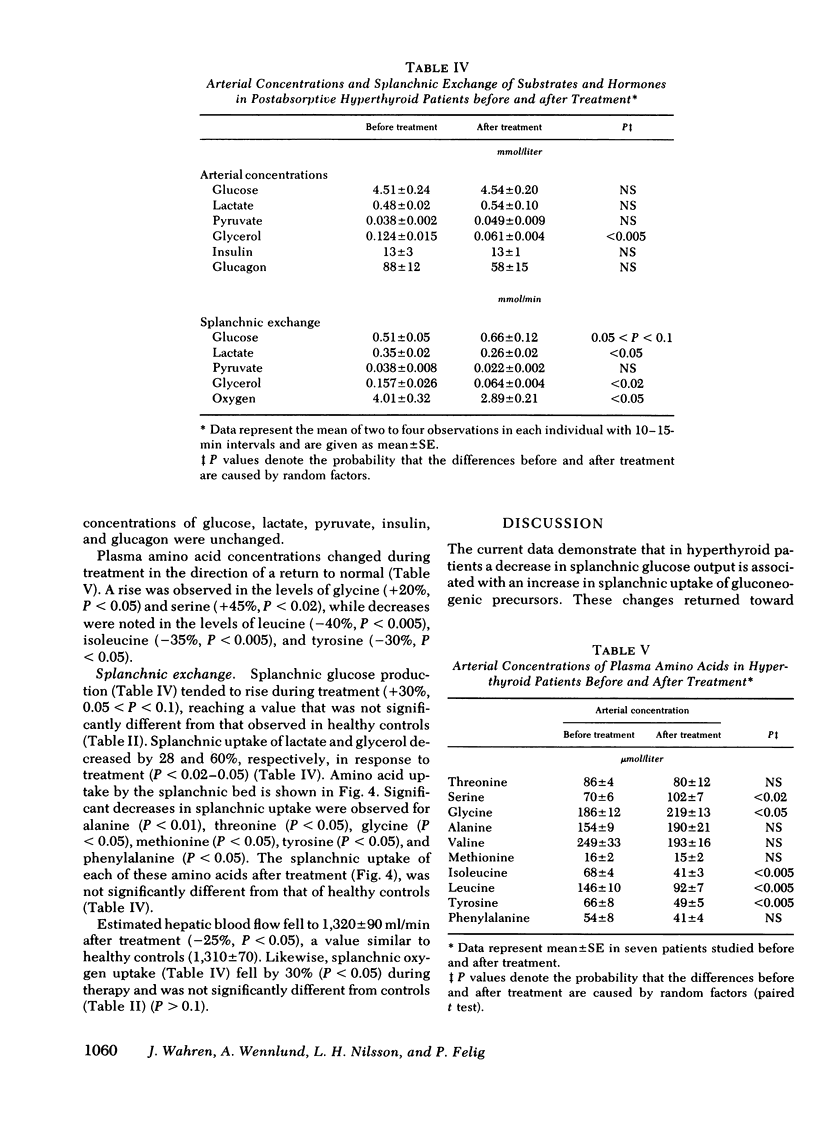
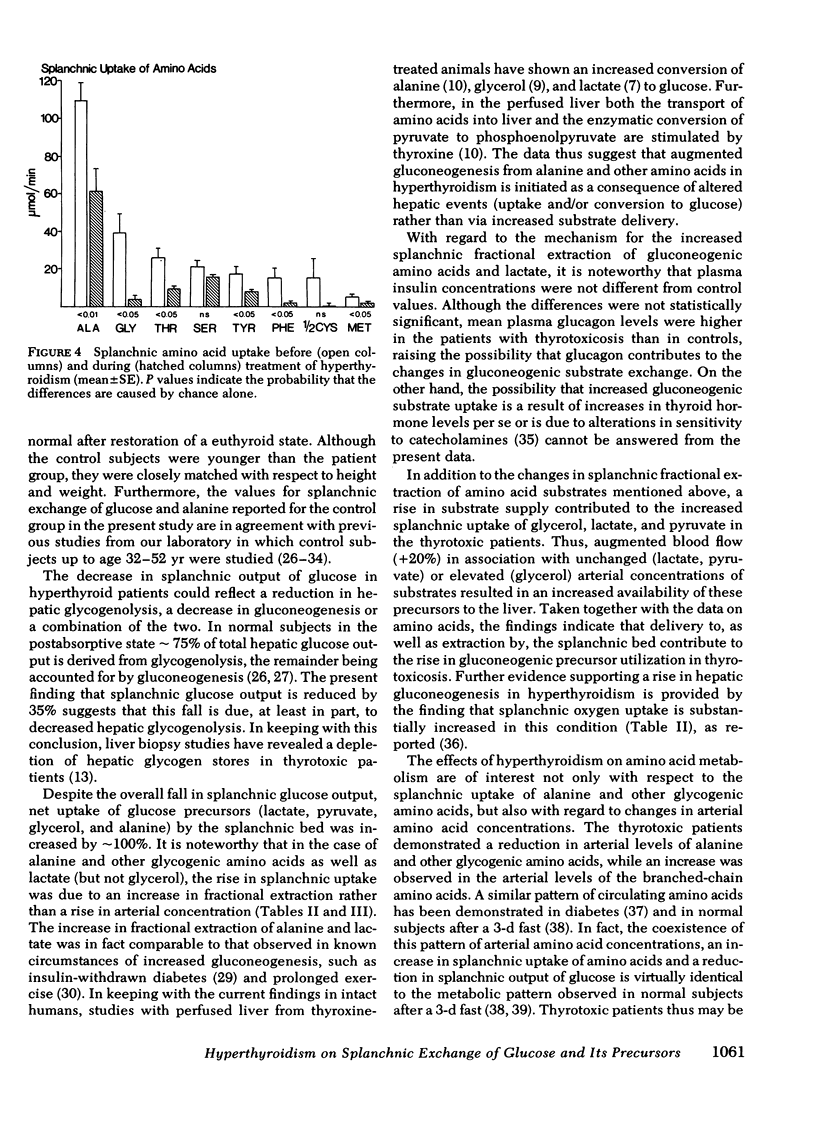
Arterial concentrations and splanchnic exchange of glucose, amino acids, lactate, pyruvate, and glycerol were determined in 14 hyperthyroid patients and 12 healthy controls. Seven of the patients were restudied after 5-12 mo of medical management at which time there was chemical and clinical evidence of a euthyroid state. The arterial level of glucose was slightly higher (+10%) in the patient group and the glycerol concentration was three times greater among the patients. The plasma levels of the glycogenic amino acids, alanine, glycine, and serine were decreased by 20-30%, while the concentrations of leucine, isoleucine, and tyrosine were increased by 20-80%. The levels of lactate and pyruvate were similar in patients and controls as were insulin and glucagon concentrations. Splanchnic glucose output in the patient group was 35% lower than in controls. However, total splanchnic uptake of glucogenic precursors was 100% higher than in controls and showed a direct linear correlation with serum triiodothyronine. Total precursor uptake could account for 75% of splanchnic glucose output in the patients, compared to 26% in controls. The increase in uptake of lactate, alanine, and other amino acids was due to a 35-80% rise in splanchnic fractional extraction plus a 20% rise in estimated hepatic blood flow. When the patients were restudied after medical treatment splanchnic exchange of glucose and glucose precursors had reverted to normal values. The present findings demonstrate that in hyperthyroidism (a) total splanchnic glucose output is reduced in relation to controls, (b) splanchnic uptake of gluconeogenic precursors is accelerated, largely due to a rise in fractional extraction of precursor substrates and to a smaller extent, as a result of an increase in hepatic blood flow, and (c) these changes revert to normal when a euthyroid state has been achieved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Wennlund A., Ostman J. Regulation of lipolysis by human adipose tissue in hyperthyroidism. J Clin Endocrinol Metab. 1979 Mar;48(3):415–419. doi: 10.1210/jcem-48-3-415. [DOI] [PubMed] [Google Scholar]
- Bartels P. D., Kristensen L. O., Heding L. G., Sestoft L. Development of ketonemia in fasting patients with hyperthyroidism. Acta Med Scand Suppl. 1979;624:43–47. doi: 10.1111/j.0954-6820.1979.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Bradley S. E., Ingelfinger F. J., Bradley G. P., Curry J. J. THE ESTIMATION OF HEPATIC BLOOD FLOW IN MAN. J Clin Invest. 1945 Nov;24(6):890–897. doi: 10.1172/JCI101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOKS J., MURRAY I. P., WAYNE E. J. Basal metabolic rate in thyrotoxicosis. Lancet. 1958 Mar 22;1(7021):604–607. doi: 10.1016/s0140-6736(58)90865-1. [DOI] [PubMed] [Google Scholar]
- Carter W. J., Shakir K. M., Hodges S., Faas F. H., Wynn J. O. Effect of thyroid hormone on metabolic adaptation to fasting. Metabolism. 1975 Oct;24(10):1177–1183. doi: 10.1016/0026-0495(75)90154-7. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Sinclair-Smith B. C., Lacy W. W. Gluconeogenesis from alanine in normal postabsorptive man. Intrahepatic stimulatory effect of glucagon. Diabetes. 1975 Jun;24(6):574–584. doi: 10.2337/diab.24.6.574. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Goldberg A. L. Thyroid hormones control lysosomal enzyme activities in liver and skeletal muscle. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1369–1373. doi: 10.1073/pnas.75.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R. T., Foster L. B. Radioimmunoassay of thyroxine in unextracted serum, by a single-antibody technique. Clin Chem. 1973 Sep;19(9):1063–1066. [PubMed] [Google Scholar]
- Felig P., Marliss E., Ohman J. L., Cahill C. F., Jr Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970 Oct;19(10):727–728. doi: 10.2337/diab.19.10.727. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Brundin T. Splanchnic glucose and amino acid metabolism in obesity. J Clin Invest. 1974 Feb;53(2):582–590. doi: 10.1172/JCI107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. N., Ball E. G. Studies on the metabolism of adipose tissue. XX. The effect of thyroid status upon oxygen consumption and lipolysis. Biochemistry. 1967 Mar;6(3):637–647. doi: 10.1021/bi00855a001. [DOI] [PubMed] [Google Scholar]
- Freedland R. A., Krebs H. A. The effect of thyroxine treatment on the rate of gluconeogenesis in the perfused rat liver. Biochem J. 1967 Sep;104(3):45P–45P. [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hüfner M., Hesch R. D. A comparison of different compounds for TBG-blocking used in radioimmunoassay for tri-iodothyronine. Clin Chim Acta. 1973 Feb 28;44(1):101–107. doi: 10.1016/0009-8981(73)90165-4. [DOI] [PubMed] [Google Scholar]
- Landsberg L., Young J. B. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978 Jun 8;298(23):1295–1301. doi: 10.1056/NEJM197806082982306. [DOI] [PubMed] [Google Scholar]
- MYERS J. D., BRANNON E. S., HOLLAND B. C. A correlative study of the cardiac output and the hepatic circulation in hyperthyroidism. J Clin Invest. 1950 Aug;29(8):1069–1077. doi: 10.1172/JCI102338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menahan L. A., Wieland O. The role of thyroid function in the metabolism of perfused rat liver with particular reference to gluconeogenesis. Eur J Biochem. 1969 Aug;10(1):188–194. doi: 10.1111/j.1432-1033.1969.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Fineberg E. S. Starvation-induced alterations of circulating thyroid hormone concentrations in man. Metabolism. 1976 Jan;25(1):79–83. doi: 10.1016/0026-0495(76)90162-1. [DOI] [PubMed] [Google Scholar]
- Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Glucose-turnover values and futile-cycle activities obtained with 14C- and 3H-labelled glucose. Biochem J. 1979 Aug 15;182(2):565–575. doi: 10.1042/bj1820565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay I. D. Muscle dysfunction in hyperthyroidism. Lancet. 1966 Oct 29;2(7470):931–934. doi: 10.1016/s0140-6736(66)90536-8. [DOI] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- SATOYOSHI E., MURAKAMI K., KOWA H., KINOSHITA M., NOGUCHI K., HOSHINA S., NISHIYAMA Y., ITO K. MYOPATHY IN THYROTOXICOSIS. WITH SPECIAL EMPHASIS ON AN EFFECT OF POTASSIUM INGESTION ON SERUM AND URINARY CREATINE. Neurology. 1963 Aug;13:645–658. doi: 10.1212/wnl.13.8.645. [DOI] [PubMed] [Google Scholar]
- Sestoft L., Bartels P. D., Fleron P., Folke M., Gammeltoft S., Kristensen L. O. Influence of thyroid state on the effects of glycerol on gluconeogenesis and energy metabolism in perfused rat liver. Biochim Biophys Acta. 1977 Aug 25;499(1):119–130. doi: 10.1016/0304-4165(77)90234-3. [DOI] [PubMed] [Google Scholar]
- Singh S. P., Snyder A. K. Effect of thyrotoxicosis on gluconeogenesis from alanine in the perfused rat liver. Endocrinology. 1978 Jan;102(1):182–187. doi: 10.1210/endo-102-1-182. [DOI] [PubMed] [Google Scholar]
- Wahren J., Efendić S., Luft R., Hagenfeldt L., Björkman O., Felig P. Influence of somatostatin on splanchnic glucose metabolism in postabsorptive and 60-hour fasted humans. J Clin Invest. 1977 Feb;59(2):299–307. doi: 10.1172/JCI108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971 Dec;50(12):2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J. Quantitative aspects of blood flow and oxygen uptake in the human forearm during rhythmic exercise. Acta Physiol Scand Suppl. 1966;269:1–93. [PubMed] [Google Scholar]


