Abstract
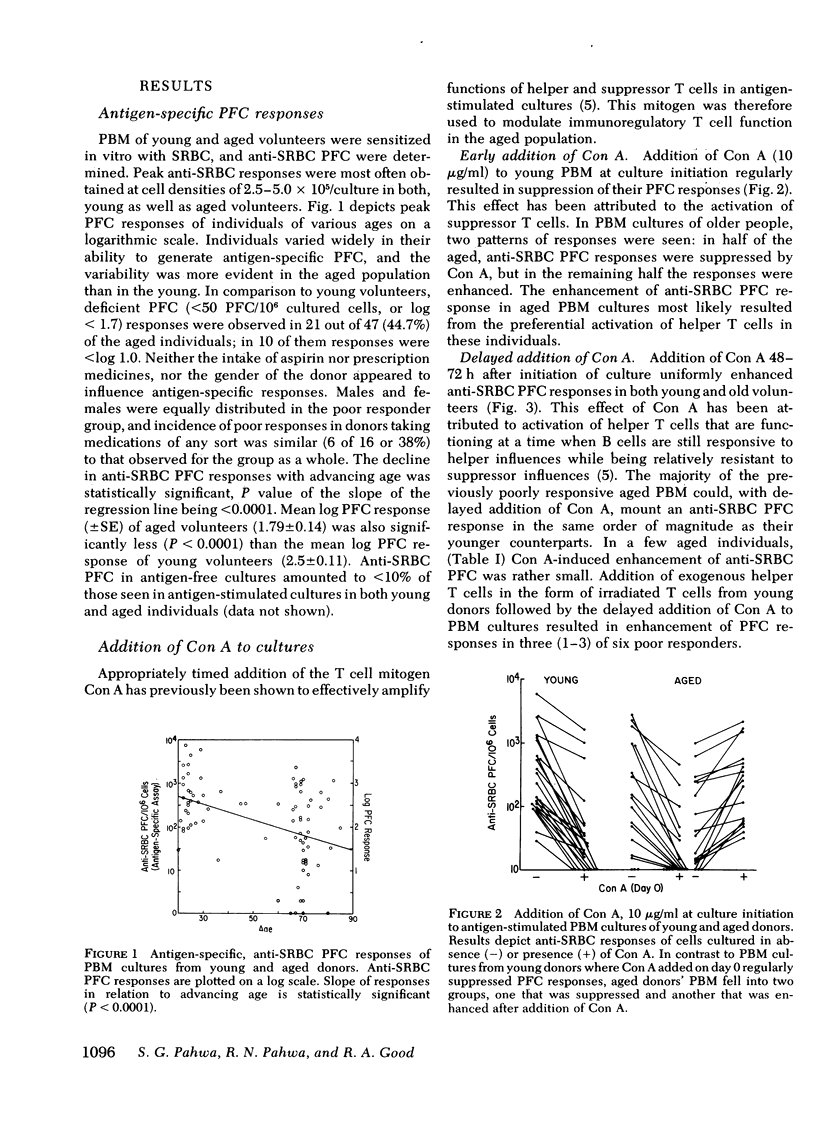
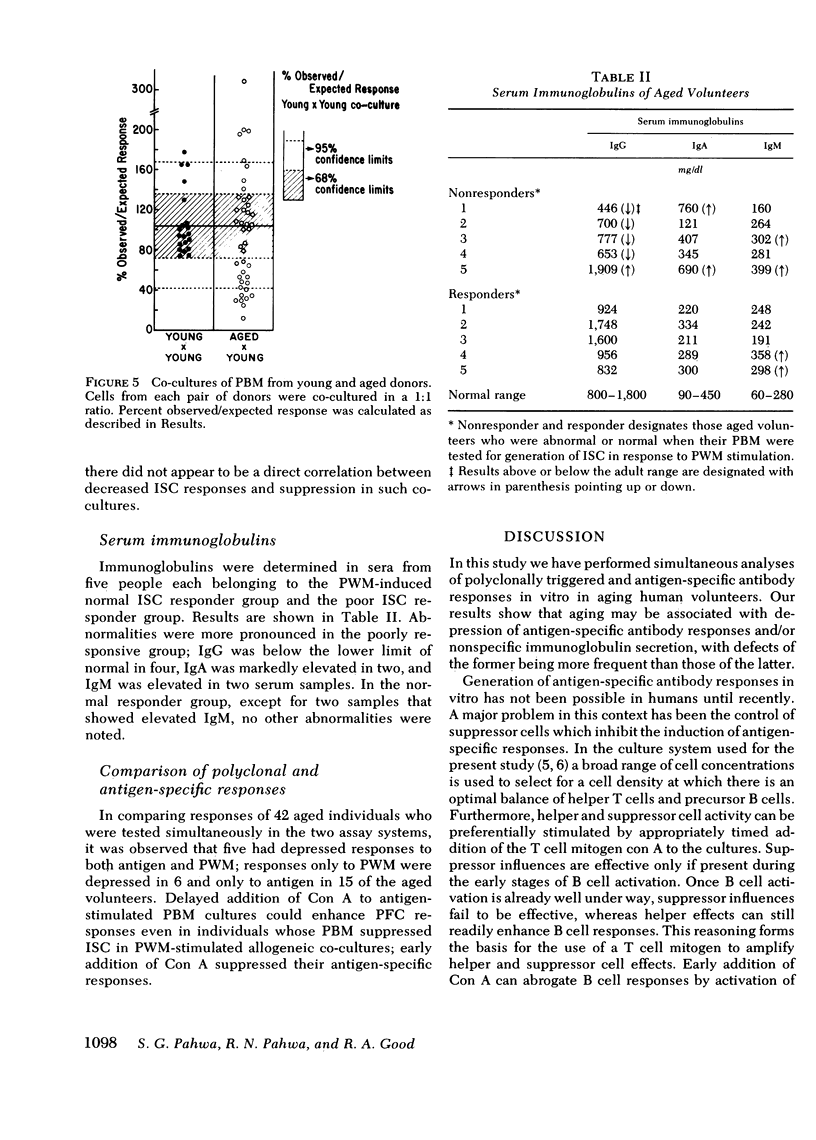
Induction of antigen-specific and non-specific (polyclonal) humoral immune responses in vitro was investigated in peripheral blood mononuclear cells of aged (65-85 yr) and young (20-30 yr) volunteers. In vitro immunization of lymphocytes with antigen (sheep erythrocytes) was performed in a recently described microculture system, and anti-sheep erythrocyte plaque forming cells were quantitated in a direct hemolytic plaque assay. Immunoglobulin secreting cells, induced polyclonally with pokeweed mitogen, were quantitated in a reverse hemolytic plaque assay. Significant depressions of antigen-specific as well as polyclonal responses were noted in relation to advancing age. Antigen-specific responses were more frequently depressed than polyclonal responses. T cell mitogen concanavalin A (Con A) was used to amplify functions of autologous immunoregulatory T cells. Addition of 10 microgram/ml Con A to lymphocytes of young donors at culture initiation resulted in activation of suppressor cells and abrogated antigen-specific responses. Delayed addition of Con A, on the other hand, enhanced responses, presumably because of activation of helper T cells. Similar manipulations of lymphocyte cultures from aged donors showed failure of Con A to suppress antigen-specific responses in approximately half of the responders. In many nonresponders, responses within normal range were elicited by the delayed addition of Con A to their lymphocyte cultures. Deviations beyond the range of expected responses were noted in 32.5% of the co-cultures between pokeweed mitogen stimulated young and aged cells. Our findings suggest that age-related deficiencies of B cell function are frequently associated with dysfunction of immunoregulatory T cells and are only occasionally due to intrinsic defects of B cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antel J. P., Weinrich M., Arnason B. G. Circulating suppressor cells in man as a function of age. Clin Immunol Immunopathol. 1978 Jan;9(1):134–141. doi: 10.1016/0090-1229(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Barrett D. J., Stenmark S., Wara D. W., Ammann A. J. Immunoregulation in aged humans. Clin Immunol Immunopathol. 1980 Oct;17(2):203–211. doi: 10.1016/0090-1229(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Basten A. Immune function in aged mice. IV. Loss of T cell and B cell function in thymus-dependent antibody responses. Eur J Immunol. 1978 Aug;8(8):552–558. doi: 10.1002/eji.1830080803. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Fazekas de St Groth B., Basten A., McKenzie I. F. Immune function in aged mice. V. Role of suppressor cells. J Immunol. 1980 Jan;124(1):52–58. [PubMed] [Google Scholar]
- Cantor H., Gershon R. K. Immunological circuits: cellular composition. Fed Proc. 1979 Jun;38(7):2058–2064. [PubMed] [Google Scholar]
- Cobleigh M. A., Braun D. P., Harris J. E. Age-dependent changes in human peripheral blood B cells and T-cell subsets: correlation with mitogen responsiveness. Clin Immunol Immunopathol. 1980 Feb;15(2):162–174. doi: 10.1016/0090-1229(80)90028-8. [DOI] [PubMed] [Google Scholar]
- Delfraissy J. F., Galanaud P., Dormont J., Wallon C. Age-related impairment of the in vitro antibody response in the human. Clin Exp Immunol. 1980 Jan;39(1):208–214. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Finelt M., Hoffmann M. K. A human monocyte function test: release of B-cell differentiation factor (BDF). Clin Immunol Immunopathol. 1979 Mar;12(3):281–288. doi: 10.1016/0090-1229(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Innes J. B., Weksler M. E. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976 Oct 1;144(4):1037–1048. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Subpopulations of human T lymphocytes. X. Alterations in T, B, third population cells, and T cells with receptors for immunoglobulin M (Tmu) or G (Tgamma) in aging humans. J Immunol. 1979 Apr;122(4):1214–1219. [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Heidrick M. L., Makinodan T. Presence of impairment of humoral immunity in nonadherent spleen cells of old mice. J Immunol. 1973 Nov;111(5):1502–1506. [PubMed] [Google Scholar]
- Hoffmann M. K. Antigen-specific induction and regulation of antibody synthesis in cultures of human peripheral blood mononuclear cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1139–1143. doi: 10.1073/pnas.77.2.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Fauci A. S. Inhibition of polyclonal B-cell activation by suppressor monocytes in patients with sarcoidosis. Clin Exp Immunol. 1978 Jun;32(3):554–562. [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Inomata K., Kotegawa S., Saito T., Kuroki M., Mitsuya H., Hisamitsu S. Age-related changes in the subsets and functions of human T lymphocytes. J Immunol. 1978 Nov;121(5):1773–1780. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Mitsuya H., Fujiwara H. Age-related changes in suppressor functions of human T cells. J Immunol. 1979 Oct;123(4):1586–1593. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Mitsuya H., Fujiwara H., Tsuda H. Age-related decline in the in vitro and in vivo syntheses of anti-tetanus toxoid antibody in humans. J Immunol. 1980 Nov;125(5):2347–2352. [PubMed] [Google Scholar]
- Makinodan T., Albright J. W., Good P. I., Peter C. P., Heidrick M. L. Reduced humoral immune activity in long-lived old mice: an approach to elucidating its mechanisms. Immunology. 1976 Dec;31(6):903–911. [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Heidrick M. L., Nordin A. A. Immunodeficiency and autoimmunity in aging. Birth Defects Orig Artic Ser. 1975;11(1):193–198. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Koski I., Dooley N., Blaese R. M. Artifactual plaque formation in vitro and in vivo to passive transfer of specific antibody. J Immunol. 1976 Apr;116(4):1016–1019. [PubMed] [Google Scholar]
- Naor D., Bonavida B., Robinson R. A., Shibata I. N., Percy D. E., Chia D., Barnett E. V. Immune response of New Zealand mice to trinitrophenylated syngeneic mouse red cells. Eur J Immunol. 1976 Nov;6(11):783–789. doi: 10.1002/eji.1830061106. [DOI] [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. I. Characterization of intrinsic deficiencies. J Immunol. 1972 Feb;108(2):403–412. [PubMed] [Google Scholar]
- Schrater A. F., Goidl E. A., Thorbecke G. J., Siskind G. W. Production of auto-anti-idiotypic antibody during the normal immune response to TNP-ficoll. I. Occurrence in AKR/J and BALB/c mice of hapten-augmentable, anti-TNP plaque-forming cells and their accelerated appearance in recipients of immune spleen cells. J Exp Med. 1979 Jul 1;150(1):138–153. doi: 10.1084/jem.150.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre D., Segre M. Humoral immunity in aged mice. II. Increased suppressor T cell activity in immunologically deficient old mice. J Immunol. 1976 Mar;116(3):735–738. [PubMed] [Google Scholar]
- Singhal S. K., Roder J. C., Duwe A. K. Suppressor cells in immunosenescence. Fed Proc. 1978 Apr;37(5):1245–1252. [PubMed] [Google Scholar]
- Waldmann T. A., Blaese R. M., Broder S., Krakauer R. S. Disorders of suppressor immunoregulatory cells in the pathogenesis of immunodeficiency and autoimmunity. Ann Intern Med. 1978 Feb;88(2):226–238. doi: 10.7326/0003-4819-88-2-226. [DOI] [PubMed] [Google Scholar]


