Abstract
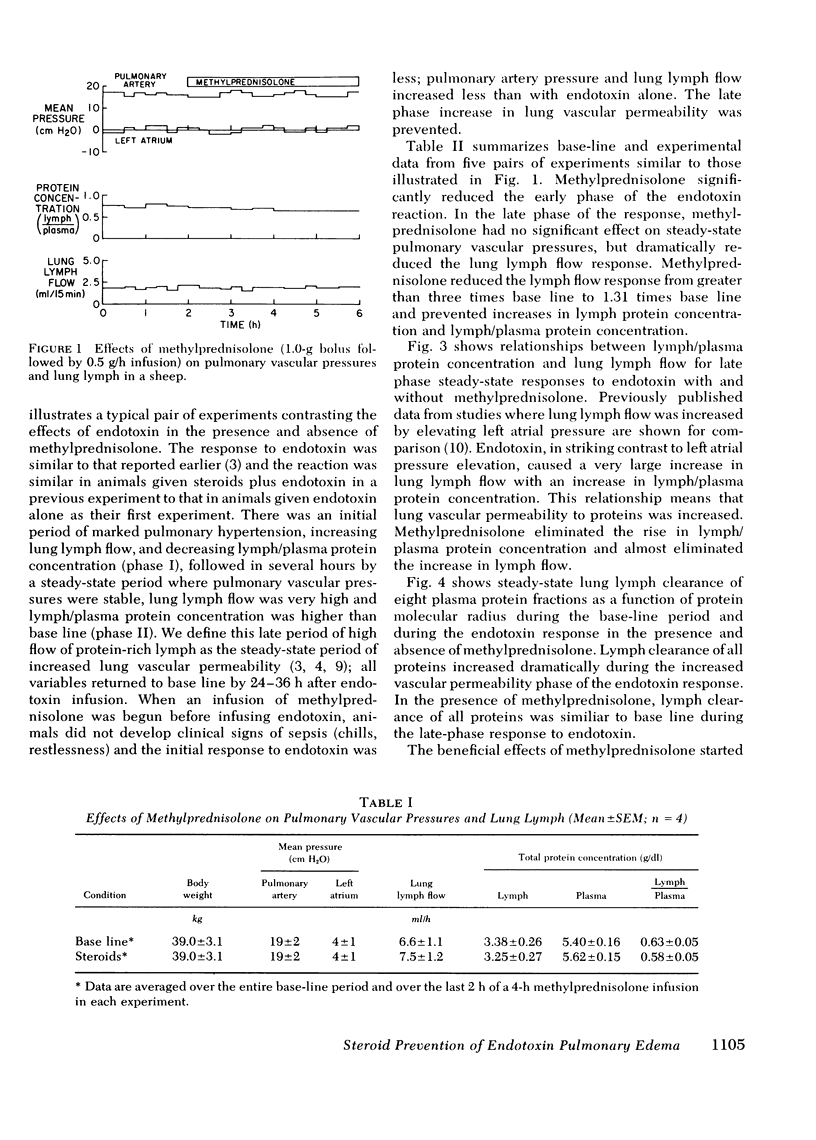
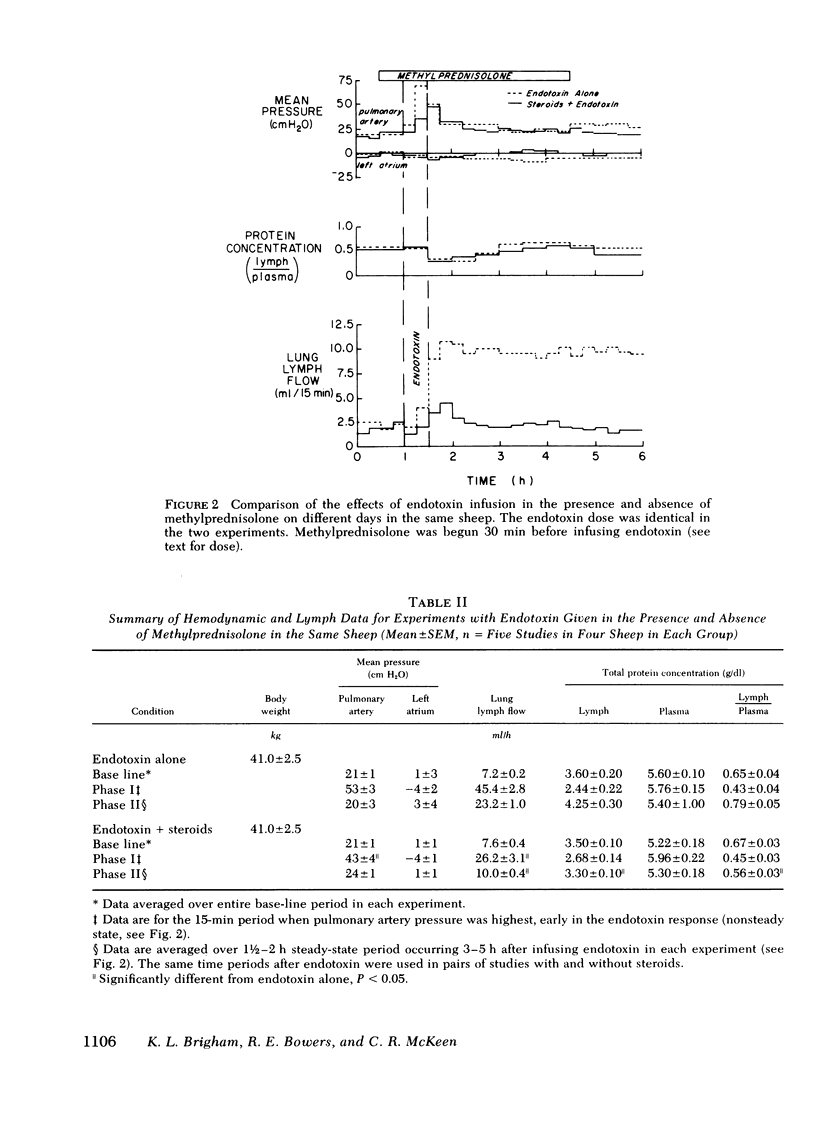
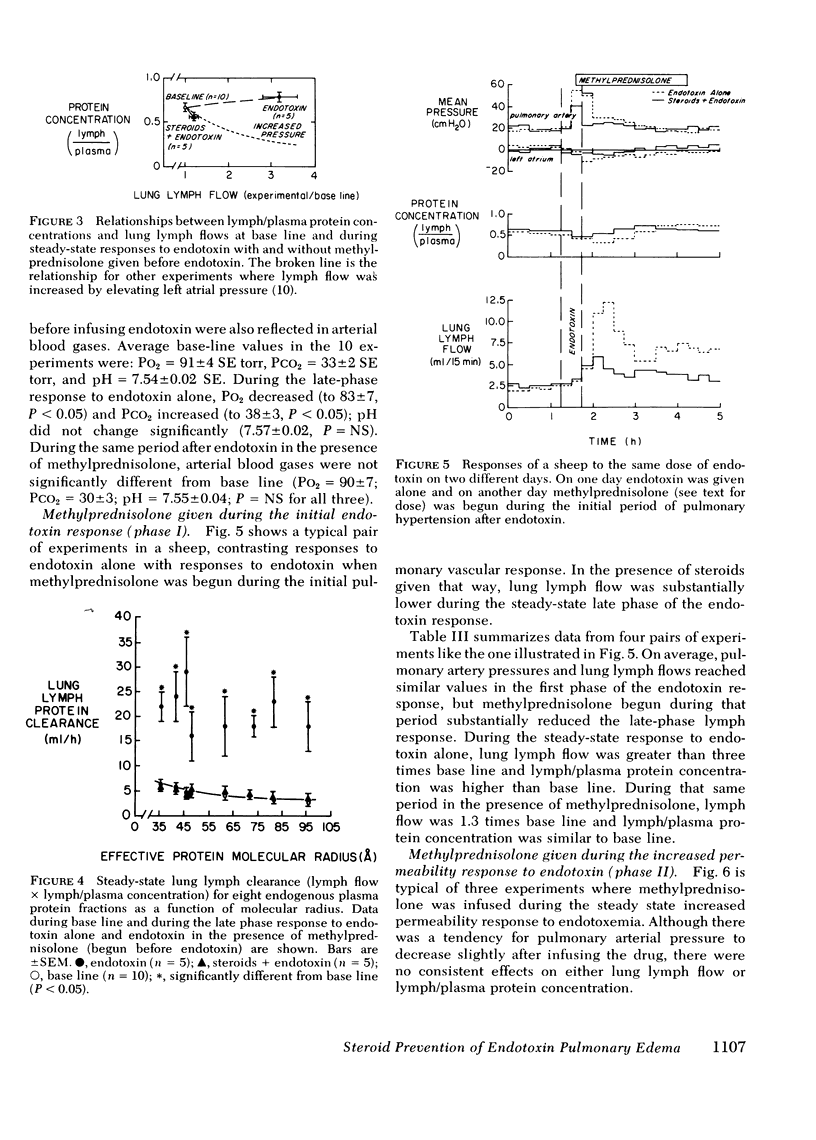
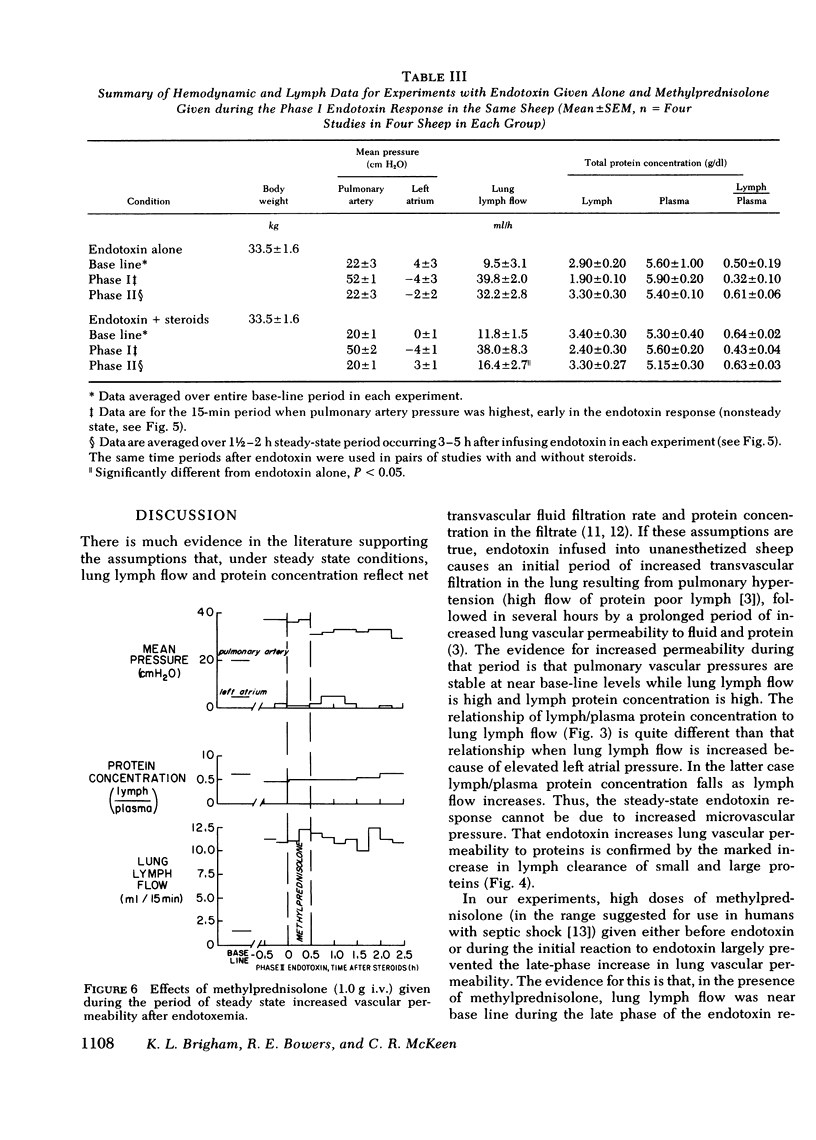
To see whether methylprednisolone would affect the pulmonary vascular response to endotoxemia, we studied responses to endotoxemia in the presence and absence of methylprednisolone in the same chronically instrumented, unanesthetized sheep. Infusion of Escherichia coli endotoxin (0.70-1.33 μg/kg) caused an initial period of marked pulmonary hypertension followed several hours later by a long period of increased vascular permeability when pulmonary vascular pressures were near base line (base-line pulmonary artery pressure (PPa) = 21±1 cm H2O SE, left atrial pressure (Pla) = 1±3; experimental PPa = 20±3, Pla = 3±4; P = NS), lung lymph flow (˙Qlym) was high (base-line ˙Qlym = 7.2±0.2 ml/h; experimental ˙Qlym = 23.2±1.0; P < 0.05) and lymph/plasma protein concentration (L/P) was high (base-line L/P = 0.65±0.04; experimental L/P = 0.79±0.05; P < 0.05). When methylprednisolone (1.0 g + 0.5 g/h i.v.) was begun 30 min before the same dose of endotoxin was infused, the initial pulmonary hypertension was less and the late phase increase in lung vascular permeability was prevented (experimental PPa = 24±1, Pla = 1±1, ˙Qlym = 10.0±0.4; L/P = 0.56±0.03). ˙Qlym and L/P were significantly (P < 0.05) lower than with endotoxin alone. Methylprednisolone began during the initial pulmonary hypertensive response to endotoxin also prevented the late phase increase in lung vascular permeability, but the drug had no effect once vascular permeability was increased. We conclude that large doses of methylprednisolone given before or soon after endotoxemia prevent the increase in lung vascular permeability that endotoxin causes, but do not reverse the abnormality once it occurs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigham K. L., Bowers R. E., Owen P. J. Effects of antihistamines on the lung vascular response to histamine in unanesthetized sheep. Diphenhydramine prevention of pulmonary edema and increased permeability. J Clin Invest. 1976 Aug;58(2):391–398. doi: 10.1172/JCI108483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Bowers R., Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979 Aug;45(2):292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Woolverton W. C., Blake L. H., Staub N. C. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974 Oct;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burka J. F., Flower R. J. Effects of modulators of arachidonic acid metabolism on the synthesis and release of slow-reacting substance of anaphylaxis. Br J Pharmacol. 1979 Jan;65(1):35–41. doi: 10.1111/j.1476-5381.1979.tb17331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer D. M., Niederhauser U., Piper P. J., Sirois P. Release of mediators of anaphylaxis: inhibition of prostaglandin synthesis and the modification of release of slow reacting substance of anaphylaxis and histamine. Br J Pharmacol. 1978 Jan;62(1):61–66. doi: 10.1111/j.1476-5381.1978.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisman S. E., DuBuy J. B., Woodward C. L. Experimental gram-negative bacterial sepsis: prevention of mortality not preventable by antibiotics alone. Infect Immun. 1979 Aug;25(2):538–557. doi: 10.1128/iai.25.2.538-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., White J. G., Craddock P. R., Jacob H. S. Corticosteroids inhibit complement-induced granulocyte aggregation. A possible mechanism for their efficacy in shock states. J Clin Invest. 1979 Apr;63(4):798–803. doi: 10.1172/JCI109365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Wu S. J., Celic L., Weaver L. J., Solliday N. Increased proportion of active sheep erythrocyte rosette-forming lymphocytes and plasma rosette enhancement in sarcoidosis. Am Rev Respir Dis. 1979 Mar;119(3):383–389. doi: 10.1164/arrd.1979.119.3.383. [DOI] [PubMed] [Google Scholar]
- McKeen C. R., Brigham K. L., Bowers R. E., Harris T. R. Pulmonary vascular effects of fat emulsion infusion in unanesthetized sheep. Prevention by indomethacin. J Clin Invest. 1978 May;61(5):1291–1297. doi: 10.1172/JCI109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaysen G., Nicolaysen A., Staub N. C. A quantitative radioautographic comparison of albumin concentration in different dized lymph vessels in normal mouse lungs. Microvasc Res. 1975 Sep;10(2):138–152. doi: 10.1016/0026-2862(75)90002-3. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Hammarström S. Nomenclature for leukotrienes. Prostaglandins. 1980 May;19(5):645–648. doi: 10.1016/0090-6980(80)90099-4. [DOI] [PubMed] [Google Scholar]
- Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976 Sep;184(3):333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Vreim C. R., Snashall P. D., Demling R. H., Staub N. C. Lung lymph and free interstitial fluid protein composition in sheep with edema. Am J Physiol. 1976 Jun;230(6):1650–1653. doi: 10.1152/ajplegacy.1976.230.6.1650. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Piper P. J. The action of chemically pure SRS-A on the microcirculation in vivo. Prostaglandins. 1980 May;19(5):779–789. doi: 10.1016/0090-6980(80)90174-4. [DOI] [PubMed] [Google Scholar]


